Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ram Krishna | -- | 3922 | 2022-11-09 11:36:41 | | | |
| 2 | Rita Xu | Meta information modification | 3922 | 2022-11-10 02:38:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Krishna, R.; Ansari, W.A.; Soumia, P.S.; Yadav, A.; Jaiswal, D.K.; Kumar, S.; Singh, A.K.; Singh, M.; Verma, J.P. Biotechnological Interventions in Tomato for Drought Stress Tolerance. Encyclopedia. Available online: https://encyclopedia.pub/entry/33738 (accessed on 07 February 2026).
Krishna R, Ansari WA, Soumia PS, Yadav A, Jaiswal DK, Kumar S, et al. Biotechnological Interventions in Tomato for Drought Stress Tolerance. Encyclopedia. Available at: https://encyclopedia.pub/entry/33738. Accessed February 07, 2026.
Krishna, Ram, Waquar Akhter Ansari, P. S. Soumia, Akhilesh Yadav, Durgesh Kumar Jaiswal, Sudhir Kumar, Achuit Kumar Singh, Major Singh, Jay Prakash Verma. "Biotechnological Interventions in Tomato for Drought Stress Tolerance" Encyclopedia, https://encyclopedia.pub/entry/33738 (accessed February 07, 2026).
Krishna, R., Ansari, W.A., Soumia, P.S., Yadav, A., Jaiswal, D.K., Kumar, S., Singh, A.K., Singh, M., & Verma, J.P. (2022, November 09). Biotechnological Interventions in Tomato for Drought Stress Tolerance. In Encyclopedia. https://encyclopedia.pub/entry/33738
Krishna, Ram, et al. "Biotechnological Interventions in Tomato for Drought Stress Tolerance." Encyclopedia. Web. 09 November, 2022.
Copy Citation
Tomato production is severely affected by abiotic stresses (drought, flood, heat, and salt) and causes approximately 70% loss in yield depending on severity and duration of the stress. Drought is the most destructive abiotic stress and tomato is very sensitive to the drought stress, as cultivated tomato lack novel gene(s) for drought stress tolerance. Only 20% of agricultural land worldwide is irrigated, and only 14.51% of that is well-irrigated, while the rest is rain fed. This scenario makes drought very frequent, which restricts the genetically predetermined yield. Primarily, drought disturbs tomato plant physiology by altering plant–water relation and reactive oxygen species (ROS) generation.
drought
tomato
Solanum lycopersicum
transgenic
1. Introduction
Cultivated tomato, Solanum lycopersicum [1] (old name: Lycopersicon esculentum Mill), originated and domesticated in Western South America and Central America from Mexico to Peru [2]. Tomato is a member of division Magnoliophyte, class Magnoliopsida, sub-class Asteridae, order Solanales, and family Solanaceae. Tomato is unquestionably the most popular garden crop worldwide [3], botanically classified as fruit (berry) but often consumed as vegetable (raw, cooked and/or in processed form), next to potato [4][5]. Although the cultivated tomato is a tropical plant, it requires moderate growing conditions and is widely adapted to different climates, thus making it cosmopolitan. China, USA, India, Turkey, Egypt, and Italy are some of the major tomato-producing countries [5]. India ranks third among the top tomato-producing countries, and it is cultivated in 6.34 million ha, with a total production of 12,433,200 tonnes and average productivity of 19,598 kg/ha [5]. The largest tomato-producing states in India include Maharashtra, Bihar, Karnataka, Uttar Pradesh, Odisha, Andhra Pradesh, Madhya Pradesh, and Assam [6]. Worldwide, more than a thousand cultivars of tomato are grown, selected based on fruit size, shapes, and growth pattern in different environments [3]. Plants of tomato are diploid (2n = 2x = 24), determinate to indeterminate growth habit, herbaceous with bisexual flowers, annual to perennial, self-pollinated, and are commonly propagated by seeds. Generally, seedlings of 4–5 weeks old are transplanted, prior to which hardening should be performed by withholding water for up to 4–5 days [7][8]. With proper water supply, tomato can be cultivated in different soil types, such as clay, black soil, and red soil; nonetheless, organic-matter-rich sandy loam soil is ideal. Tomato can also tolerate moderate saline and acidic soils. Temperature of 15–27 °C is found to be optimum for its cultivation; however, day temperatures exceeding 38 °C may adversely affect fruit set. In general, tomato is a self-pollinated crop; however, 30% cross-pollination has been recorded in some cases [8][9]. Most tomato genotypes have compound leaves, pinnately dissected with 5–9 leaflets on petioles. The inflorescence is a cyme with monochasial or dichotomous branching patterns. Flowers are bisexual and usually yellow in colour; the male part of flower is the stamens, consisting of anther borne on a filament. Anthers join laterally to form a flask-shaped cone with a prolonged sterile tip at the apex. The female flower part is the pistil, consisting of stigma, style, and ovary, and is situated in the flower’s centre, encircled by the stamens. Though tomato is commonly classified as a vegetable, it is really a fruit; berry. Most cultivated varieties, except cherry tomatoes, are either bilocular or multilocular with 4–5 locules [10]. Fruits come in a variety of shapes (globose, round, oval, flattened, elongated, or heart-shaped) and colours (golden, yellow, red, pink, purple, green, striped to white) [11]. Tomato fruits are rich in vitamin A, vitamin C, vitamin E, potassium, dietary fibre, β-carotene, and lycopene. Upon ripening, fruits develop a characteristic deep-red colour due to the pigment lycopene [12]. Among dietary carotenoids, lycopene is one of the most powerful antioxidants that protects against free radicals and is also known to prevent cancer [12]. Besides its nutritional and therapeutic properties, tomato is used for its distinct flavour and as a food colourant. Tomato fruits are also used to make a range of value-added products, such as whole-peeled tomatoes, diced products, paste, sauce, juice, and soups [13][14]. Tomato’s projected genome size is 900 megabases (Mb), containing 34,727 protein-coding genes [15]. Genes are mostly located in the euchromatin region, which accounts for less than a quarter of total genomic DNA [16]. Relatively small genome size makes tomato a genetic model for crop improvement [8] and, also, a suitable system for studying functional genomics, proteomics, and metabolomics. Further, it has been widely used for studies of various biotic and abiotic responses due to availability of various resistant genes and adaptation to a wide environmental condition and photoperiod insensitivity [17][18][19][20][21]. The tomato genome consortium sequenced the whole tomato genome as a key model plant for speeding genomic research in the Solanaceae family because it has the same haploid set of chromosomes and conserved genome structure (a high level of synteny) as other Solanaceous plants. Tomato was the first transgenic food (Flavr Savr Tomato) crop released for commercial cultivation in 1992 in U.S.A., genetically engineered to increase the shelf-life of fruits [22]. Being a model crop for molecular study and genetic manipulation, tomato is the highest studied crop among the vegetable crops. To date, numerous transgenes were transferred in tomato and evaluated against the biotic (virus, bacteria, fungi, and insect) and abiotic (drought, salt, heat, cold, and water logging) stress tolerance and quality improvement. Among abiotic stresses, drought is the most important stress. Drought is the inadequacy of water, which restricts tomato growth and yield [14][23]. Globally, only 14.51 percent agricultural land is well irrigated and the rest is moderately to highly drought susceptible (FAO, 2012). Drought stress primarily affects the crops by generating reactive oxygen species (ROS), which disturbs the cellular homeostasis [14][23]. In order for normal growth and development, crop must constantly adapt to climatic conditions. The water loss management in crop and detoxification of ROS are the key strategies in drought stress management. In tomato, for drought stress management, numerous transgenic tomatoes were developed.
