
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mattia Bartoli | + 900 word(s) | 900 | 2020-11-30 08:50:17 |
Video Upload Options
We overviewed the complex chemical behavior of bismuth during the transformation of its compounds to oxide and bismuth oxide phase transitions.
1. Introduction
Nanosized oxides and related materials have raised more and more interest in the scientific community due to their cost-effective fabrication processes [1][2], high stability [3] and versatility in terms of morphology [4][5]. Furthermore, the high atomic number of bismuth brings about a high energy radiation attenuation larger than that of lead at an almost negligible risk of toxicity [6]. The combinations of bismuth properties represent a unique chance to exploit singularly or simultaneously cytotoxicity and diagnostic effects.
2. Bismuth Oxide and Related Materials: Productive Strategies
Nowadays, bismuth is mainly produced as a side product of lead streams and could be isolated through the Betterton–Kroll process [7] or through an electrochemical procedure known as Betts electrolytic process [8]. It is obtained in a highly purified form for those applications where it is used as a replacement for lead.
Commonly, bismuth is used in form of halide, oxo-halide, nitrate and oxides derivatives. Bismuth halides (BiX3, X = F, Cl, Br, I) are generally prepared by treating bismuth oxide in a watery medium by adding the specific HX acid. Bismuth trihalides are bipyramidal molecular species in the gas phase with angle X-Bi-X in the range 96–100° [9]. In the solid phase, they show a variety of different structures based on the halogen present in the crystals. BiF3 shows a pseudo-ionic structure with tricapped trigonal prismatic motive where bismuth atoms are surrounded by nine fluoride atoms, while the other halides show bicapped trigonal prism crystals. Bismuth oxide halides (BiOX) are formed by partial hydrolysis of bismuth halides. BiOF and BiOI can also be made by heating the corresponding halides in the air. BiOX have complex layer lattice structures [10] and, when heated up to 600 °C, BiOCl or BiOBr are decomposed by forming Bi24031X10 [11].
Moving on, bismuth can easily be produced as bismuth nitrate. Firstly, it is recovered as Bi(NO3)3×5H2O through crystallization after hydrolysis of Bi2O3 by using concentrated nitric acid. If a diluted acid is used is possible to recover the basic salt BiO(NO3). BiO(NO3) could be also produced by precipitation treating Bi(NO3)3×5H2O at 150 °C with butanol forming road-like structures as reported by Liu et al.[12]. As clearly enlighten by Briand and Burford [13] the hydrolysis of Bi(NO3)3×5H2O could lead to a plethora of different compounds. Furthermore, several attempts were reported in the literature [14][15][16][17][18] pursuing the thermal oxodehydration of Bi(NO3)3×5H2O with the formation of a series of complex species as summarized in Figure 1.
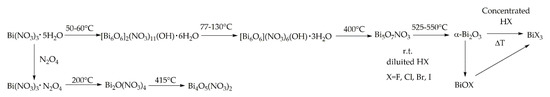
Figure 1. Comprehensive scheme of chemical evolution of Bi(NO3)3×5 H2O.
An interesting study was reported by Tanveer et al. [4] about the transition from Bi(NO3)3×5H2O to Bi5O7NO3 showing how it is possible to isolate a species of Bi5O7NO3 tailored on the surface with β-Bi2O3.
Bismuth oxides are the other deeply studied class of bismuth compounds and they present four different phases [19] as reported in Figure 2.

Figure 2. Scheme of phase transition of bismuth oxide.
At room temperature, monoclinic α-Bi2O3 is the common stable phase with a polymeric-distorted layered structure composed of pentacoordinate bismuth atoms enclosed into pseudo-octahedral units. At a temperature higher than 710 °C, α phase is converted into the cubic δ phase that has a defective structure with random oxygen vacancies [20]. The β phase and several oxygen-rich forms are closely related to the δ phase. In particular, the vacancy structures of highly defected bismuth oxides some sites filled with O−2 together with Bi(III) and Bi (V) sites. Bismuth oxide γ-phase shows also a cubic structure but it is highly unstable and hard to synthesize without supporting it onto other oxides or metallic species [21]. The other two polymorphic metastable bismuth oxide phases are known as the ω phase stable at temperatures higher than 800 °C [22] and the ε phase isolated in 2006 by Cornei and co-workers [23].
Bismuth(V) oxides are less stable than Bi(III) but several studies reported their preparation as lithium [24] or sodium [25] salt derivatives.
Bismuth derivatives were also studied for the production of colloidal phases. Kiran et al. [26] synthesized a bismuth-substituted cobalt ferrite with a nominal formula CoFe2−0.1Bi0.1O4 quite active for the reduction of 4-nitrophenol to 4-aminophenol in a watery solution of sodium borhydride. Metal bismuth nanoparticles were produced by Petsom et al.[27] showing that the size of the nanoparticles can be tuned by adding different amounts of ionic and non-ionic surfactants.
Furthermore, several organometallic species of bismuth such as subgallate [28] and subsalicylate[29] have found use in medical applications that will be more thoroughly discussed in the next sections and briefly summarized in Table 1.
