
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adrián Rabadán | + 2938 word(s) | 2938 | 2020-12-04 07:18:40 | | | |
| 2 | Peter Tang | -222 word(s) | 2716 | 2020-12-08 13:33:37 | | |
Video Upload Options
This entry is about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel.
1. Introduction
The almond is the most cultivated nut in the world, where the estimated annual production exceeds 3 million tons [1]. Most of the world's production is concentrated in three regions, which include California, the Mediterranean Basin and the Middle East, although almond cultivation is also increasing in the Southern Hemisphere, in countries such as Australia or Chile.
Almond tree, Prunus dulcis, belongs, taxonomically, to the Amygdalus subgenus inside the Prunus genus, the Rosaceae family and the order Rosales [2]. Its cultivars are classified depending on the hardness of the shell. Soft and medium-hard shell cultivars, like Non Pareil and Guara, respectively, show low resistance to attacks by pests and are more susceptible to rancid oxidation, but show high kernel yields (55% and 35–40%, respectively) [3]. On the other hand, hard shell varieties present the lowest kernel yield (< 25%), but they maintain in a better way the organoleptic and commercial characteristics, highlighting the importance of Marcona and Desmayo Largueta cultivars. Physical parameters are useful for cultivar determination even when the nuts are grown in the same conditions.
From the botanic point of view, the almond tree nut is a drupe. It is formed by the evolution of the ovary walls, which develop into the pericarp (hull), an outer layer that is formed by a pulpy and very fibrous tissue, that can be divided into the exocarp (thin and pubescent) and the mesocarp (thickest); and a lignified interior layer that creates a heavy to less heavy coat, the endocarp (shell). At maturity, the pulpy mesocarp dries and opens by its ventral suture, releasing the lignified endocarp. The seed, which constitutes the edible kernel and the commercial part of the nut, occupies the inner part, surrounded by the endocarp. The kernel contains the embryo coated by the teguments [2].
Almond consumption has been found to be associated with many health benefits [4], especially related to the reduction of the cardiovascular diseases risk, but also with effects on other pathologies, such as hypertension, diabetes mellitus or metabolic syndrome. These activities are generally attributed to the lipid fraction, where the fatty acid profile has a predominant role, but also minor compounds such as polyphenols and phytosterols may be involved. Moreover, recent studies have explored the effect of other nutritional compounds like fiber on gut microbiota [5] or the antioxidant capacity of the protein fraction [6].
2. Chemical Composition of Almond Kernel
The main fractions that can be found in almond kernels, other than water, are the lipid fraction, the protein fraction, carbohydrates and the mineral fraction. A numerous group of compounds called phytochemicals should also be added, because even though they appear in low quantities, they have a main role in almond quality. The proportion of these compounds changes according to the cultivars, the cultivation system and the geographical origin [7][8][9][10][11][12] (Figure 1).
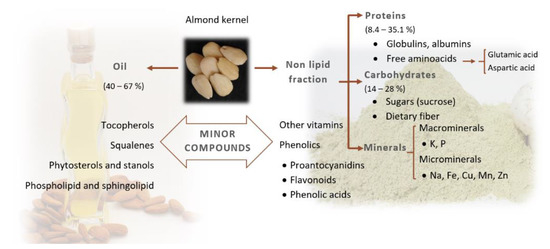
Figure 1. Chemical composition of almond kernel.
Precise knowledge about almond kernel composition is of great interest from the commercial, industrial and nutritional points of view, especially taking into account the variability that exists between different cultivars. In Yada et al. [8], total lipid values between 40 and 67 g/100 g of dry almond weight and between 35 and 66 g/100 g of almond fresh weight (f.w.) were reported. Almond oil is mainly composed of mono- and diunsaturated fatty acids [13]. In the case of total proteins (considering a conversion N factor of 5.18), the values oscillated between 14 and 61 g/100 g of almond fresh weight, and in the case of soluble sugars, values between 1.8 and 7.6 g/100 g of dry almond and between 2.5 and 12 g/100 g of fresh almond have been reported.
Regarding phenotypic correlations, a negative correlation was found between the oil and the total protein content [14]. This interdependence can be explained biochemically, because both fractions are formed during the ripening process from carbohydrates, which are abundant in the early stages of seed development but decrease over the ripening process [15].
In the existing literature, a clear evolution of the topic treatment can be observed. The first works about the chemical composition of almond kernels started appearing in the 1950s [16][17], providing data about the main fractions, without discrimination between cultivars and origins. In the decades of the 1970s and 1980s, works about the chemical composition appeared, referring to defined cultivars and providing information about fatty acids, amino acids, mineral salts and soluble sugars compositions [15][18][19]. Works studying the influence and effect of different labor systems, the place of origin and the harvest year on almond chemical composition also appeared.