2. Drought Stress
Any altered physiological condition that tends to disrupt plants’ homeostatic equilibrium is termed as stress [9][13][19][24][25]. Homeostasis is, in turn, a fixed value for metabolism under standard conditions, which is hardly attained by plants, as most of them are exposed to various types of stresses [24]. Drought, heat, cold, salt, high temperature and light, oxidative stress, heavy metal toxicity, radiations, and UV light are some of the constant threats to plants worldwide. The effect of biotic and abiotic factors on plants is determined by the quantity, intensity, duration, and methods of application on plants. Furthermore, plants have evolved a wide range of physiological and biochemical changes to fit and adapt to a variety of environmental challenges. Nevertheless, globally abiotic stresses are considered as the primary factor for the crop loss which roughly estimates to ~70% of yield reduction [2][24][25]. Water deficit is the most critical abiotic stress that affects many physiological parameters of plants, particularly photosynthetic capability. During drought, dehydration of the plant cells and tissues causes diminished plant growth and productivity (Figure 1). Thus, water deficit works as a limiting factor in agriculture for production by inhibiting the crop from performing its genetic yield potential [20]. Drought is characterised by inadequate availability of water, due to precipitation variability, evaporation, and soil moisture holding capacity; high water requirement by plants or a combination of all these factors restricts the plants from achieving their full genetic potential. Metrologically, drought is described as a period of below-normal precipitation that limits plant productivity in an agricultural or natural system [26]. Nearly two thirds of geographical area of India receives low (>1000 mm) or erratic rainfall. Out of 140 million hectares of cultivated area, about 68% is vulnerable to drought stress and ~34% is classified as “severe”, where the drought frequency is nearly periodic. Drought stress affects around 68% of the 140 million hectares of cultivated land, with ~34% categorised as “severe”, indicating the drought frequency being nearly periodic. Among the abiotic stresses, drought is the most severe and pervasive environmental factor [9][13][24]. Plants do expedite the detoxification and repair processes in response to stress (Figure 2), which may include alterations in the expression of ROS scavenging enzymes, late embryogenesis abundant (LEA)/dehydrin-type genes, and the synthesis of molecular chaperones, proteinases, and other detoxification proteins [9][27][28].
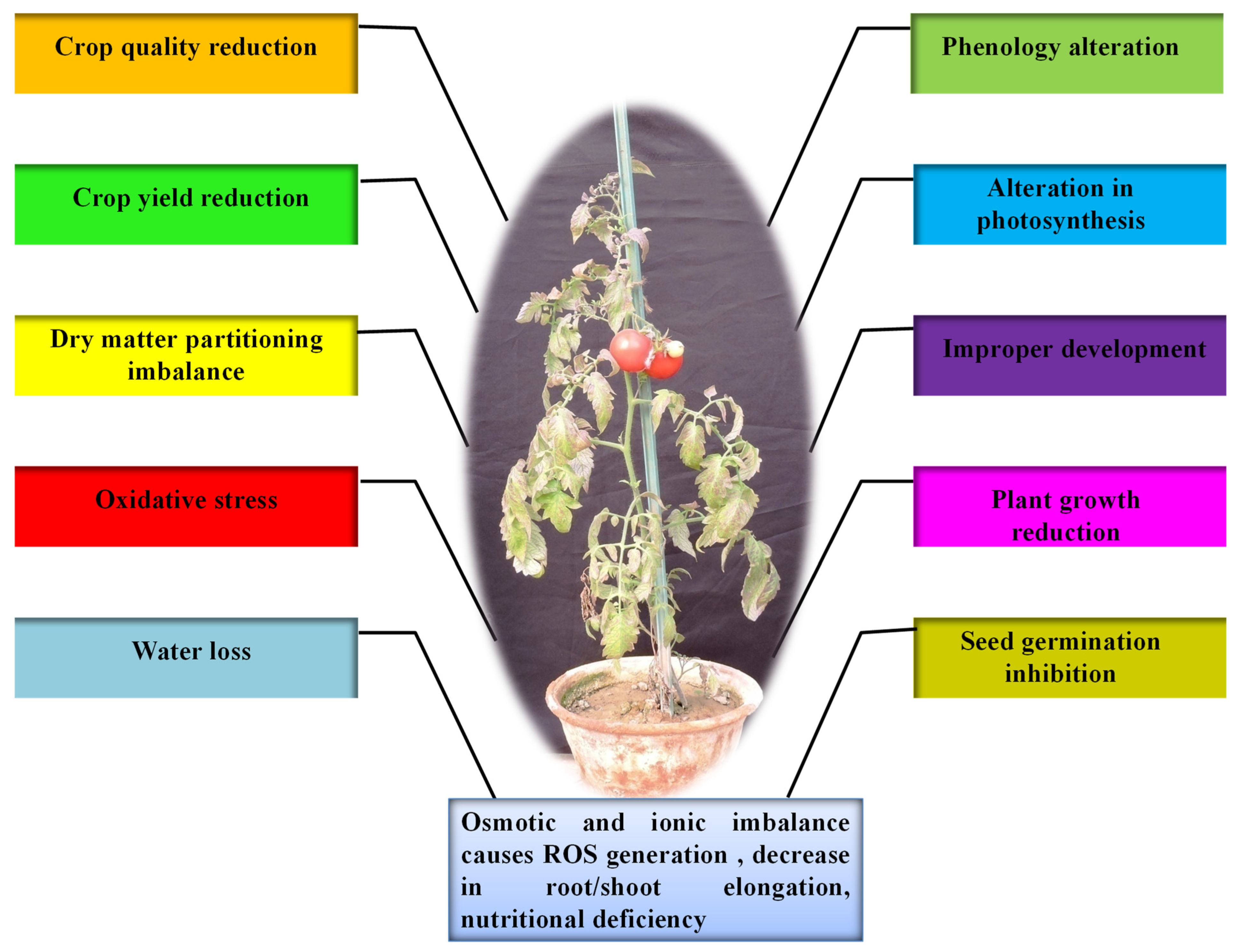
Figure 1. The overall impact of drought stress on tomato plant growth.
