Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naffisah Othman | -- | 1114 | 2022-11-10 03:11:23 | | | |
| 2 | Vivi Li | -8 word(s) | 1106 | 2022-11-10 08:54:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Othman, N.; Ismail, Z.; Selamat, M.I.; Kadir, S.H.S.A.; Shibraumalisi, N.A. Polychlorinated Biphenyls (PCBs) Pollution in the Air. Encyclopedia. Available online: https://encyclopedia.pub/entry/33792 (accessed on 03 March 2026).
Othman N, Ismail Z, Selamat MI, Kadir SHSA, Shibraumalisi NA. Polychlorinated Biphenyls (PCBs) Pollution in the Air. Encyclopedia. Available at: https://encyclopedia.pub/entry/33792. Accessed March 03, 2026.
Othman, Naffisah, Zaliha Ismail, Mohamad Ikhsan Selamat, Siti Hamimah Sheikh Abdul Kadir, Nur Amirah Shibraumalisi. "Polychlorinated Biphenyls (PCBs) Pollution in the Air" Encyclopedia, https://encyclopedia.pub/entry/33792 (accessed March 03, 2026).
Othman, N., Ismail, Z., Selamat, M.I., Kadir, S.H.S.A., & Shibraumalisi, N.A. (2022, November 10). Polychlorinated Biphenyls (PCBs) Pollution in the Air. In Encyclopedia. https://encyclopedia.pub/entry/33792
Othman, Naffisah, et al. "Polychlorinated Biphenyls (PCBs) Pollution in the Air." Encyclopedia. Web. 10 November, 2022.
Copy Citation
Polychlorinated biphenyls (PCBs) were widely used in industrial and commercial applications, until they were banned in the late 1970s as a result of their significant environmental pollution. PCBs in the environment gained scientific interest because of their persistence and the potential threats they pose to humans. Traditionally, human exposure to PCBs was linked to dietary ingestion.
inhalation
indoor
outdoor
air
non-dietary
polychlorinated biphenyls
1. Introduction
Polychlorinated biphenyls (PCBs) are a group of toxic environmental pollutants categorized as persistent organic pollutants (POPs). The commercial production of PCBs started in 1929. Their main use was in electrical and hydraulic equipment and construction materials. Because of their significant adverse effects on human well-being and ecosystems, the manufacturing of PCBs was banned in the United States in 1979 under the US Toxic Substance Control Act [1]. Nevertheless, production continued elsewhere. As a result, the Stockholm Convention was established by the United Nations Environment Programme in May 2001, with the aim of eliminating PCB-containing products by 2028 in an environmentally sound manner [2]. Following this effort, the international total ban of PCBs came into effect on 17 May 2004.
There is still a long way to go, as only 3 million tons of PCB products had been eliminated as of 2015. Meanwhile, 80% of PCBs (17 million tons) are still in the environment, highlighting that the risk of exposure has not yet been eliminated [3]. Even with the production of PCBs banned in most countries, they continue to pose a threat due to their environmental persistence and bioaccumulation, raising global concerns. PCBs are still slowly and continuously being released by products that were manufactured before the ban and that are dumped as waste into the environment [4][5]. They are found in the ambient air and the food chain, and can be transmitted to humans through the ingestion of contaminated food products, inhalation, or transdermal exposure. PCBs have been detected in various food samples, and human exposure has been reported in several countries worldwide [6][7][8].
2. Polychlorinated Biphenyls (PCBs)
Polychlorinated biphenyls (PCBs) are a group of synthetic aromatic chemicals. They consist of a biphenyl structure that is bound to hydrogen and chlorine atoms according to the chemical formula C12HxCly, where x and y range from 1 to 10, and x + y = 10. The chemical structure of chlorinated biphenyls is shown in Figure 1. According to IUPAC, there are 209 PCB congeners, which differ by the number of hydrogen atoms substituted by chlorine atoms and their position on the biphenyl rings. This variance in PCB molecule determines the physical and chemical properties and toxicity.
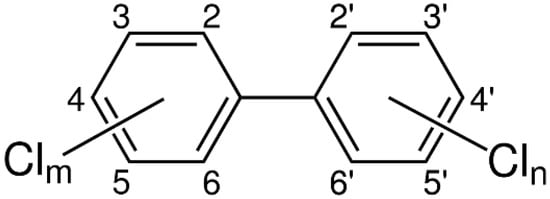
Figure 1. Chemical structure of PCBs (m and n denote number of chlorine atoms on each ring).