From 1990, a step forward can be observed, related to the use of advanced statistical treatments, in such a way that not only composition data are given, but it is also tried to bring together genotypes that have a similar response and present close values to commercial effects [7][20][21][22]. In addition, food origin determination appears as a main target in food quality control and safety [23].
In the new century, the approach to the chemical composition of almond kernels, which can be applied to the rest of nuts, is focused on minority components, called phytochemicals. It has been shown that almond kernels present a wide range of substances with high nutritional value or with effects on health on one side [24][25][26] and with antioxidant effects on the other [27][28][29]. The interest aroused by these substances has boosted the development of new methods for their determination, increasing exponentially the published articles about them. Another important source of data is due to the recent interest in almond oil extraction as virgin edible oil [11][12][13][30][31][32][33][34][35]. In this sense, almond oil has been widely characterized, but oil extraction industries generate a by-product derived from the grinding of the pressing cake, which originates a partially defatted flour where the non-lipidic fraction takes on a special relevance. These flours have been reported to have promising uses in the culinary industry to enhance the nutritional properties of various products [36][37], or in mushroom cultivation, where it can be added as a nutritional supplement [34].
3. Protein Fraction of Almond Kernel
Almond kernel is a protein-rich food (second fraction in importance after the lipid fraction), but its content presents differences depending on the cultivar, weather conditions and cultivation area [9][14][16][32][34][38][39][40][41][42].
Table 1 shows the protein content of almond kernel samples with different origins found in relevant published articles. The percentage of variation ranges from 8.4%, found in Spanish samples [14], to 35.1%, found in Moroccan samples [42]. The differences in the protein content found in different samples may be related to the methods used in the analysis. To calculate the protein content, typically a specific conversion factor of nitrogen to protein of 5.18 is used [43], since amandine, which is the dominant protein in almond, is a globulin that contains 19.3% of nitrogen [44]. However, other studies use the general conversion factor (6.25), based on the nitrogen content of most common proteins, which could lead to overestimate the protein content. This point could explain some discrepancies found within the data. For this reason, data regarding total nitrogen would be more useful to compare samples from different origins.
Table 1. Macronutrients content (%) of almond samples with different origins.
|
Nutrient |
Range of Variability (g/100 g) |
Origin |
Source of Variability Studied |
References |
||
|
V * |
E ** |
Ap *** |
||||
|
Protein, total (N × 5.18) |
||||||
|
|
16.4–22.1 |
USA |
- |
- |
- |
[38] |
|
|
18.5–24.0 |
California |
Yes |
Yes |
- |
[9] |
|
|
20.7–23.3 |
USA |
Yes |
- |
- |
[45] |
|
|
15.8–25.1 |
Spain |
Yes |
Yes |
- |
[18] |
|
|
14.5–29.2 |
Spain |
Yes |
Yes |
- |
[39] |
|
|
8.4–24.7 |
Spain |
Yes |
- |
- |
[14] |
|
|
14.1–26.5 |
Spain |
- |
- |
- |
[3] |
|
|
21.0–24.0 |
Portugal |
Yes |
Yes |
- |
[46] |
|
|
9.6–28.5 |
France, Italy and Greece |
Yes |
Yes |
- |
[41] |
|
|
20.0–32.8 |
Spain and Morocco |
Yes |
Yes |
- |
[39] |
|
|
14.1–35.1 |
Morocco |
Yes |
Yes |
- |
[42] |
|
|
16.7–31.5 |
Turkey |
Yes |
Yes |
- |
[47] |
|
|
12.7–16.3 |
Turkey |
Yes |
- |
- |
[40] |
|
|
20.4–25.8 |
Turkey |
Yes |
Yes |
- |
[48] |
|
|
11.52 ± 1.1 |
Nigeria |
- |
- |
- |
[49] |
|
|
23.8 |
India |
- |
- |
- |
[50] |
|
|
20.0 |
South Africa |
- |
- |
- |
[51] |
|
|
17.36–23.02 |
Serbia |
Yes |
- |
Yes |
[52] |
|
Carbohydrates, total |
||||||
|
|
14–21 |
Portugal |
Yes |
Yes |
- |
[46] |
|
|
23.6–27 |
USA |
Yes |
- |
- |
[45] |
|
|
28 |
Nigeria |
- |
- |
- |
[53] |
|
|
28.0 |
South Africa |
- |
- |
- |
[51] |
|
Sugars, soluble |
||||||
|
|
2.6 |
Turkey |
Yes |
- |
- |
[54] |
|
|
7.9 |
Spain |
Yes |
- |
- |
[15] |
|
|
1.74–4.31 |
Greece |
Yes |
- |
Yes |
[55] |
|
Sucrose |
||||||
|
|
2.5–5.1 |
California |
Yes |
Yes |
- |
[9] |
|
|
1.42–3.62 |
Greece |
Yes |
- |
Yes |
[55] |
|
|
1.15–2.22 |
Portugal |
Yes |
- |
- |
[56] |
|
|
3.67–7.09 |
Spain |
- |
- |
Yes |
[57] |
|
|
1.21–3.08 |
Portugal |
Yes |
- |
- |
[58] |
|
Starch |
||||||
|
|
0.4–1.4 |
Italy |
- |
- |
- |
[59] |
|
Fiber, total dietary |
||||||
|
|
9.8 |
California |
- |
- |
- |
[60] |
|
|
7.9–16 |
California |
Yes |
Yes |
- |
[9] |
|
|
3.3–8.6 |
Spain |
Yes |
Yes |
- |
[22] |
|
|
4.73–6.01 |
Spain |
- |
- |
Yes |
[57] |
|
|
11–14 |
Italy |
- |
- |
- |
[59] |
Font i Forcada et al. [61] found that two quantitative trait loci (QTL) controlled the total protein content. The first marker LG6, located in the lowest part of the almond linkage groups, had a logarithm of the odds (LOD) values of 3.21 and explained a phenotypic variance of 17%. The second QTL was found in the lowest part of LG7 and had a similar effect, with an LOD of 3.18 explaining a phenotypic variance of 16.6%.