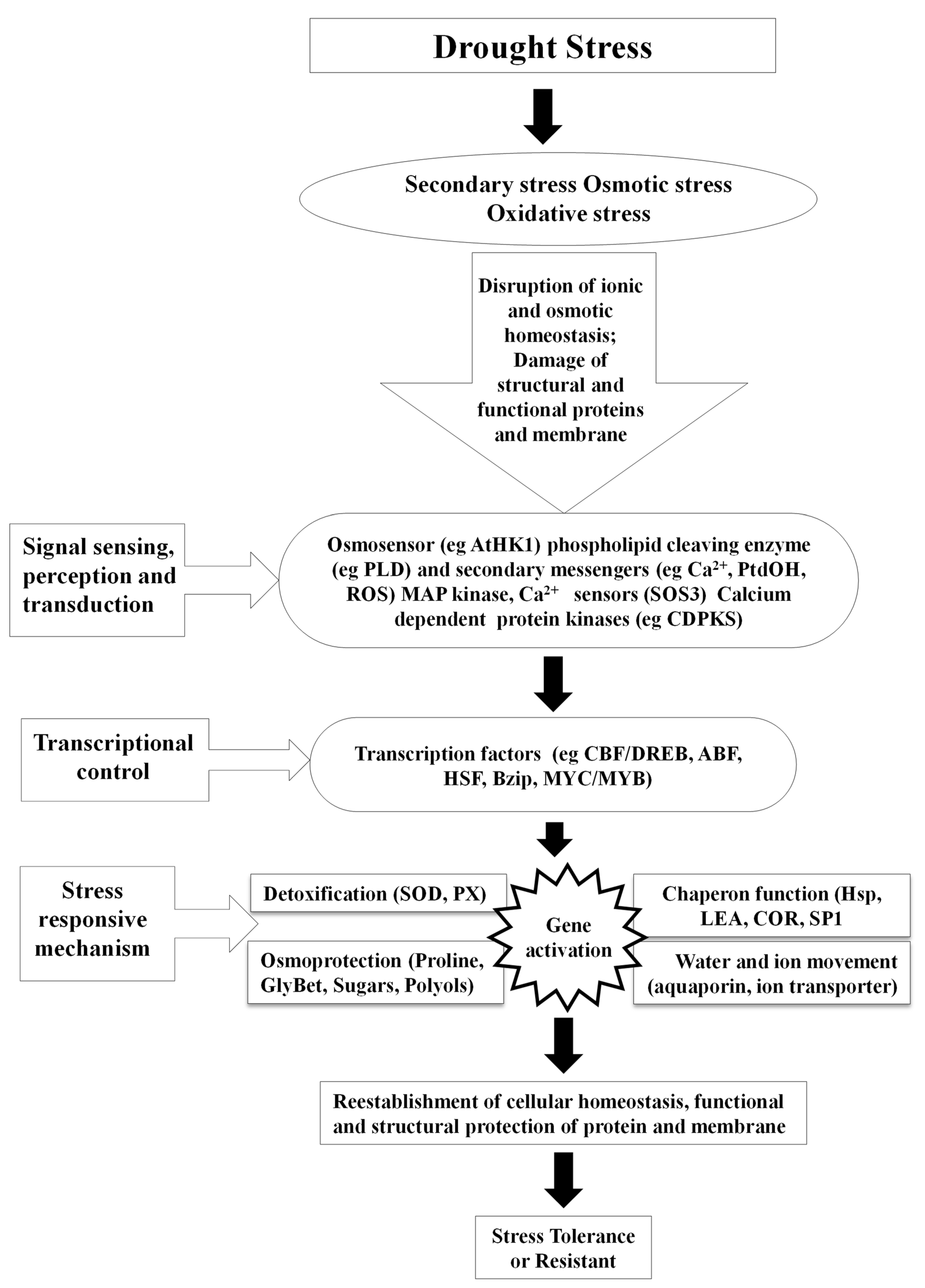
Figure 2. Mechanism involved in drought stress tolerance.
2.1. Drought-Responsive Transcription Factor Genes in Tomato
Drought stress causes a rise in the levels of endogenous ABA in plants [29], and a concomitant altered numerous gene expression [30] in cultivated tomato leaves. Wild relatives of S. lycoperscum are resistant to many biotic and abiotic stresses. S. pennellii (syn. L. pennellii), a wild relative of tomato, grows and reproduces with minimal water, higher water use efficiency, and resistance to wilt [31]. Martin and Thorstenson [32] used carbon isotope composition and season-long water use efficiency measures to confirm drought resistance in S. pennellii. Kahn et al. [33] discovered and isolated four tomato genes (le4, le16, le20, and le25) in response to increased levels of endogenous ABA. Kahn et al. [33] investigated the expression of these genes in S. pennellii to gain a better idea of their function during drought stress. All four ABA-sensitive S. lycopersicum genes had homologous counterparts in S. Pennellii. In S. pennellii, the increase usually occurs after a prolonged duration of drought compared to that in S. lycopesocum.
