| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mădălina Oprea | + 2018 word(s) | 2018 | 2020-12-02 03:32:02 |
Video Upload Options
Tissue engineering is an interdisciplinary field that combines principles of engineering and life sciences to obtain biomaterials capable of maintaining, improving, or substituting the function of various tissues or even an entire organ. In virtue of its high availability, biocompatibility, and versatility, cellulose was considered a promising platform for such applications. The combination of cellulose with graphene or graphene derivatives leads to the obtainment of superior composites in terms of cellular attachment, growth and proliferation, integration into host tissue, and stem cell differentiation toward specific lineages.
1. General Aspects Concerning Tissue Engineering
In recent decades, the rapidly aging population, environmental stressors, frequent cases of traumatic injuries, and chronic diseases lead to a growing interest in the revolutionary domain of tissue engineering (TE) [1][2][3]. Tissue engineering evolved from the field of biomaterials; its purpose is to combine scaffolds, cells, and biologically active molecules to obtain multifunctional materials that restore, maintain, or improve damaged tissues or an entire organ. Some examples of Food and Drug Administration (FDA)-approved engineered tissues include artificial skin and cartilage, but they have limited use in human medicine due to several yet unknown aspects regarding their long term biocompatibility [4][5][6][7][8][9]. Bioactive scaffolds, cell therapy, smart drug delivery systems, and wound healing mats are some representative examples of the research topics approached by TE. In addition to medical applications, non-therapeutic findings include the use of tissues as biosensors to detect chemical or biological threats or the development of organs-on-a-chip for toxicity screening of experimental medication [4][8][10].
Porous three-dimensional (3D) scaffolds are an important component of tissue engineering. These constructs are used to provide an appropriate environment for tissue and organ regeneration. Biological scaffolds (e.g., fibrin, amniotic membrane, and perfusion-decellularized organs) are an accessible option because they already contain a broad spectrum of signaling molecules with an important role in the processes of cellular morphogenesis and function development [11][12]. However, their composition is strongly related to their source of origin, therefore they have poor reproducibility. Biomaterials-based scaffolds have the advantage that they can be tailored to meet specific requirements, the result being a controllable environment in which stem cells and growth factors can be incorporated to recreate various tissues [4][5][13]. Considering the response of the body’s immune system, it is recommended that scaffolds replicate the native extracellular matrix (ECM) of different tissues, in terms of physical structure, chemical composition, and biological functionality [13][14][15][16]. Biocompatibility, non-immunogenicity, and non-toxicity are mandatory features of biomaterials-based scaffolds. Their design and mechanical properties are also important because they should have the ability to enhance cell migration, proliferation, and differentiation, by presenting appropriate biomechanical, biophysical, and biochemical signals, in vivo, while maintaining their shape and integrity [17][18].
2. Characteristics Recommending Cellulose and Graphene for Applications in Tissue Engineering
Cellulose remarks itself among the biomaterials used for scaffold production due to its high availability and renewability. Cellulose is mainly extracted from plant cell walls. The nano-scaled forms of plant cellulose—cellulose nanofibers (CNFs) and cellulose nanocrystals (CNCs), are obtained following specific mechanical and chemical treatments (e.g., ball milling, enzymatic or chemical hydrolysis, TEMPO-mediated oxidation) [3][19]. In virtue of its natural origin, cellulose has native biocompatibility and negligible cytotoxicity [20]. Some issues were posed related to the inflammatory effect and oxidative stress caused by the cellular uptake of the nano-scaled forms of cellulose [21] but studies showed that both cellulose nanocrystals and cellulose nanofibers presented a non-immunogenic and non-cytotoxic character when different mammalian cell lines were exposed to CNCs suspensions [22] or CNFs membranes [23].
Cellulose can also be produced by certain microorganisms, in this case being called bacterial cellulose (BC). BC is considered to be the most biocompatible form of cellulose because it lacks biogenic impurities such as lignin and hemicellulose and only mild chemical treatments are required to ensure its purity [24]. The nanofibrillar porous structure of bacterial cellulose is similar to the extracellular matrix, thus making BC one of the most recommended materials for tissue engineering scaffolds [14][25][26]. Previous studies highlighted that BC has the ability to reduce the inflammatory response and increase the rate of tissue regeneration when it is used as a wound dressing [25][26][27]. Due to its excellent mechanical properties, BC was considered a promising material for the development of vascular grafts, dental implants, artificial skin, and blood vessels [25][28][29][30][31][32].