Nitrogen total content of almond samples has shown different percentages: 3% [15], 4.06% [62], 4.23% [54] and 4.62% [63].
4. Carbohydrates in Almond Kernel
The carbohydrates from almond kernel are soluble sugars, starch and other polysaccharides such as celluloses and hemicelluloses that are non-digestible, but they have physical effects in the intestinal tract with benefits for human health [8]. The total carbohydrates content ranged from 14% to 28% (Table 1). The sugars that can be found in almond kernel, although not found in high concentrations, are enough to provide the sweet flavor to almonds.
5. Mineral Fraction of Almond Kernel
5.1. Ashes
Mineral content is sometimes expressed as the ash content, which is the inorganic residue that remains after the incineration of the plant tissues. Almond kernels contain approximately 3 g ash/100 g of fresh weight [64][65]. These values may vary depending on the study considered (Table 2), between 2.3% [9] and 5.0% [51].
Table 2. Average value or range of main mineral elements (macro- and microminerals) found in almond kernel in the literature (mg/100 g).
|
Ash (g/100 g) |
K |
P |
Ca |
Mg |
S |
Cl |
Na |
Fe |
Cu |
Mn |
Zn |
Origin |
Reference |
|
|
435 |
577 |
298 |
299 |
587 |
|
2.27 |
3.4 |
0.96 |
1.36 |
3.04 |
Spain |
[7] |
|
2.69–3.6 |
821 |
585 |
275 |
281 |
130 |
14 |
10.8 |
4 |
1.2 |
1.6 |
3.8 |
Spain |
[15] |
|
|
618–785 |
345–507 |
88–124 |
242–285 |
|
|
4.7–15.5 |
3.5–5.3 |
1–1.6 |
1.1–1.7 |
3.4–3.9 |
Spain |
[18] |
|
2.74–3.05 |
1373.8 |
873.8 |
243.2 |
351 |
|
|
32.6 |
23.4 |
1 |
|
5 |
Turkey |
[40] |
|
|
1546–1685 |
253–259 |
640–678 |
447–494 |
|
|
|
5.5–6.5 |
2.4–2.6 |
3.8 |
7.6–8.0 |
|
[63] |
|
|
1050 |
300 |
467 |
30 |
|
|
|
7.0 |
0.5 |
|
3.4 |
Italy |
[64] |
|
3.03–4.66 |
1677–2051 |
404–800 |
98–187 |
361–513 |
|
|
5.66–10.38 |
3.98–14.6 |
1.60–2.30 |
2.90–3.39 |
7.78–8.84 |
Turkey |
[54] |
|
- |
465–1235 |
119–748 |
160 -663 |
100–333 |
|
|
|
|
|
|
|
France, Italy and Greece |
[41] |
|
2.3–3.4 |
543–902 |
364–548 |
198–373 |
224–303 |
|
|
|
2.58–4.47 |
0.46–1.57 |
1.31–3.98 |
2.02–4.03 |
California |
[9] |
|
3.29–4.66 |
679–986 |
584–697 |
250–332 |
325–381 |
|
|
9.20–16.06 |
6.08–10.62 |
2.02–3.97 |
2.52–4.76 |
4.80–9.53 |
Turkey |
[48]* |
|
5.0 |
|
|
539.2 |
542.4 |
|
|
|
7.15 |
2.37 |
2.58 |
4.97 |
South Africa |
[51] |
|
|
|
133.25 |
450.0 |
|
|
|
|
6.25 |
|
|
|
India |
[66] |
The sum of mineral elements is sensibly lower than the ash content, with percentages around 60%, which is fundamentally explained because the oxygen associated with these minerals is not counted in the ashes obtained by calcination [15]. According to Esteban [18], the percentge of all minerals, excluding nitrogen, represents between 51.3% and 55.2% of total ash content.