However, only three of the four genes (le76, le20, and le2.5) were expressed in S. pennellii in response to exogenous ABA treatment. The expression patterns of these genes in S. pennellii were usually parallel to those in S. lycopersicum, indicating a similar but undetermined function in both genotypes rather than having genes that are responsible for improved drought tolerance in S. pennellii. Members of the abscisic-acid-responsive element binding protein (AREB)/abscisic acid element binding factor (ABF) subfamily of basic leucine zipper (bZIP) transcription factors have been involved in the response to ABA and abiotic stress. In Arabidopsis, AREB1 and AREB2 functional evaluation against ABA, drought, and salinity stress conditions are revealed [34][35]; however, the tomato AREB function and transcriptional regulation remain unknown [36]. Yaez et al. [37] identified a basic leucine zipper (bZIP) transcription factor in Solanum lycopersicum (SlAREB1), Solanum chilense (ScAREB1), and Solanum peruvianum (SpAREB1). The three proteins’ deduced amino acid sequences were 97% similar and exhibited strong homology with the ABF/AREB subfamily of transcription factors which was demonstrated in other plant species, such as Arabidopsis (ABF2, 54% similar) and tobacco (PHI-2, 50% similar). The induced SlAREB1 expression was reported in S. lycopersicum by abscisic acid, cold, and drought. For a quick investigation of genes controlled by SlAREB1 in tomato and tobacco, a simple transient expression assay using Agrobacterium-mediated transformation was performed. Tobacco leaves expressing SlAREB1 exhibited upregulation of stress-responsive genes, such as rd29B, LEA ERD10B, TAS14, PHI-2, and trehalose-6-phosphate phosphatase [37]. These findings imply that bZIP has a function in abiotic stress response in Solanum species. In the case of S. lycopersicum, [38] Orellana et al. identified SlAREB1 and SlAREB2 are induced in leaf as well as in root tissue by drought and salinity, but the effect on SlAREB1 was more than that on SlAREB2. The SlAREB1 overexpression in transgenic tomato plants improved salt and water stress tolerance compared to non-transgenic tomato plants. SlAREB1 target gene microarray and cDNA-amplified fragment length polymorphism (AFLP) analyses revealed that genes coding for oxidative-stress-related proteins, lipid transfer proteins (LTPs), transcription regulators, and LEA proteins were found to be upregulated in SlAREB1-overexpressing tomato lines, especially in aerial tissue. The findings indicate that bZIP transcription factor (SlAREB1) is associated with ABA signal pathway that contributes to abiotic stress, as well as in pathogen response [38]. Hsieh et al. [36] found AREB gene, a SlAREB transcription factor, using a tomato cDNA microarray. Electrophoretic mobility shift tests revealed that the chimeric DNA-binding domain of SlAREB proteins can bind to the promoter regions of Atrd29A and SlLAP. However, an ABA-dependent post-translational alteration is required for the SlAREB protein trans-activation [36].
SlAREB constitutive expression improves tolerance to drought and salt stressors in Arabidopsis and tomato plants, preserving PSII, membrane integrity, and water availability in plants. Stress-related genes AtCOR47, Atrd29A, and SlCI7-like dehydrin were regulated by SlAREB overexpression in A. thaliana and tomato plants subjected to abiotic and ABA stress. Hsieh et al. [36] found that SlAREB regulates some genes involved in stress response and its overexpression improves plant tolerance to drought and salt stress. Islam and Wang [39] isolated full-length LeDREB3 cDNA from tomato, investigated the pattern of expression in tomato under various abiotic stresses, and suggested that the LeDREB3 gene may be responsible for tomato plant stress tolerance. LeDREB3 is present in duplicate copies in the tomato genome and has considerable sequence match to DREB proteins belonging to various species of plant family. The LeDREB3 constitutive expression reported in all the tested organs, which was especially higher in flower. Drought, low temperature, salt, and H2O2 were shown to probably enhance LeDREB3 expression, which is expected to provide tolerance to these stresses. However, they did not evaluate its constitutive transgenic expression in tomato or any other plant species.
Investigating the ethylene response factor (ERF) protein demonstrates how it affects the expression of downstream genes in plants under stress by interacting with the GCC box, dehydration-responsive element, and C-repeat [40]. By inhibiting antisense TERF2/LeERF2, the ethylene-inducible ERF protein TERF2/LeERF2 demonstrates the positive control of ethylene production in tomatoes [41]. According to Zhang and Huang [40], increased freeze tolerance in tomato and tobacco is associated with the control of TERF2/LeERF2, and cold gradually triggers the expression of TERF2/LeERF2. Similar to Arabidopsis CBF1–3 genes, tomato encodes LeCBF1–3 (L. esculentum CBF1–3), three homologs that are tandemly present within the genome [42]. However, only tomato LeCBF1 gene is reported to be induced by cold. LeCBF1-3 transcripts did increase in response to mechanical agitation, similar to Arabidopsis CBF1-3, but not in response to ABA, drought, or high salt. LeCBF1 is a homolog of the functional proteins CBF1-3 in Arabidopsis, and its constitutive overexpression in transgenic Arabidopsis increased the expression of CBF-targeted genes and improved tolerance to freezing. Tomato harbours an absolute CBF cold response pathway, according to Zhang et al. [43]. However, the tomato CBF regulon differs from that of Arabidopsis and seems to be substantially smaller and less functionally diverse. A number of dehydration-responsive elements-binding proteins (DREBs) in plants have been identified, and it has been suggested that both abiotic and biotic stressors can activate them. Recently, Guo and Wang [44] isolated a DREB gene from tomato and designated it as LeDREB2.
LeDREB2 is classified in DREB family with the AP2 group. Being a single-copy gene of the tomato genome, it expresses strongly in young leaves and roots but shows weak expression in shoots and mature leaves. Various environmental stresses, such as cold and draught, induce the transcription of LeDREB2. Different studies demonstrate the expression analysis inducing the transcription of LeDREB2 gene due to various stresses (H2O2, salt, ABA, and methyl viologen) in tomato. Guo and Wang [44] found that LeDREB2 gene is a DREB transcription factor involved with oxidative and abiotic stress in tomato. Recently, Li et al. [45] cloned SlDREB from cultivated tomato M82, a transcription factor, and found that it plays a negative role in architecture of tomato plant, and elevates tolerance against drought. Expression profiles indicate that SlDREB expression is mainly expressed in leaf and stem and is unable to induce by abscisic acid (ABA) but is suppressed by ethylene and GA. The activity of yeast assay exhibited that SlDREB exclusively binds to dehydration responsive element/C-repeat (DRE/CRT) of the SlCPS promoter region. When constitutively overexpressed, the SlDREB altered plant morphology by regulating leaf and internode elongation, and the consequent dwarfism of tomato plants could be reversed by adding gibberellic acid (GA3) exogenously. The findings of Hsieh et al. [36] showed constitutive overexpression of SlAREB in tomato induced dwarfism that could be alleviated by exogenous GA3 treatment, which were comparable to findings of [45]. Transcript level analysis of transgenic plants disclosed that SlDREB overexpression resulted in dwarf phenotype by downregulating key GA biosynthesis genes involved, such as ent-copalyldiphosphate synthase (SlCPS) and GA 20-oxidases (SlGA20ox1, -2, and -4), thereby reducing endogenous GA levels of application in transgenic plants.