Pure cellulose lacks solubility in common organic solvents. This represents an issue when it comes to processing techniques where a stable polymeric solution is required to prepare the final material (e.g., electrospinning, phase inversion). Cellulose was dissolved so far only in mixtures of highly toxic solvents (e.g., ionic liquids, carbon disulfide, N-methyl-morpholine-N-oxide, dimethylformamide) [33]; however, for biomedical applications such as tissue engineering, they are not recommended because even small traces of such solvents could cause a substantial biocompatibility decrease. Consequently, cellulose derivatives were developed, their improved dissolution ability in less noxious solvents, or even water, encouraging their use as an alternative to pure cellulose [3]. Moreover, cellulose derivatives maintain the biocompatibility features of pristine cellulose presenting mild or no foreign body reaction during in vivo assays [34].
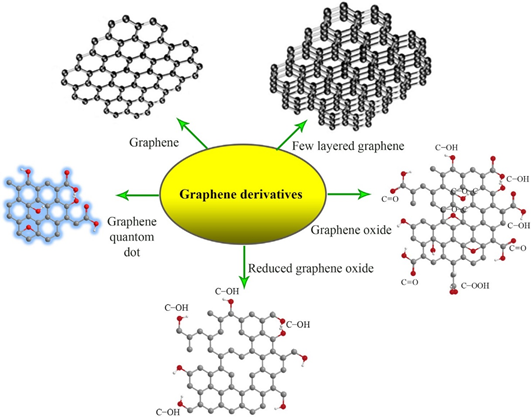
Graphene (GE) is an allotrope of carbon produced by top-down (e.g., mechanical or chemical exfoliation of graphite, chemical synthesis) or bottom-up techniques (e.g., chemical vapor deposition, pyrolysis, epitaxial growth) [35]. GE has some unique properties such as high specific surface area, superior electrical and thermal conductivity, and excellent mechanical properties. The free π electrons and reactive sites, generated by the plane carbon-carbon bonds in GE’s aromatic structure, ensure it a facile surface functionalization [36][37]. However, the hydrophobicity and strong interactions between sheets hinder the dispersion of GE in aqueous or organic environments [38][39]. This issue was solved with the development of graphene derivatives such as graphene oxide (GO) and reduced graphene oxide (rGO) which possess specific surface groups that allow them to be effectively dispersed in a wide range of solvents or to be incorporated in polymeric matrices (Figure 1) [40][41]. For example, graphene oxide presents hydroxyl functional groups on the upper and bottom surface as well as carboxylic groups on the edges. This chemical structure is characterized by a hydrophilic character that enables GO’s dispersion in water and polar solvents and facilitates hydrogen bonding with polymeric matrices [42]. Reduced graphene oxide is characterized by a lower hydrophilicity and oxygen content but an enhanced electrical conductivity. It was showed that the addition of rGO in polymer composites can increase thermal stability, improve the bioactivity and mechanical properties, and also provide an appropriate medium for electrical stimulation procedures [40][43][44]. A special type of graphene derivative is represented by graphene quantum dots (GQDs). This nano-scaled form of graphene is made up of one or a few GE layers, with lateral dimensions smaller than 10 nm (Figure 1). GQDs photoluminescence and quantum confinement effect recommend them for applications in bioimagistics, biosensors, and photocatalysis devices [45].
Figure 1. The chemical structures of graphene and its derivatives [46]. Reproduced with copyright permission.
Generally, graphene and its derivatives are considered biocompatible and non-cytotoxic, still, their preparation method highly influences the in vivo and in vitro tests because the residual solvents and reagents used during the synthesis procedures can interact with cells and tissues, thus inducing cytotoxicity and oxidative stress. The hydrazine used to obtain reduced graphene oxide was found to be particularly noxious for human mesenchymal stem cells (hMSCs) [47]. Eco-friendly reduction methods were developed to diminish these residual chemicals-induced adverse effects. For example, Erdal et al. used a microwave-induced hydrothermal reaction and caffeic acid (Caf), a green reducing agent, to produce nanosized reduced graphene oxide (n-rGO), starting from commercial α-cellulose. Cellulose was treated with an aqueous solution of H2SO4 in a microwave device. The material was kept at 180 °C and a pressure of 40 bar, for 2 h, under nitrogen flow. Following this treatment, black solid carbon spheres were obtained. The spheres were further dispersed in concentrated nitric acid (HNO3), ultrasonicated 30 min at 45 °C, and heated at 90 °C for 30 min, under magnetic stirring to obtain nGO. The ultrasonication time is a decisive factor in obtaining materials with uniform and reproducible properties [48]. The green reduction process was performed by placing an aqueous suspension of nGO and Caf in the microwave device, using the same conditions as in the case of carbon spheres synthesis [49]. The resulting n-rGO was incorporated in polycaprolactone (PCL) matrices for the production of bioactive and bioresorbable composites [50], 3D scaffolds with drug delivery ability [51], and macroporous scaffolds with applications in bone tissue engineering [52].