The phenyl rings can rotate around the C-C bond, but with an increasing number of chlorines in the ortho positions (2, 2, 6, 6), this rotation is hampered. With no ortho-chlorines, the two phenyls can align co-planarly [9]. The plane between the phenyls as the C-C bond attain increased double bond characteristics is stabilized by the aromatic system. However, with an increased number of chlorine atoms in the ortho position, the likelihood that planarity will be established decreases. This is significant for the toxicity of the individual congener, as the toxicity of co-planar non-ortho congeners have a “dioxin-like” toxicity [10]. Mono-ortho congeners may also be somewhat co-planar, but if there are two or more chlorines in the ortho position, co-planarity is not possible.
3. Why Concern about PCBs
PCBs are among the 12 initial POPs called the “dirty dozen” under the Stockholm Convention [11], listed in Annex A (elimination) and Annex C (unintentional production). They are of global concern due to their (1) persistence in the environment, (2) long-distance travel in the atmosphere, (3) bioaccumulation, and (4) biomagnification in the food chain. It is a global issue, as everyone is likely to have some amount of PCB in their body from either the ingestion of contaminated food, inhalation, or dermal exposure to a polluted environment. Thus, PCBs can significantly impact animal and human health and the environment.
The consumption of contaminated food is often regarded as the major source of human PCB exposure, and there have been limited studies on the role of air pollution as an inhalational pathway. Because of the high PCB concentrations in some animals, dietary exposure has traditionally been prioritized in studies over dermal and inhalation exposure. However, the inhalation route of PCB exposure is often overlooked. A study reported decreased PCB concentrations in food, highlighting that inhalation may be an essential route of exposure [12]. Airborne emissions of PCB may result in inhalation exposure levels comparable to, and occasionally more significant than, dietary ingestion [13].
Atmospheric PCB is a significant contributor to the total body burden, as evidenced by elevated blood levels of lower-chlorinated PCBs (dominant congeners) in the air by 40% among occupants of contaminated buildings [14]. For people living in buildings with significant PCB levels in the building materials, the bulk of their overall PCB burden comes from exposures in their homes, nearly 40 years after the Danish ban on the use of PCBs in construction products [15]. Following exposure, PCB can bioaccumulate and persist in the human body for up to 12 years [16]. Many lower-chlorinated congeners are likewise endocrine-disruptive [17] and carcinogenic [18]. This indicates that inhalation and dermal absorption are significant routes of exposure to PCBs in the air [19]. This entry discussed the occurrence of PCBs in the air as a source of inhalation exposure and the subsequent impacts on human health in light of the increased interest in this study area.
4. Occurrence of PCBs in the Air
The primary source of PCBs in the atmosphere is the volatilization of PCB-containing products in landfills that are disposed of as waste [20][21]. The widespread use of PCBs in commercial and industrial products and their inappropriate disposal have created severe environmental contamination. Today, PCBs can still be released into the environment from the following sources: (1) poorly maintained hazardous waste sites that contain PCBs; (2) illegal or improper dumping of PCB wastes into landfills that are not designed to handle hazardous waste; (3) accidental spills and leaks during the transport of the chemical; (4) leaks or fires from electrical transformers, capacitors, or other products containing PCBs; and (5) waste incineration and open burning in landfills that emit PCBs during the combustion process [1].
Once in the environment, PCBs do not readily break down. Instead, they undergo chemical biotransformation that allow them to continue cycling between air, water, and soil for an extended period. PCBs in the air are generally lower-chlorinated (≤5 chlorine atoms), and are mainly taken up by humans via inhalation and dermal resorption. In contrast to high-chlorinated congeners (>5 chlorine atoms), lower chlorinated PCBs exhibit a faster elimination rate from the body and a lower environmental persistence. Figure 2 shows the sources of PCB in the air, human exposure and possible health impacts.