2.2. Genetic Engineering of Tomato for Improved Drought Stress Tolerance
In the last century, efforts through classical breeding have resulted in improved agronomic traits, as well as nutritional value of cultivated tomato. However, traditional breeding has not been very successful in enhancing drought tolerance in tomato cultivars due to the limited genetic variation within S. lycopersicum species. It is evaluated that just ~5% of the complete genetic variability inside the tomato family can be found within S. lycopersicum (100 Tomato Genome Sequencing Consortium, 2014). The genes related to different required agricultural traits, including drought resistance, do not exist in this cultivated species [13]. However, fortunately, related wild tomato species, including S. pimpinellifolium, S. chilense, S. peruvianum, S. pennellii, and S. hirsutum, have shown to be a rich source of the genes and traits needed to increase resistance to various abiotic stresses. Due to the intricacy of abiotic stress tolerance mechanisms, transferring these traits from wild relatives of S. lycopersicum is challenging and takes a lot of effort; therefore, they have not been fully utilised [13]. The genetic base for resistance and tolerance against abiotic stress in species of wild tomato are hereditary as quantitative traits; thus, it is also unlikely that a single gene from wild tomato species expressed in cultivated species will confer drought tolerance [46]. For such reasons, developing stress-tolerant transgenic tomato taking regulatory genes from related or distant species is a workable approach to improve drought tolerance of tomato. Therefore, genetic engineering is a relatively quick, precise, and frequently successful method of improving abiotic stress resistance in many plant species.
Considering the problem of drought stress in tomato, attempts have been made to enhance drought tolerance by incorporating single genes from distant plant species [8][19][20][24][27][47], as well as some microbes, governing drought and other water-deficit stress tolerance. Constitutive overexpression of Arabidopsis CBF1 in tomato brought about upgraded plant resistance to cold, dry season, and salt burdens. In any case, this upgraded resistance included some significant pitfalls, causing decreased plant development and yield [19]. The transgenic tomatoes showed improved resilience to dry season, cold, and salt stress when a related gene (CBF1) was expressed using an ABA/stress-inducible ARBC1 promoter from the barley HAV22 gene. Additionally, the use of the inducible promoter eliminated the negative effects of the ectopic expression of CBF1 on plant growth and yield [19][27]. By ensuring various proteins, boiling stable proteins (BSP), have been involved in parching resistance against drought stress. Drought stress resistance of transformed tomato plants was attempted with a special 66-kD BSP from Populus tremula using polyethylene glycol (PEG) study, biomass investigation, proline assay, and electrolyte leakage measurement. These plants displayed slightly increased drought stress tolerance [48]. S. lycopersicum was found to contain the basic leucine zipper (bZIP) transcription factor SlAREB1 [37]. Uncomplicated transitory expression analysis was implemented for fast study of genes controlled by SlAREB1 in tobacco and tomato recommended regarding the group of bZIP that performs a role in abiotic stress reaction in the Solanum genus. Zhang et al. [49] developed marker-free selectable transgenic tomato plants exhibiting increased resistance to drought, cold, and oxidative stresses, which constitutively expressed AtIpk2b, an inositol polyphosphate 6-/3-kinase gene from A. thaliana. Tobacco osmotin gene driven by CaMV35S promoter was transferred to tomato and physiological analysis at T1 and T2 generations was used to test for resistance against drought and salt stress. Increased resistance to drought and salt stress was observed during NaCl stress and when water was withheld [47]. Tomato plants were transformed with the mannitol-1-phosphate dehydrogenase (mtlD; EC1.1.1.17) gene from bacteria, which is controlled by the CaMV35S promoter. Drought, cold, and salinity resistance study showed that transgenic lines perform a better resistance against abiotic stresses [50]. The SlAREB1-overexpressing transgenic tomato plants exhibited increased resistance against drought and salt stress, as analysed by various physiological parameters, such as relative water content, damage by lipoperoxidation, and chlorophyll fluorescence [38].
By offering the codA gene expressing a choline oxidase from Arthrobacter globiformis, Goel et al. [51] modified tomato for increased resistance to salt and water stress with the capacity to assimilate glycine betaine. The codA transgenic plants demonstrated increased resistance to salt stress during seed germination and ensuing development of young seedlings compared to wild varieties of plants. Developed codA transgenic plants had uncovered more elevated levels of relative water content, chlorophyll concentration, and proline level. Rai et al. [19][24] developed transgenic tomato cv. Kashi Vishesh (H-86) for improved drought tolerance by using the BcZAT12 gene coding sequence regulated through Bclea1a promoter. BcZAT12 expresses a C2H2 type zinc finger protein that is known to confer multiple abiotic stress tolerance to plants. Analysis of RWC, EL, CCI, H2O2 and superoxide anion level, and antioxidant enzyme activities suggested that BcZAT12 tomato transformants had increased levels of drought tolerance. Transgenic plants with the A. thaliana transcription factor ATHB-7 gene inserted displayed reduced stomatal density and increased tolerance to drought stress [52]. However, AtATHB-7 was established to be expressed increasingly under drought stress; thus, it performs as a negative growth regulator. The integration of drought-stress-tolerant genes from unrelated species into tomato has been the focus of intense research efforts from scientists all over the world (Table 1) [47][48][49][50][51][52][53][54].