5.2. Macrominerals
Macrominerals refer to those minerals that are needed in quantities higher than 100 mg/day. On the other hand, those that are needed in small quantities are called microminerals, oligo elements or trace elements. Table 2 shows the average value of the main mineral elements (macrominerals and microminerals) found in almond kernel.
Potassium is the major element in all studies, except the one carried out by Prats [7], followed by phosphorus. Both elements represent 70% of the mineral fraction, not counting nitrogen. The next in importance are calcium and magnesium with very close values, in such a way that in some samples, one is higher and in others the opposite happens [15][18]. Globally, the mean magnesium values are higher than calcium values, and both represent half the phosphorus content, or even less [15].
Sulfur also appears in high amounts, although it is an element that is not commonly analyzed in comparison with the previous ones. Its values vary greatly depending on the study, probably due to the different methods applied for its determination. Prats [7] found higher values, comparable to phosphorus values. Macronutrients aggregation, not counting nitrogen, represents large percentages which are almost identical between cultivars, ranging from 98.0% to 98.7% of total minerals. Among Chinese wild almond species, potassium contents between 534 and 663 mg/100 g, calcium contents between 80 and 229 mg/100 g and magnesium contents between 194 and 239 mg/100 g have been found [67].
5.3. Microminerals or Trace Elements
Main microminerals or trace elements found in almond kernel are sodium, chlorine, iron, copper, manganese and zinc (Table 2). Sodium and chlorine are those that appear in higher proportion [15][16][54], followed by iron and zinc contents, which also show important values. In this case, as it happened with calcium and magnesium, for some authors, the content of iron is higher, and for others, the zinc content, but generally the quantity, is lower than 5.5 mg/100 g. Nevertheless, attention should be paid to the high contents in iron and zinc found by Ozcan et al. [40] and Aslantas et al. [54], respectively. Among Chinese wild almond species, iron contents between 4.6 and 6.0 mg/100 g and zinc contents between 4.1 and 5.6 mg/100 g have been found [67].
Other elements found in almond kernel, although in minor concentrations, include molybdenum that ranges from 4 to 30 µg/100 g, boron which ranges between 0.18 and 2.9 mg/100 g [15][16][68], chromium ranging between 0.04 [69] and 0.17 mg/100 g [68], aluminum ranging between 0.83 [69] and 2.2 mg/100 g [68], nickel with 0.034 mg/100 g [69] and selenium with 0.004 mg/100 g [51].
Some references to toxic heavy metals have also been found [50][51][69]. Even though some heavy metals such as cobalt, copper, chromium, manganese and nickel are needed for humans in small proportions, others may be carcinogenic or toxic, affecting the central nervous system (manganese, mercury, lead, arsenic), kidney or liver (mercury, lead, cadmium, copper), or the skin, bones or teeth (nickel, cadmium, copper, chromium).
6. Phytochemical Compounds of Almond Kernel
Phytochemicals, also known as bioactive compounds, are mainly additional nutritional compounds that can be found in certain foods, and that show an important and interesting physiological activity with positive effects on human health, which makes them very valuable elements for the scientific community and the food industry.
Several thousands of phytochemicals have been reported, some of them having a strong antioxidant activity (catechin, quercetin, tannin, ellagic acid, chlorogenic acid, cyanidin, etc.) [70], which are added to the already known antioxidant nutrients (vitamins A, C, E, selenium, etc.).
Phytochemicals comprise the following chemical groups: carotenoids, phenolic compounds, organosulfur compounds, some nitrogen compounds and alkaloids. Bolling et al. [71] added a carbohydrates group to this classification, the phytates, and together with the carotenoids, they include other unsaponifiable compounds of the lipid fraction.
7. Volatile Compounds
Seventeen aroma compounds were detected in raw almonds [72], including six aldehydes, two ketones, two nitrogen-containing compounds, one sulfur-containing compound, two acids, one furanone and three unknown compounds. Six of these compounds were quantitated in raw almonds, where vanillin with 830 ng/g was the most abundant and acetic acid (137 ng/g) and nonanal (72 ng/g) were found in high abundance.
Ojeda-Amador et al. [35] analyzed volatile compounds which are related to sensory notes, such as fruit/banana (hexanol), oily/green–sweet (hexanal), fruity (pentanol) and bitter almonds (benzaldehyde). The most important family found in all the varieties studied was that of aldehydes (1.35–7.52 mg/kg). Benzaldehyde was the main aldehyde (52–74% of total), followed by hexanal (0–10%).
Alcohols were the second major family, accounting for 14% to 30% of the total volatiles. Hexanol was the main contributor and was most abundant in "Marcona" (1.89 mg/kg). Acids (mainly acetic acid), hydrocarbons, ketones and terpenes showed close concentrations to each other, indicating about 0.30 mg/kg for each family.