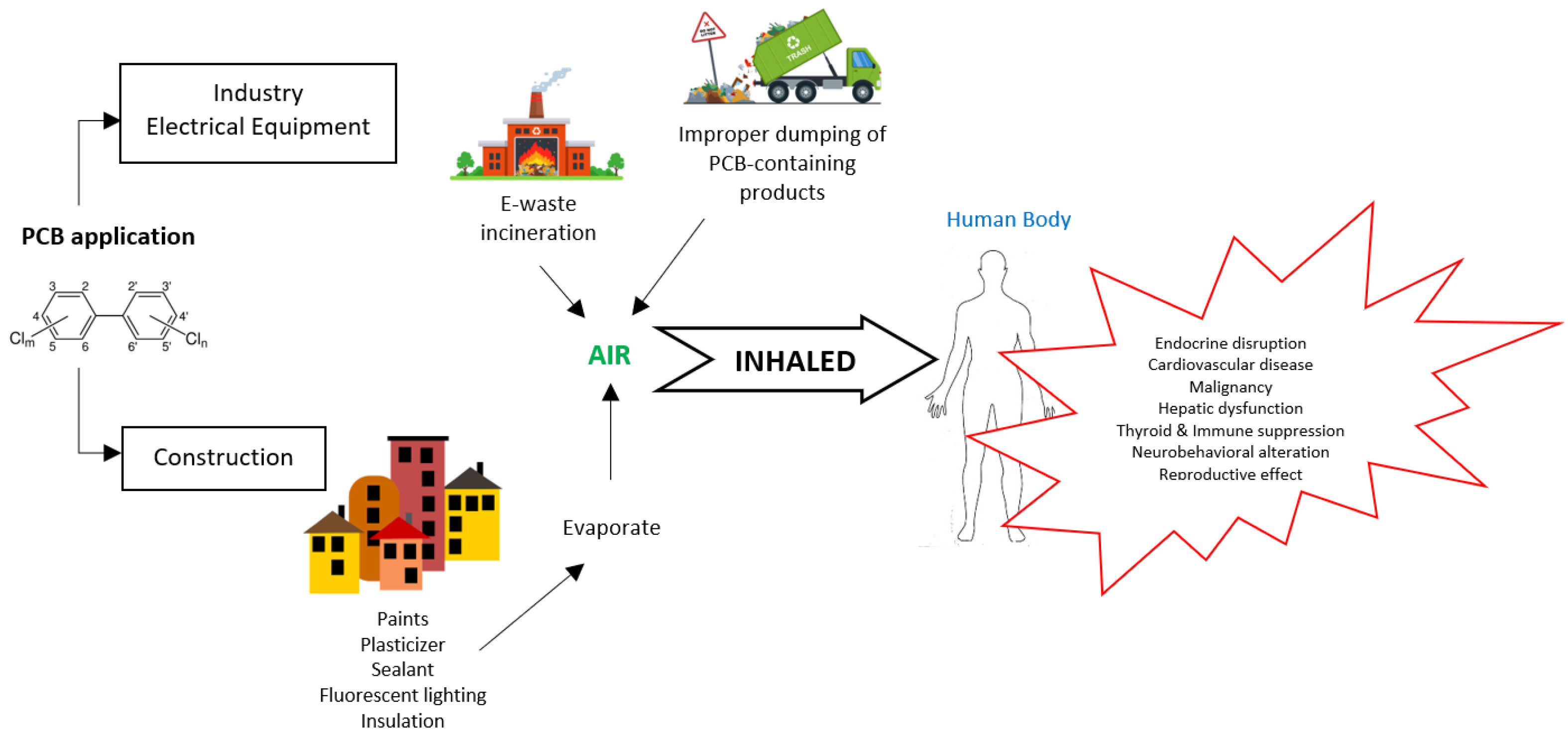
Figure 2. Sources of PCB in the air, human exposure, and possible health impacts.
References
- US EPA. Polychlorinated Biphenyls (PCBs). Available online: https://www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls-pcbs (accessed on 18 October 2021).
- UNEP. PCB a Forgotten Legacy? Available online: https://www.unep.org/explore-topics/chemicals-waste/what-we-do/persistent-organic-pollutants/pcb-forgotten-legacy (accessed on 18 October 2021).
- Stockholm Convention. PCB Overview. Available online: http://chm.pops.int/Implementation/IndustrialPOPs/PCB/Overview/tabid/273/Default.aspx (accessed on 18 October 2021).
- Yu, H.; Liu, Y.; Shu, X.; Ma, L.; Pan, Y. Assessment of the spatial distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban soil of China. Chemosphere 2020, 243, 125392.
- Peng, Y.; Wu, J.; Luo, X.; Zhang, X.; Giesy, J.P.; Mai, B. Spatial distribution and hazard of halogenated flame retardants and polychlorinated biphenyls to common kingfisher (Alcedo atthis) from a region of South China affected by electronic waste recycling. Environ. Int. 2019, 130, 104952.
- Harmouche-Karaki, M.; Mahfouz, Y.; Salameh, P.; Matta, J.; Helou, K.; Narbonne, J.F. Patterns of PCBs and OCPs exposure in a sample of Lebanese adults: The role of diet and physical activity. Environ. Res. 2019, 179 Pt B, 108789.