Table 1. Transgenes used for development of drought stress tolerance, their function, and mechanism of action.
| S. No. | Gene | Function | Mechanism of Action | Reference |
|---|---|---|---|---|
| 1 | CBF1 | Stress-inducible transcription factors | Expression of stress-responsive gene | [55] |
| 2 | H1-S, drought induced linker histone | DNA packaging and organisation of chromosomes in the nucleus | Modulation of mechanisms related to the stomatal function | [56] |
| 3 | AtCBF-1 | Stress-inducible transcription factors | Expression of stress-responsive gene | [57] |
| 4 | H+-pyrophosphatase | Facilitate auxin fluxes | Enhance pyrophosphate-driven cation transport into root vacuolar fractions |
[58] |
| 5 | bspA | Protein protection | Enhance desiccation tolerance by protecting proteins in membranes and cytosol | [48] |
| 6 | coda | Accumulation of glycine betaine | Osmolyte accumulation to protect against oxidative damage. | [59] |
| 7 | LeNCED1 | Increase in abscisic acid (ABA) accumulation |
Stomatal closure and increased water-use efficiency (WUE) | [59] |
| 8 | Osmotin | Stress-responsive multifunctional protein | Osmotin provides protection via different mechanisms related with programmed cell death | [47] |
| 9 | PtADC | Induce the stress-responsive gene | Improves dehydration and drought tolerance | [60] |
| 10 | DREBs/ CBFs; ABF3 |
Stress-induced transcription factors | Enhanced expression of downstream stress-related genes confers drought tolerance. | [19] |
| 11 | ZAT12 | Stress-induced transcription factors | Enhanced expression of downstream stress-related genes confers drought | [27] |
| 12 | AtGAMT1 | Suppress gibberellin | GAMT1 overexpression inhibited the expansion of leaf epidermal cells. | [61] |
| 13 | SlNAC4 | Stress-responsive transcription factor | Modulation of ABA-independent signaling networks | [62] |
| 14 | GalUR | GalUR encodes Lgalactono- 1,4-lactone as a precursor of ascorbic acid |
Ascorbic acid detoxifies superoxide anion radical and hydroxyl radical and also plays a crucial role in scavenging ROS |
[63] |
| 15 | EgDREB1 | Enhances the expression of DRE/CRT and DRE/CRT-containing genes | Elevated level of antioxidative enzymes scavenge the superoxide radical and accumulation of nonenzymatic antioxidants maintains the cell basic structure under drought and cold stress | [64] |
| 16 | CcHRD | AP2/ERF-like tanscritpion factor | Regulate many pathways involved in stress tolerance | [65] |
| 17 | SlMAPK1 | Encodes for mitogen-activated protein kinases | SlMAPK1 improves drought stress tolerance by activating antioxidant enzymes, reducing oxidative damage, and modulating transcription of stress-related genes. | [66] |
| 18 | SiDHN | Encodes for Dehydrins (DHNs) commonly hydrophilin LEA proteins |
Increased chlorophyll a and b, carotenoid and relative water, proline and soluble sugar content and improve photosynthetic efficiency and suppress the formation of malondialdehyde H2O2 and O2 |
[67] |
| 19 | MdSWEET17 | Sugar transporters | Enhances accumulation of sugars, such as glucose and fructose, which act as osmoprotestants and carbon source under drought stress | [68] |
| 20 | SlSAMS1 | S-adenosylmethionine synthetase (SAMS) | SlSAMS1 modulates the production of polyamines and H2O2 and maintains the cellular homeostatasis | [69] |
| 21 | AtDREB1A and BcZAT12 | Encodes for transcription factors | Independent expression of AtDREB1A and BcZAT12 gene enhances drought tolerance in tomato | [14][23] |
| 22 | SlGATA17 | Improves phenylpropanoid biosynthesis pathway activity | DNA-binding domain of GATA TFs regulates many pathways in plants and enhances drought stress tolerance |
[70] |
| 23 | CsECR | Encodes for enoyl-CoA reductase (ECR) enzyme, which is involved in biosynthesis of cuticular waxes and catalyses the last step of very long-chain fatty acids (VLCFAs) elongation | Ectopic overexpression of CsECR increased the contents of total waxes and aliphatic wax leaves and fruits of the transgenic tomato and improves drought tolerance | [71] |
References
- Linnaeus, C. Species Planatarium, 1st ed.; Holmiae: Stockholm, Sweden, 1753.
- Bauchet, G.; Causse, M. Genetic Diversity in Tomato (Solanum lycopersicum) and Its Wild Relatives. Genet. Divers. Plants 2012, 133, 162.
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tissue Organ Cult. 2015, 120, 881–902.
- Causse, M.; Zhao, J.; Diouf, I.; Wang, J.; Lefebvre, V.; Caromel, B.; Génard, M.; Bertin, N. Genomic designing for climate-smart tomato. In Genomic Designing of Climate-Smart Vegetable Crops; Springer: Cham, Switzerland, 2020; pp. 47–159.
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database; FAO: Rome, Italy, 2018.
- National Horticultura Board. 2018. Available online: https://nhb.gov.in/statistics/Publication/Horticulture%20Statistics%20at%20a%20Glance-2018.pdf (accessed on 13 October 2022).
- Foolad, M.R. Genome Mapping and Molecular Breeding of Tomato. Int. J. Plant Genom. 2007, 2007, 64358.
- Rai, G.K. Biochemical and Molecular Analysis of AtDREB1A/CBF3 Transcription Factor in Transgenic Tomato under Drought Stress. Ph.D. Thesis, Banaras Hindu University, Varanasi, India, 2012.
- Rai, A.C.; Singh, M.; Shah, K. Engineering drought tolerant tomato plants over-expressing BcZAT12 gene encoding a C2H2 zinc finger transcription factor. Phytochemistry 2013, 85, 44–50.
- Bhatnagar-Mathur, P.; Devi, M.J.; Reddy, D.S.; Lavanya, M.; Vadez, V.; Serraj, R.; Yamaguchi-Shinozaki, K.; Sharma, K.K. Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Rep. 2007, 26, 2071–2082.
- Atherton, J.; Rudich, J. (Eds.) The Tomato Crop: A Scientific Basis for Improvement; Springer: Berlin/Heidelberg, Germany, 2012.
- Sestito, R.; Palozza, P. Lycopene and Down-regulation of Cyclin D1, pAKT and pBad. In Lycopene: Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2019; p. 133.
- Krishna, R.; Karkute, S.G.; Ansari, W.A.; Jaiswal, D.K.; Verma, J.P.; Singh, M. Transgenic tomatoes for abiotic stress tolerance: Status and way ahead. 3 Biotech 2019, 9, 143.
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Singh, A.K.; Verma, J.P.; Singh, M. Co-overexpression of AtDREB1A and BcZAT12 increases drought tolerance and fruit production in double transgenic tomato (Solanum lycopersicum) plants. Environ. Exp. Bot. 2021, 184, 104396.
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. TOMATOMA: A Novel Tomato Mutant Database Distributing Micro-Tom Mutant Collections. Plant Cell Physiol. 2011, 52, 283–296.
- Van der Hoeven, R.; Ronning, C.; Giovannoni, J.; Martin, G.; Tanksley, S. Deductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing. Plant Cell 2002, 14, 1441–1456.