8. Conclusions
Almond kernel contains a considerable amount of good-quality proteins, mainly globulins, essential minerals and fiber with a low content in sugars, in addition to many phytochemicals with potential health benefits. The presence of large variability in nutritive compounds has been reported, although most pre- and postharvest factors may have a significant effect on their content. However deeper studies about drying, blanching, storage or roasting processes and genetic, agricultural and environmental conditions are necessary to clarify their influence on the quality and quantity of almond phytochemicals.
As regards the bibliography consulted, practically no work has been found focused on the study of the phenotypic correlations that occur between the different components of the almond.
The complexity in the phytochemical composition makes the use of standard methods for extracting and quantifying almond phytochemicals difficult. Non-conventional extraction techniques are gaining major interest, especially methods based on microwave, supercritical fluids and ultrasound, combined with well-known and safe solvents such as ethanol, water and ethanol−water mixtures. Other methods such as sonication and hydrolysis are barely cited in scientific papers.
In another direction, more studies are needed to understand the impact of almond processing on protein and AA digestibility. Furthermore, increasing efforts to establish a new method for assessing protein quality, based on the Digestible Indispensable Amino Acid Score (DIAAS) system, are necessary.
The valorization of non-lipid compounds from almond has been scarcely treated in the scientific literature. Most papers focus on compounds identification and quantification and rarely on industrial extraction methods, as opposed to oil extraction. However, the nutritional composition of the non-lipid fraction of almond kernel makes the by-products obtained in the oil extraction process interesting candidates for food applications, to be used as a source of protein, fiber and minerals.
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 5 November 2020).
- Felipe, A. El Almendro. I. El Material Vegetal; Mira Editores: Zaragoza, Spain, 2000.
- Salazar, D.; Melgarejo, P. El Cultivo del Almendro; Mundi-Prensa: Madrid, Spain, 2002.
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus dulcis Mill. D. A. webb): A source of nutrients and health-promoting compounds. Nutrients 2020, 12, 672, doi:10.3390/nu12030672.
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and their effect on gut microbiota, gut function and symptoms in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2020, 12, 2347, doi:10.3390/nu12082347.
- Siddiqui, I.; Husain, Q.; Azam, A. Exploring the antioxidant effects of peptides from almond proteins using PAni-Ag-GONC conjugated trypsin by improving enzyme stability & applications. Int. J. Biol. Macromol. 2020, 158, 150–158, doi:10.1016/j.ijbiomac.2020.04.188.
- Prats, M.S. Caracterización Quimiométrica de Diez Variedades de Almendra Cultivadas en Diferentes Localidades. Ph.D. Thesis, University of Alicante, Alicante, Spain, 2000.
- Yada, S.; Lapsley, P.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480, doi:10.1016/j.jfca.2011.01.007.
- Yada, S.; Huang, G.; Lapsley, K. Natural variability in the nutrient composition of California-grown almonds. J. Food Compos. Anal. 2013, 30, 80–85, doi:10.1016/j.jfca.2013.01.008.
- Kodad, O.; Estopañán, G.; Juan, T.; Alonso, J.M.; Espiau, M.T.; Rafel Socias i Company. Oil content, fatty acid composition and tocopherol concetration in the spanish almond genebank collection. Sci. Horicult. 2014, 177, 99–107, doi:10.1016/j.scienta.2014.07.045.
- Rabadán, A.; Álvarez-Ortí, M.; Gómez, R.; de Miguel, C.; Pardo, J.E. Influence of genotype and crop year in the chemometrics of almond and pistachio oils. J. Sci. Food Agric. 2017, 98, 2402–2410, doi:10.1002/jsfa.8732.
- Rabadán, A.; Pardo, J.E.; Gómez, R.; Álvarez-Ortí, M. Influence of temperature in the extraction of nut oils by means of screw pressing. LWT 2018, 93, 354–361, doi:10.1016/j.lwt.2018.03.061.
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Almond virgin oil: Parameters of regulated physicochemical quality and stability. Riv. Ital. Sostanze Grasse 2016, 93, 237–243.
- Font i Forcada, C.; Kodad, O.; Juan, T.; Estopañan, G.; Rafel Socias i Company. Genetic variability and pollen effect on the transmission of the chemical components of the almond kernel. Span. J. Agric. Res. 2011, 9, 781–789.
- Saura, F.; Cañellas, J.; Soler, L. La Almendra. Composición, Variedades, Desarrollo y Maduración; Instituto Nacional de Investigaciones Agrarias (INIA): Madrid, Spain, 1988.
- Casares, R.L.H. Chemical data on the raises of Málaga. An. Bromatol. 1952, 4, 411–419.
- Hall, A.M. The nutritive value of fresh and roasted, California-grown Nonpareil almonds. J. Agric. Food Chem. 1958, 6, 377–382, doi:10.1021/jf60087a008.