The size and oxidation status of GE are also important for cytotoxicity evaluation. GE and GE derivatives may disrupt cell membranes by direct contact. Moreover, small-sized GO has a high potential of being internalized into cells via endocytosis and could cause apoptosis at high concentrations [53]. Surface modification with biocompatible molecules or the insertion in biopolymer matrices were the main solutions proposed to minimize the potential cytotoxic character of GE and GE derivatives. Besides, after incorporation in a polymer matrix, the carbonaceous fillers provide cellular binding sites and, in the case of GO, the oxygenated surface groups increase the hydrophilic character, thus improving cellular adhesion [47].
GE and its derivatives have a demonstrated ability to promote stem cell differentiation processes, particularly adipogenesis and osteogenesis by enhancing the adsorption of differentiation factors and cell adhesion [54]. Graphene-induced osteogenesis was found to be related to the activation of the mechanosensitive integrin-focal adhesion kinase (FAK) axis and also to GE’s capacity of promoting the paracrine release of pro-osteogenic molecules in its surroundings, as well as enhancing their delivery to the sites of action [55]. According to recent studies, graphene oxide and reduced graphene oxide are pro-angiogenic. The mechanisms for GO and rGO induced angiogenesis include the intracellular formation of reactive oxygen and nitrogen species as well as activation of specific serum antibodies (e.g., phospho-eNOS, phospho-Akt) [56]. The potential of reduced graphene oxide (rGO) to enhance angiogenesis was evaluated by Chakraborty et al. using polyvinyl alcohol/carboxymethyl cellulose (PVA/CMC) scaffolds loaded with different concentrations of rGO nanoparticles. Primary biocompatibility studies included in vitro alamarBlue cytotoxicity assays on three different cell lines—fibroblasts NIH3T3, endothelial-like cells (ECV304), and endothelial cells (EA.hy926). The scaffolds showed no toxicity toward the analyzed cell lines, the cellular viability being similar to the control group. It was concluded that when incorporated inside a scaffold, rGO does not present cytotoxicity even if it is used in concentrations higher than the cytotoxicity threshold in free solution (100 ng/mL). The composite scaffolds were implanted in chick chorioallantoic membrane (CAM) models to study their influence on the neovascularization process. Two days following implantation, the number and wall thickness of the blood vessels were substantially increased, compared to the untreated control. Moreover, angiogenesis and arteriogenesis were enhanced as the rGO concentration in the composites increased, whereas neat PVC/CMC scaffolds showed no bioactivity [57].
As a result of their remarkable properties, cellulose/graphene composites were extensively researched particularly for biomedical applications. Cellulose is a versatile, highly available, biodegradable, and biocompatible material, these characteristics recommending it as a low cost, sustainable alternative to petroleum-based plastics or other types of natural polymers used in tissue engineering. Cellulose/graphene composites designed for the TE field can be divided into two main categories—composites where specific types of cellulose (e.g., cellulose paper [58], bacterial cellulose [59], cellulose derivatives [60]) are employed as polymer matrices in which the carbonaceous fillers are dispersed to improve their mechanical characteristics and biocompatibility, or, composite fillers based on CNCs or CNFs combined with GE or GE derivatives that are used to synergistically reinforce other polymer matrices [21] (e.g., polylactic acid—PLA [61][62], polybutylene succinate—PBS [63], polyacrylamide—PAM [38], polycaprolactone—PCL [64]). Composite membranes with graphene [65][66] were also studied for applications in the hemodialysis field [67][68]. The use of GO for reinforcing cellulose acetate membranes led to an increase of bovine serum albumin (BSA) retention from 80% to over 96% [69][70]. The synergistic effect between GO and carbon nanotubes (CNT) used for the preparation of composite cellulose acetate membranes with potential applications in hemodialysis showed good results in the retention of BSA and hemoglobin [71]. These results are due to both the presence of GO, which has a high surface adsorption ability of the proteins that need to be separated [72][73] and also to the weak chemical interactions that emerge between the delocalized electrons on the surface of graphene and the non-participating electrons from the functional groups of the polymer [74][75].
References
- Profire, L.; Constantin, S.M. Nanomaterials in Tissue Engineering. In Polymeric Nanomaterials in Nanotherapeutics, Vasile, C., Ed. Elsevier: 2019, Chapter 12, pp. 421-436.