- Ravenscroft, J.; Schell, L.M. Patterns of PCB exposure among Akwesasne adolescents: The role of dietary and inhalation pathways. Environ. Int. 2018, 121 Pt 1, 963–972.
- Rusin, M.; Dziubanek, G.; Marchwinska-Wyrwal, E.; Cwielag-Drabek, M.; Razzaghi, M.; Piekut, A. PCDDs, PCDFs and PCBs in locally produced foods as health risk factors in Silesia Province, Poland. Ecotoxicol. Environ. Saf. 2019, 172, 128–135.
- ATSDR. Toxicological Profile for Polychlorinated Biphenyls (PCBs); ATSDR: Atlanta, GA, USA, 2014.
- ATSDR. Polychlorinated Biphenyls (PCBs) Toxicity; ATSDR: Atlanta, GA, USA, 2014.
- UNEP. Toward Elimination of PCB. Available online: https://www.unep.org/explore-topics/chemicals-waste/what-we-do/persistent-organic-pollutants/toward-elimination-pcb? (accessed on 18 October 2021).
- Saktrakulkla, P.; Lan, T.; Hua, J.; Marek, R.F.; Thorne, P.S.; Hornbuckle, K.C. Polychlorinated Biphenyls in Food. Environ. Sci. Technol. 2020, 54, 11443–11452.
- Norstrom, K.; Czub, G.; McLachlan, M.S.; Hu, D.; Thorne, P.S.; Hornbuckle, K.C. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ. Int. 2010, 36, 855–861.
- Egsmose, E.L.; Bräuner, E.V.; Frederiksen, M.; Mørck, T.A.; Siersma, V.D.; Hansen, P.W.; Nielsen, F.; Grandjean, P.; Knudsen, L.E. Associations between plasma concentrations of PCB 28 and possible indoor exposure sources in Danish school children and mothers. Environ. Int. 2016, 87, 13–19.
- Meyer, H.W.; Frederiksen, M.; Goen, T.; Ebbehoj, N.E.; Gunnarsen, L.; Brauer, C.; Kolarik, B.; Muller, J.; Jacobsen, P. Plasma polychlorinated biphenyls in residents of 91 PCB-contaminated and 108 non-contaminated dwellings-an exposure study. Int. J. Hyg. Environ. Health 2013, 216, 755–762.
- Esser, A.; Ziegler, P.; Kaifie, A.; Kraus, T.; Schettgen, T. Estimating plasma half-lives of dioxin like and non-dioxin like polychlorinated biphenyls after occupational exposure in the German HELPcB cohort. Int. J. Hyg. Environ. Health 2021, 232, 113667.
- Aminov, Z.; Haase, R.; Rej, R.; Schymura, M.J.; Santiago-Rivera, A.; Morse, G.; DeCaprio, A.; Carpenter, D.O. Diabetes Prevalence in Relation to Serum Concentrations of Polychlorinated Biphenyl (PCB) Congener Groups and Three Chlorinated Pesticides in a Native American Population. Environ. Health Perspect. 2016, 124, 1376–1383.
- Lerro, C.C.; Jones, R.R.; Langseth, H.; Grimsrud, T.K.; Engel, L.S.; Sjodin, A.; Choo-Wosoba, H.; Albert, P.; Ward, M.H. A nested case-control study of polychlorinated biphenyls, organochlorine pesticides, and thyroid cancer in the Janus Serum Bank cohort. Environ. Res. 2018, 165, 125–132.
- Schettgen, T.; Alt, A.; Preim, D.; Keller, D.; Kraus, T. Biological monitoring of indoor-exposure to dioxin-like and non-dioxin-like polychlorinated biphenyls (PCB) in a public building. Toxicol. Lett. 2012, 213, 116–121.
- Li, M.; Zhou, Y.; Wang, G.; Zhu, G.; Zhou, X.; Gong, H.; Sun, J.; Wang, L.; Jinsong, L. Evaluation of atmospheric sources of PCDD/Fs, PCBs and PBDEs around an MSWI plant using active and passive air samplers. Chemosphere 2021, 274, 129685.
- Zhu, M.; Yuan, Y.; Yin, H.; Guo, Z.; Wei, X.; Qi, X.; Liu, H.; Dang, Z. Environmental contamination and human exposure of polychlorinated biphenyls (PCBs) in China: A review. Sci. Total Environ. 2021, 805, 150270.
More
Information
Subjects:
Meteorology & Atmospheric Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
15 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