- Esteban, R.M. Estudio Comparativo de la Calidad Nutritiva de Variedades de Almendra del S.E. y N.E. español; Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA): Madrid, Spain, 1985.
- Salvo, F.A. Composizionee dell’olio di mandorle. Nota III: Variazione di alcuni parametri chimici e chimico-ficici durante la conservazione. Riv. Ital. Sostanze Grasse 1986, 63, 37–40.
- García, C.G. Major fatty acid composition of 19 almond cultivars of different origins. A chemometric approach. J. Agric. Food Chem. 1996, 44, 1751–1755, doi:10.1021/jf950505m.
- Martín, M.L. Comparative study on the triglyceride composition of almond kernel oil. A new basis for cultivar chemometric characterization. J. Agric. Food Chem. 1999, 47, 3688–3692, doi:10.1021/jf981220n.
- Kodad, O. Criterios de Selección y de Evaluación de Nuevas Obtenciones Autocompatibles en un Programa de Mejora Genética del Almendro (Prunus amygdalus Batsch). Ph.D. Thesis, University of Lleida, Lleida, Spain, 2006.
- Barile, D.; Coïsson, J.D.; Arlorio, M.; Rinaldi, M. Identification of production area of Ossolano Italian cheese with chemometric complex approach. Food Control. 2006, 17, 197–206, doi:10.1016/j.foodcont.2004.10.016.
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B. Almond allergens: Molecular characterization, detection, and clinical relevance. J. Agric. Food Chem. 2012, 60, 1337–1349, doi:10.1021/jf2044923.
- Xie, L.; Bolling, B.W. Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC–MS. Food Chem. 2014, 148, 300–306, doi:10.1016/j.foodchem.2013.10.057.
- Vanamala, J. Food systems approach to cancer prevention. Crit. Rev. Food Sci. Nutr. 2017, 57, 2573–2588, doi:10.1080/10408398.2015.1028023.
- Bolling, B. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825, doi:10.1016/j.foodchem.2010.03.068.
- Zhu, Y.W. Lipophilic antioxidant content of almonds (Prunus dulcis): A regional and varietal study. J. Food Compos. Anal. 2015, 39, 120–127, doi:10.1016/j.jfca.2014.12.003.
- Csakvari, A.C.; Lupitu, A.; Bungău, S.; Gîtea, M.A.; Gîtea, D.; Ţiţ, D.M.; Copolovici, D. Fatty acids profile and antioxidant activity of almond oils obtained from six Romanian varieties. Farmacia 2019, 67, 882–887, doi:10.31925/farmacia.2019.5.19.
- Maestri, D.; Martínez, M.; Bodoira, R.; Rossi, Y.; Oviedo, A.; Pierantozzi, P. Variability in almond oil chemical traits from traditional cultivars and native genetic resources from Argentina. Food Chem. 2015, 170, 55–61, doi:10.1016/j.foodchem.2014.08.073.
- Sena-Moreno, E.; Pardo, J.E.; Pardo-Giménez, A.; Gómez, R.; Alvarez-Ortí, M. Differences in Oils from Nuts Extracted by Means of Two Pressure Systems. Int. J. Food Prop. 2016, 19, 2750–2760, doi:10.1080/10942912.2016.1144068.
- Roncero, J.M.; Alvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Virgin almond oil: Extraction methods and composition. Grasas Aceites 2016, 67, e143, doi:10.3989/gya.0993152.
- Martínez, M.L.; Bordón, M.G.; Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Maestri, D.M. Walnut and almond oil screw-press extraction at industrial scale: Effects of process parameters on oil yield and quality. Grasas Aceites 2017, 68, e216, doi:10.3989/gya.0554171.
- Pardo-Giménez, A.; Carrasco, J.; Roncero, J.M.; Álvarez-Ortí, M.; Zied, D.C.; Pardo, J.E. Recycling of the biomass waste defatted almond meal as a novel nutritional supplementation for cultivated edible mushrooms. Acta Sci. Agron. 2018, 40, e39341, doi:10.4025/actasciagron.v40i1.39341.
- Ojeda-Amador, R.; Fregapane, G.; Salvador, M. Chemical Characterization of Virgin Almond and Hazelnut Oils and Their By-Products. Eur. J. Lipid Sci. Technol. 2019, 121, 1–10.
- Barreira, J.C.M.; Nunes, M.A.; da Silva, B.V.; Pimentel, F.B.; Costa, A.S.G.; Alvarez-Ortí, M.; Pardo, J.E.; Oliveira, M.B.P.P. Almond cold-pressed oil by-product as ingredient for cookies with potential health benefits: Chemical and sensory evaluation. Food Sci. Hum. Wellness 2019, 8, 292–298, doi:10.1016/j.fshw.2019.07.002.