Table 1. Summary of main properties of bismuth and related compounds.
|
Bismuth Species |
Advantages |
Issues |
|
Metallic bismuth |
§ Easy to synthesized § High size control § Highest concentration of radiopaque atoms |
§ High cytotoxicity for low average size particles § Only spherical shaped § Neat surfaces without any functional groups |
|
Organometallic bismuth |
§ Hydrosoluble § High cellular uptaking § High stability |
§ Low concentration of radiopaque atoms § Fast excretion § Could trespass the hematoencephalic barrier[30] |
|
Bismuth nitrates |
§ High shape tunability § Highly tailoring surface |
§ Fast hydrolysis in watery phase under mild conditions § Difficult to isolated as pure compounds § Difficult to predict the correct active species |
|
Bismuth halide and oxohalides |
§ Easily synthesizable § Photocatalytic activity |
§ Oxidizable § Hygroscopic § Highly acidic |
|
Bismuth oxides |
§ Highly stable § Easy to synthesized § High size control § Poor cytotoxicity § Good cellular uptake § Photocatalytic activity |
§ Highly hydrophobic § Phase impurities § Surface defects |
References
- Karen Barrera-Mota; Monserrat Bizarro; Micaela Castellino; Alberto Tagliaferro; Aracely Hernández; Sandra E. Rodil; Spray deposited β-Bi2O3 nanostructured films with visible photocatalytic activity for solar water treatment. Photochemical & Photobiological Sciences 2015, 14, 1110-1119, 10.1039/c4pp00367e.
- Pravin Jagdale; Micaela Castellino; Françoise Marrec; Sandra E. Rodil; Alberto Tagliaferro; Nano sized bismuth oxy chloride by metal organic chemical vapour deposition. Applied Surface Science 2014, 303, 250-254, 10.1016/j.apsusc.2014.02.158.
- M.A. Meitl; T.M. Dellinger; P. V. Braun; Bismuth–Ceramic Nanocomposites with Unusual Thermal Stability via High-Energy Ball Milling. Advanced Functional Materials 2003, 13, 795-799, 10.1002/adfm.200304433.
- Tanveer A. Gadhi; Simelys Hernàndez; Micaela Castellino; Pravin Jagdale; Thomas Husak; Agileo Hernández-Gordillo; Alberto Tagliaferro; Nunzio Russo; Insights on the role of β-Bi2O3/Bi5O7NO3 heterostructures synthesized by a scalable solid-state method for the sunlight-driven photocatalytic degradation of dyes. Catalysis Today 2019, 321-322, 135-145, 10.1016/j.cattod.2017.12.038.
- Tanveer A. Gadhi; Agileo Hernández-Gordillo; Monserrat Bizarro; Pravin Vitthal Jagdale; Alberto Tagliaferro; Sandra E. Rodil; Efficient α/β-Bi 2 O 3 composite for the sequential photodegradation of two-dyes mixture. Ceramics International 2016, 42, 13065-13073, 10.1016/j.ceramint.2016.05.087.
- Narveer Singh; Kanwar Jit Singh; Kulwant Singh; Harvinder Singh; Comparative study of lead borate and bismuth lead borate glass systems as gamma-radiation shielding materials. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2004, 225, 305-309, 10.1016/j.nimb.2004.05.016.
- K. Mallaley; D. R. Morris; Analysis of the Kroll-Betterton Process: The Removal of Bismuth from Lead Bullion. Canadian Metallurgical Quarterly 1990, 29, 67-71, 10.1179/000844390795576175.
- E. Peters; David Dreisinger; J. A. González-Domínguez; The refining of lead by the Betts process. Journal of Applied Electrochemistry 1991, 21, 189-202, 10.1007/bf01052570.
- Chester R. Berry; Electron Diffraction from Small Crystals. Physical Review 1952, 88, 596-599, 10.1103/physrev.88.596.
- Lijun Zhao; Xiaochao Zhang; Caimei Fan; Zhenhai Liang; Peide Han; First-principles study on the structural, electronic and optical properties of BiOX (X=Cl, Br, I) crystals. Physica B: Condensed Matter 2012, 407, 3364-3370, 10.1016/j.physb.2012.04.039.
- Greenwood, N.N.; Earnshaw, A.. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012; pp. 547-596.
- Gen-Qing Liu; Hui Zhong; Xiaorong Li; Kai Yang; Fei-Fei Jia; Zhi-Peng Cheng; Li-Li Zhang; Jing-Zhou Yin; Li-Ping Guo; Hai-Yan Qian; et al. Research on nonenzymatic electrochemical sensor using HO-BiONO3 nanocomposites for glucose detection. Sensors and Actuators B: Chemical 2017, 242, 484-491, 10.1016/j.snb.2016.11.019.
- Glen G. Briand; Neil Burford; Bismuth Compounds and Preparations with Biological or Medicinal Relevance. Chemical Reviews 1999, 99, 2601-2658, 10.1021/cr980425s.