- Rellán-Álvarez, R.; Andaluz, S.; Rodríguez-Celma, J.; Wohlgemuth, G.; Zocchi, G.; Álvarez-Fernández, A.; Fiehn, O.; López-Millán, A.F.; Abadía, J. Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol. 2010, 10, 120.
- Singh, S.; Rathore, M.; Goyary, D.; Singh, R.; Anandhan, S.; Sharma, D.K.; Ahmed, Z. Induced ectopic expression of At-CBF1 in marker-free transgenic tomatoes confers enhanced chilling tolerance. Plant Cell Rep. 2011, 30, 1019–1028.
- Rai, G.K.; Rai, N.P.; Kumar, S.; Yadav, A.; Rathaur, S.; Singh, M. Effects of explant age, germination medium, pre-culture parameters, inoculation medium, pH, washing medium, and selection regime on Agrobacterium-mediated transformation of tomato. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 565–578.
- Rai, G.K.; Rai, N.P.; Rathaur, S.; Kumar, S.; Singh, M. Expression of rd29A: AtDREB1A/CBF3 in tomato alleviates drought-induced oxidative stress by regulating key enzymatic and non-enzymatic antioxidants. Plant Physiol. Biochem. 2013, 69, 90–100.
- Karkute, S.; Krishna, R.; Ansari, W.; Singh, B.; Singh, P.; Singh, M.; Singh, A. Heterologous expression of the AtDREB1A gene in tomato confers tolerance to chilling stress. Biol. Plant. 2019, 63, 268–277.
- Bruening, G.; Lyons, J. The case of the FLAVR SAVR tomato. Calif. Agric. 2000, 54, 6–7.
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Singh, A.K.; Prasad, R.; Verma, J.P.; Singh, M. Overexpression of AtDREB1 and BcZAT12 genes confers drought tolerance by reducing oxidative stress in double transgenic tomato (Solanum lycopersicum L.). Plant Cell Rep. 2021, 40, 2173–2190.
- Behera, T.K.; Krishna, R.; Ansari, W.A.; Aamir, M.; Kumar, P.; Kashyap, S.P.; Pandey, S.; Kole, C. Approaches involved in the vegetable crops salt stress tolerance improvement: Present status and way ahead. Front. Plant Sci. 2021, 12, 787292.
- Ansari, W.A.; Atri, N.; Ahmad, J.; Qureshi, M.I.; Singh, B.; Kumar, R.; Rai, V.; Pandey, S. Drought mediated physiological and molecular changes in muskmelon (Cucumis melo L.). PLoS ONE 2019, 14, e0222647.
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: Cambridge, MA, USA, 1995.
- Rai, A.C.; Singh, M.; Shah, K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol. Biochem. 2012, 61, 108–114.
- Shah, K.; Singh, M.; Rai, A.C. Effect of heat-shock induced oxidative stress is suppressed in BcZAT12 expressing drought tolerant tomato. Phytochemistry 2013, 95, 109–117.
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161.
- Hussain, S.S.; Kayani, M.A.; Amjad, M. Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol. Prog. 2011, 27, 297–306.
- Harrak, H.; Azelmat, S.; Baker, E.N.; Tabaeizadeh, Z. Isolation and characterization of a gene encoding a drought-induced cysteine protease in tomato (Lycopersicon esculentum). Genome 2001, 44, 368–374.
- Martin, B.; Thorstenson, Y.R. Stable carbon isotope composition (δ13C), water use efficiency, and biomass productivity of Lycopersicon esculentum, Lycopersicon pennellii, and the F1 hybrid. Plant Physiol. 1988, 88, 213–217.
- Kahn, T.L.; Fender, S.E.; Bray, E.A.; O’Connell, M.A. Characterization of expression of drought-and abscisic acid-regulated tomato genes in the drought-resistant species Lycopersicon pennellii. Plant Physiol. 1993, 103, 597–605.
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637.
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96.
- Hsieh, T.-H.; Li, C.-W.; Su, R.-C.; Cheng, C.-P.; Tsai, Y.-C.; Chan, M.-T. A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 2010, 231, 1459–1473.
- Yáñez, M.; Cáceres, S.; Orellana, S.; Bastías, A.; Verdugo, I.; Ruiz-Lara, S.; Casaretto, J.A. An abiotic stress-responsive bZIP transcription factor from wild and cultivated tomatoes regulates stress-related genes. Plant Cell Rep. 2009, 28, 1497–1507.
- Orellana, S.; Yanez, M.; Espinoza, A.; Verdugo, I.; Gonzalez, E.; Ruiz-Lara, S.; Casaretto, J.A. The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant Cell Environ. 2010, 33, 2191–2208.
- Islam, M.S.; Wang, M.-H. Expression of dehydration responsive element-binding protein-3 (DREB3) under different abiotic stresses in tomato. BMB Rep. 2009, 42, 611–616.
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249.
- Zhang, Y.; Liu, H.; Li, B.; Zhang, J.-T.; Li, Y.; Zhang, H. Generation of selectable marker-free transgenic tomato resistant to drought, cold and oxidative stress using the Cre/loxP DNA excision system. Transgenic Res. 2009, 18, 607–619.
- Zhang, J.Z.; Creelman, R.A.; Zhu, J.-K. From Laboratory to Field. Using Information from Arabidopsis to Engineer Salt, Cold, and Drought Tolerance in Crops. Plant Physiol. 2004, 135, 615–621.
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919.
- Guo, J.; Wang, M.-H. Expression profiling of the DREB2 type gene from tomato (Solanum lycopersicum L.) under various abiotic stresses. Hortic. Environ. Biotechnol. 2011, 52, 105–111.
- Li, J.; Sima, W.; Ouyang, B.; Wang, T.; Ziaf, K.; Luo, Z.; Liu, L.; Li, H.; Chen, M.; Huang, Y.; et al. Tomato SlDREB gene restricts leaf expansion and internode elongation by downregulating key genes for gibberellin biosynthesis. J. Exp. Bot. 2012, 63, 6407–6420.
- Labate, J.A.; Grandillo, S.; Fulton, T.; Munos, S. Tomato/Genome mapping and molecular breeding in plants. In Vegetables; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5.
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Bansal, K.C. Overexpression of osmotin gene confers tolerance to salt and drought stresses in transgenic tomato (Solanum lycopersicum L.). Protoplasma 2010, 245, 133–141.