- Rabadán, A.; Álvarez-Ortí, M.; Martínez, E.; Pardo-Giménez, A.; Zied, D.C.; Pardo, J.E. Effect of replacing traditional ingredients for oils and flours from nuts and seeds on the characteristics and consumer preferences of lamb meat burgers. LWT 2021, 136, 110307, doi:10.1016/j.lwt.2020.110307.
- Sathe, S.K. Solubilization, electrophoretic characterization and in vitro digestibility of almond (Prunus amygdalus) proteins. J. Food Biochem. 1993, 16, 249–264, doi:10.1111/j.1745-4514.1992.tb00450.x.
- Kodad, O.; Mamouni, A.; Lahlo, M.; Rafel Socias i Company. Contenido en aceite y proteína y de los caracteres físicos del fruto y de la pepita del almendro en las condiciones climáticas mediterráneas. ITEA Inf. Tec. Econ. Agrar. 2011, 107, 300–314.
- Ozcan, M.; Ünver, A.; Erkan, E.; Arslan, D. Characteristics of some almond kernel and oils. Sci. Hortic. 2011, 127, 330–333, doi:10.1016/j.scienta.2010.10.027.
- Drogoudi, P.D.; Pantelidis, G.; Bacchetta, L.; Giorgio, D.; Duval, H.; Metzidakis, I. Protein and mineral nutrient contents in kernels from 72 sweet almond cultivars and accessions grown in France, Greece and Italy. Int. J. Food Sci. Nutr. 2012, 64, 202–209, doi:10.3109/09637486.2012.728202.
- Kodad, O.; Estopañán, G.; Juan, T.; Socias i Compani, R. Protein content and oil composition of almond from moroccan seedlings: Genetic diversity, oil quality and geographical origin. J. Am. Oil Chem. Soc. 2013, 90, 243–252, doi:10.1007/s11746-012-2166-z.
- USDA Food Database U.S. Department of Agriculture. Available online: https://fdc.nal.usda.gov/download-datasets.html (accessed on 21 February 2019).
- Osborne, T.B.; Campbell, G.F. Conglutin and vitellin. J. Am. Chem. Soc. 1986, 18, 609–623.
- Ahrens, S.; Venkatachalam, M.; Mistry, A.M.; Lapsley, K.; Sathe, S.K. Almond (Prunus dulcis L.) protein quality. Plant Foods Hum. Nutr. 2005, 60, 123–128, doi:10.1007/s11130-005-6840-2.
- Barreira, J.; Casal, S.; Ferreira, I.; Peres, A.M.; Pereira, J. Supervised chemical pattern recognition in almond (Prunus dulcis) portuguese PDO cultivars: PCA- and LDA-Based triennial study. J. Agric. Food Chem. 2012, 60, 9697–9704, doi:10.1021/jf301402t.
- Askin, M.; Baltab, M.; Tekintasc, F.; Kazankayab, A.; Balta, F. Fatty acid composition affected by kernel weight in almond (Prunus dulcis Mill.) genetic resources. J. Food Compos. Anal. 2007, 20, 7–12, doi:10.1016/j.jfca.2006.06.005.
- Simsek, M.; Gulsoy, E.; Yavic, A.; Arikan, B.; Yildirim, Y.; Olmez, N.; Erdogmus, B.; Boguc, F. Fatty acid, mineral and proximate compositions of various genotypes and commercial cultivars of sweet almond from the same ecological conditions. Appl. Ecol. Environ. Res. 2018, 16, 2957–2971, doi:10.15666/aeer/1603_29572971.
- Agumbiade, S.O. Evaluation of some nutritional characteristics of inidian almond (Prunus amygdalus) nut. Pak. J. Nutr. 2006, 5, 316–318.
- Chung, K.H.; Shin, K.O.; Hwang, H.J.; Choi, K.S. Chemical composition of nuts and seeds sold in Korea. Nutr. Res. Pract. 2013, 7, 82–88, doi:10.4162/nrp.2013.7.2.82.
- Moodley, R.; Kindness, A.; Jonnalagadda, S. Elemental composition and chemical characteristics of five edible nuts (almond, Brazil, pecan, macadamia and walnut) consumed in Southern Africa. J. Environ. Sci. Health Part B 2007, 58, 585–591, doi:10.1080/03601230701391591.
- Čolić.; S.D.; Bakić, I.V.; Zagorac, D.C.; Natić, M.M.; Smailagić, A.T.; Pergal, M.V.; Pešić, M.B.; Milinčić, D.D.; Rabrenović, B.B.; Akšić, M.M. Chemical Fingerprint and Kernel Quality Assessment in Different Grafting Combinations of Almond Under Stress Condition. Sci. Hortic. 2020, 275, 109705, doi:10.1016/j.scienta.2020.109705.