- B. Lu; Y. Zhu; Synthesis and photocatalysis performances of bismuth oxynitrate photocatalysts with layered structures. Physical Chemistry Chemical Physics 2014, 16, 16509-16514, 10.1039/c4cp01489h.
- Yuxiao Yang; Huoyan Liang; Na Zhu; Yaping Zhao; Changsheng Guo; Lu Liu; New type of [Bi6O6(OH)3](NO3)3·1.5H2O sheets photocatalyst with high photocatalytic activity on degradation of phenol. Chemosphere 2013, 93, 701-707, 10.1016/j.chemosphere.2013.06.062.
- A. Zahariev; Nikolay Kaloyanov; C. Girginov; Veneta Parvanova; Synthesis and thermal decomposition of [Bi6O6(OH)2](NH2C6H4SO3)4. Thermochimica Acta 2012, 528, 85-89, 10.1016/j.tca.2011.11.003.
- Hiroshi Kodama; Synthesis of a New Compound, Bi5O7NO3, by Thermal Decomposition. Journal of Solid State Chemistry 1994, 112, 27-30, 10.1006/jssc.1994.1259.
- Shujie Yu; Gaoke Zhang; Yuanyuan Gao; Baibiao Huang; Single-crystalline Bi5O7NO3 nanofibers: Hydrothermal synthesis, characterization, growth mechanism, and photocatalytic properties. Journal of Colloid and Interface Science 2011, 354, 322-330, 10.1016/j.jcis.2010.10.012.
- Ernest M. Levin; Robert S. Roth; Polymorphism of Bismuth Sesquioxide. I. Pure Bi2O3. Journal of Research of the National Bureau of Standards Section A: Physics and Chemistry 1964, 68, 189-195, 10.6028/jres.068A.019.
- Lei, B.; Cui, W.; Sheng, J.; Wang, H.; Chen, P.; Li, J.; Sun, Y.; Dong; Synergistic effects of crystal structure and oxygen vacancy on Bi2O3 polymorphs: intermediates activation, photocatalytic reaction efficiency, and conversion pathway. Science Bulletin 2020, 65 (6), 467-476, 10.1016/j.scib.2020.01.007.
- T.M. Bruton; J.C. Brice; O.F. Hill; P.A.C. Whiffin; The flux growth of some γ-Bi2O3 crystals by the top seeded technique. Journal of Crystal Growth 1974, 23, 21-24, 10.1016/0022-0248(74)90036-0.
- A. F. Gualtieri; S. Immovilli; M. Prudenziati; Powder X-ray diffraction data for the new polymorphic compound ω-Bi2O3. Powder Diffraction 1997, 12, 90-92, 10.1017/s0885715600009490.
- Nicoleta Cornei; Nathalie Tancret; Francis Abraham; Olivier Mentré; New ε-Bi2O3Metastable Polymorph. Inorganic Chemistry 2006, 45, 4886-4888, 10.1021/ic0605221.
- N. Kumada; N. Takahashi; N. Kinomura; A.W. Sleight; Preparation and Crystal Structure of a New Lithium Bismuth Oxide: LiBiO3. Journal of Solid State Chemistry 1996, 126, 121-126, 10.1006/jssc.1996.0319.
- Ting Zhang; Yaobin Ding; Heqing Tang; Generation of singlet oxygen over Bi(V)/Bi(III) composite and its use for oxidative degradation of organic pollutants. Chemical Engineering Journal 2015, 264, 681-689, 10.1016/j.cej.2014.12.014.
- Venkat Savunthari Kiran; Shanmugam Sumathi; Comparison of catalytic activity of bismuth substituted cobalt ferrite nanoparticles synthesized by combustion and co-precipitation method. Journal of Magnetism and Magnetic Materials 2017, 421, 113-119, 10.1016/j.jmmm.2016.07.068.
- K. Petsom; Atcha Kopwitthaya; M. Horphathum; J. Kaewkhao; N. Sangwaranatee; The effect of additive chemicals on synthesis of bismuth nanoparticles. Materials Today: Proceedings 2018, 5, 14057-14062, 10.1016/j.matpr.2018.02.061.
- Vinicius Augusto Tramontina; Maria Angela Naval Machado; Getúlio Da Rocha Nogueira Filho; Sung Hyun Kim; Mário R Vizzioli; Sérgio De Toledo; Effect of bismuth subgallate (local hemostatic agent) on wound healing in rats. Histological and histometric findings.. Brazilian Dental Journal 2002, 13, 11-16.
- H. L. Dupont; Prevention of travelers' diarrhea by the tablet formulation of bismuth subsalicylate. JAMA: The Journal of the American Medical Association 1987, 257, 1347-1350, 10.1001/jama.257.10.1347.
- Roger Pamphlett; M. Stoltenberg; Jørgen Rungby; Gorm Danscher; Uptake of bismuth in motor neurons of mice after single oral doses of bismuth compounds.. Neurotoxicology and Teratology 2000, 22, 559-563, 10.1016/s0892-0362(00)00083-0.