- Roy, R.; Purty, R.S.; Agrawal, V.; Gupta, S.C. Transformation of tomato cultivar ‘Pusa Ruby’ with bspA gene from Populus tremula for drought tolerance. Plant Cell Tissue Organ Cult. 2006, 84, 56.
- Zhang, Z.; Zhang, H.; Quan, R.; Wang, X.-C.; Huang, R. Transcriptional Regulation of the Ethylene Response Factor LeERF2 in the Expression of Ethylene Biosynthesis Genes Controls Ethylene Production in Tomato and Tobacco. Plant Physiol. 2009, 150, 365–377.
- Khare, N.; Goyary, D.; Singh, N.K.; Shah, P.; Rathore, M.; Anandhan, S.; Sharma, D.; Arif, M.; Ahmed, Z. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2010, 103, 267–277.
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Murata, N.; Bansal, K.C. Transformation of tomato with a bacterial codA gene enhances tolerance to salt and water stresses. J. Plant Physiol. 2011, 168, 1286–1294.
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtílek, M.; Cellini, F.; Nedbal, L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012, 182, 79–86.
- Wang, C.; Guo, Y.; Wang, C.; Liu, H.; Niu, D.; Wang, Y.; Guo, J. Enhancement of tomato (Lycopersicon esculentum) tolerance to drought stress by plant-growth-promoting rhizobacterium (PGPR) Bacillus cereus AR156. J. Agric. Biotechnol. 2012, 20, 1097–1105.
- Hsieh, T.H.; Lee, J.T.; Yang, P.T.; Chiu, L.H.; Charng, Y.Y.; Wang, Y.C.; Chan, M.T. Heterology expression of the Arabidopsis C-repeat/dehydration response element binding Factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2002, 129, 1086–1094.
- Lee, J.-T.; Prasad, V.; Yang, P.-T.; Wu, J.-F.; Ho, T.-H.D.; Charng, Y.-Y.; Chan, M.-T. Expression of Arabidopsis CBF1 regulated by an ABA/stress inducible promoter in transgenic tomato confers stress tolerance without affecting yield. Plant Cell Environ. 2003, 26, 1181–1190.
- Scippa, G.S.; Griffiths, A.; Chiatante, D.; Bray, E.A. The H1 histone variant of tomato, H1-S, is targeted to the nucleus and accumulates in chromatin in response to water-deficit stress. Planta 2000, 211, 173–181.
- Hsieh, T.-H.; Lee, J.-T.; Charng, Y.-Y.; Chan, M.-T. Tomato Plants Ectopically Expressing Arabidopsis CBF1 Show Enhanced Resistance to Water Deficit Stress. Plant Physiol. 2002, 130, 618–626.
- Park, S.; Gilmour, S.J.; Grumet, R.; Thomashow, M.F. CBF-dependent and CBF-independent regulatory pathways contribute to the differences in freezing tolerance and cold-regulated gene expression of two Arabidopsis ecotypes locally adapted to sites in Sweden and Italy. PLoS ONE 2018, 13, e0207723.
- Wu, L.; Chen, X.; Ren, H.; Zhang, Z.; Zhang, H.; Wang, J.; Wang, X.C.; Huang, R. ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta 2007, 226, 815–825.
- Wang, J.; Sun, P.-P.; Chen, C.-L.; Wang, Y.; Fu, X.-Z.; Liu, J.-H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914.
- Nir, I.D.O.; Moshelion, M.; Weiss, D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Env. 2014, 37, 113–123.
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A New Tomato NAC (NAM/ATAF1/2/CUC2) Transcription Factor, SlNAC4, Functions as a Positive Regulator of Fruit Ripening and Carotenoid Accumulation. Plant Cell Physiol. 2014, 55, 119–135.
- Liao, X.; Guo, X.; Wang, Q.; Wang, Y.; Zhao, D.; Yao, L.; Wang, S.; Liu, G.; Li, T. Overexpression of Ms DREB 6.2 results in cytokinin-deficient developmental phenotypes and enhances drought tolerance in transgenic apple plants. Plant J. 2017, 89, 510–526.
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm drought inducible DREB1 induced expression of DRE/CRT- and non-DRE/CRT-containing genes in lowland transgenic tomato under cold and PEG treatments. Plant Physiol. Biochem. 2017, 112, 129–151.
- Guo, X.; Zhang, L.; Zhu, J.; Wang, A.; Liu, H. Christolea crassifolia HARDY gene enhances drought stress tolerance in transgenic tomato plants. Plant Cell Tissue Organ Cult. 2017, 129, 469–481.
- Wang, L.; Zhao, R.; Li, R.; Yu, W.; Yang, M.; Sheng, J.; Shen, L. Enhanced drought tolerance in tomato plants by overexpression of SlMAPK1. Plant Cell Tissue Organ Cult. 2018, 133, 27–38.
- Guo, X.; Zhang, L.; Wang, X.; Zhang, M.; Xi, Y.; Wang, A.; Zhu, J. Overexpression of Saussurea involucrata dehydrin gene SiDHN promotes cold and drought tolerance in transgenic tomato plants. PLoS ONE 2019, 14, e0225090.
- Lu, J.; Sun, M.-H.; Ma, Q.-J.; Kang, H.; Liu, Y.-J.; Hao, Y.-J.; You, C.-X. MdSWEET17, a sugar transporter in apple, enhances drought tolerance in tomato. J. Integr. Agric. 2019, 18, 2041–2051.
- Zhang, X.; Bao, Z.; Gong, B.; Shi, Q. S-adenosylmethionine synthetase 1 confers drought and salt tolerance in transgenic tomato. Environ. Exp. Bot. 2020, 179, 104226.
- Zhao, T.; Wu, T.; Pei, T.; Wang, Z.; Yang, H.; Jiang, J.; Zhang, H.; Chen, X.; Li, J.; Xu, X. Overexpression of SlGATA17 promotes drought tolerance in transgenic tomato plants by enhancing activation of the phenylpropanoid biosynthetic pathway. Front. Plant Sci. 2021, 12, 634888.
- Liu, D.; Guo, W.; Guo, X.; Yang, L.; Hu, W.; Kuang, L.; Huang, Y.; Xie, J.; Liu, Y. Ectopic Overexpression of CsECR from Navel Orange Increases Cuticular Wax Accumulation in Tomato and Enhances Its Tolerance to Drought Stress. Front. Plant Sci. 2022, 13, 924552.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
10 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