- Akpambang, V.; Amoo, I.; Izuagie, A. Comparative compositional analysis on two varieties of melon (Colocynthis citrullus and Cucumeropsis edulis) and a variety of almond (Prunus amygdalus). Res. J. Agric. Biol. Sci. 2008, 4, 639–642, doi:10.1007/s10578-014-0515-x.
- Aslantas, R.; Guleryuz, M.; Turan, M. Some chemical contents of selected almond (Prunus amygdalus Batsch) types. Cah. Options Méditerr. 2001, 56, 347–350.
- Kazantzis, I.; Nanos, G.; Stavroulakis, G. Effect of harvest time and storage conditions on almond kernel oil and sugar composition. J. Sci. Food Agric. 2003, 83, 354–359, doi:10.1002/jsfa.1312.
- Barreira, J.; Pereira, J.; Oliveira, M.; Ferreira, I. Sugars profiles of different chestnut (Castanea sativa Mill.) and almond (Prunus dulcis) cultivars by HPLC-RI. Plant Foods Hum. Nutr. 2010, 65, 38–43, doi:10.1007/s11130-009-0147-7.
- Sánchez-Bel, P.; Egea, I.; Martınez-Madrid, M.; Flores, B.; Romojaro, F. Influence of irrigation and organic/inorganic fertilization on chemical quality of almond (Prunus amygdalus cv. Guara). J. Agric. Food Chem. 2008, 56, 10056–10062, doi:10.1021/jf8012212.
- Oliveira, I.; Meyer, A.; Afonso, S.; Aires, A.; Goufo, P. Phenolic and fatty acid profiles, α‐tocopherol and sucrose contents, and antioxidant capacities of understudied Portuguese almond cultivars. J. Food Biochem. 2019, 43, e12887, doi:10.1111/jfbc.12887.
- Ruggeri, S.; Cappelloni, M.; Gambelli, L.; Carnovale, E. Chemical composition and nutritive value of nuts grown in Italy. Ital. J. Food Sci. 1998, 10, 243–252.
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270, doi:10.1128/AEM.00739-08.
- Font i Forcada, C.; Fernández i Martí, A.; Rafel Socias i Company. Mapping quantitative trait loci for kernel composition in almond. BMC Genet. 2012, 13, 47, doi:10.1186/1471-2156-13-47.
- Venkatachalam, M.; Sathe, S. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714, doi:10.1021/jf0606959.
- Barbera, G.; Di Marco, L.; La Mantia, T.; Schirra, M. Effect of rootstock on productive and qualitative response of two almond varieties. Acta Hortic. 1994, 373, 129–134, doi:10.17660/ActaHortic.1994.373.17.
- Schirra, M.; Mulas, M.; Nieddu, G.; Virdis, F. Mineral content in Texas almonds during fruit growth and ripening. Acta Hortic. 1994, 373, 207–214, doi:10.17660/ActaHortic.1994.373.29.
- Martínez, M.L.; Penci, M.C.; Marin, M.A.; Ribotta, P.D.; Maestri, D.M. Screw press extraction of almond (Prunus dulcis Miller): Oil recovery and oxidative stability. J. Food Eng. 2013, 119, 40–45, doi:10.1016/j.jfoodeng.2013.05.010.
- Sudhakar, P.; Priyanka, K.; Peter, A.E.; Sandeep, B.V.; Rajeswari, M.; Rao, B.; Sujatha, P. A study on the proximate composition and nutritive value of local tree almonds, Prunus amygdalus. Ann. Plant Sci. 2018, 7, 2363–2372, doi:10.21746/aps.2018.7.6.13.
- Jing, Z.; Cheng, J.; Guo, C.; Wang, X. Seed traits, nutrient elements and assessment of genetic diversity for almond (Amygdalus spp.) endangered to China as revealed using SRAP markers. Biochem. Syst. Ecol. 2013, 49, 51–57, doi:10.1016/j.bse.2013.03.015.
- Ozcan, M.M. Determination of the mineral compositions of some selected oil-bearing seeds and kernels using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). Grasas Aceites 2006, 57, 211–218, doi:10.3989/gya.2006.v57.i2.39.
- Cabrera, C.; Lloris, F.; Gimenez, R.; Olalla, M.; Lopez, M.C. Mineral content in legumes and nuts: Contribution to the Spanish dietary intake. Sci. Total Environ. 2003, 308, 1–14, doi:10.1016/S0048-9697(02)00611-3.
- Alasalvar, C.; Pelvan, E. Fat-soluble bioactives in nuts. Eur. J. Lipid Sci. Technol. 2011, 113, 943–949, doi:10.1002/ejlt.201100066.
- Bolling, B.; Chen, C.Y.; McKay, D.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275, doi:10.1017/S095442241100014X.
- Erten, E.; Cadwallader, K. Identification of predominant aroma components of raw, dry roasted and oil roasted almonds. Food Chem. 2017, 217, 244–253, doi:10.1016/j.foodchem.2016.08.091.