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Ann. Rev. Chem. Biomol. Eng. 2011, 2, 403-430, doi:10.1146/annurev-chembioeng-061010-114257.
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683, doi:https://doi.org/10.1016/j.carbpol.2020.116683.
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H., et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, doi:10.3389/fbioe.2020.00083.
- Elitok, M.S.; Gunduz, E.; Gurses, H.E.; Gunduz, M. Tissue Engineering: Towards Development of Regenerative and Transplant Medicine A2 - Barh, Debmalya. In Omics Technologies and Bio-Engineering, Azevedo, V., Ed. Academic Press: 2018, Chapter 20, pp. 471-495.
- Steffens, D.; Braghirolli, D.I.; Maurmann, N.; Pranke, P. Update on the main use of biomaterials and techniques associated with tissue engineering. Drug Discov. Today 2018, https://doi.org/10.1016/j.drudis.2018.03.013.
- Frey, B.M.; Zeisberger, S.M.; Hoerstrup, S.P. Tissue Engineering and Regenerative Medicine - New Initiatives for Individual Treatment Offers. Transfus. Med. Hemother. 2016, 43, 318-319, doi:10.1159/000450716.
- Katari, R.; Peloso, A.; Orlando, G. Tissue Engineering and Regenerative Medicine: Semantic Considerations for an Evolving Paradigm. Front. Bioeng. Biotechnol. 2014, 2, 57, doi:10.3389/fbioe.2014.00057.
- Tobita, M.; Konomi, K.; Torashima, Y.; Kimura, K.; Taoka, M.; Kaminota, M. Japan's challenges of translational regenerative medicine: Act on the safety of regenerative medicine. Regenerative Therapy 2016, 4, 78-81, doi:https://doi.org/10.1016/j.reth.2016.04.001.
- Saul, J.M.; Williams, D.F. Hydrogels in Regenerative Medicine A2 - Modjarrad, Kayvon. In Handbook of Polymer Applications in Medicine and Medical Devices, Ebnesajjad, S., Ed. William Andrew Publishing: Oxford, 2011, Chapter 12, pp. 279-302.
- Tominac Trcin, M.; Dekaris, I.; Mijović, B.; Bujić, M.; Zdraveva, E.; Dolenec, T.; Pauk-Gulić, M.; Primorac, D.; Crnjac, J.; Špoljarić, B., et al. Synthetic vs natural scaffolds for human limbal stem cells. Croat. Med. J. 2015, 56, 246-256, doi:10.3325/cmj.2015.56.246.
- Goh, S.-K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E., et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760-6772, doi:https://doi.org/10.1016/j.biomaterials.2013.05.066.
- Castells-Sala, C.; Alemany-Ribes, M.; Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Aloy-Reverté, C.; Caballero-Camino, J.; Márquez-Gil, A.; Semino, C.E. Current applications of tissue engineering in biomedicine. J. Biochips Tiss. Chips 2013, S2, 1.
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mat. Sci. Eng.: C 2018, 82, 372-383, doi:https://doi.org/10.1016/j.msec.2016.11.121.
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530-544, doi:https://doi.org/10.1016/j.biotechadv.2017.05.006.
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1-12, doi:https://doi.org/10.1016/j.actbio.2015.11.007.
- Oprea, M.; Panaitescu, D.; Nicolae, C.-A.; Gabor, R.; Frone, A.; Raditoiu, V.; Trusca, R.; Casarica, A. Nanocomposites from functionalized bacterial cellulose and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polym. Degrad. Stab. 2020, 179, 109203, doi:10.1016/j.polymdegradstab.2020.109203.
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue engineered scaffolds in regenerative medicine. World J. Plast. Surg. 2014, 3, 3-7.
- Frone, A.N.; Chiulan, I.; Panaitescu, D.M.; Nicolae, C.A.; Ghiurea, M.; Galan, A.-M. Isolation of cellulose nanocrystals from plum seed shells, structural and morphological characterization. Mater. Lett. 2017, 194, 160-163, doi:https://doi.org/10.1016/j.matlet.2017.02.051.
- Credou, J.; Berthelot, T.J.J.o.M.C.B. Cellulose: from biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767-4788, doi: https://doi.org/10.1039/C4TB00431K.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392-392, doi:10.3389/fchem.2020.00392.
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano LIFE 2012, 2, 1241006, doi: https://doi.org/10.1142/S1793984412410061.
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym, 2018, 201, 87-95, doi:https://doi.org/10.1016/j.carbpol.2018.08.056.
- Khan, S.; Ul-Islam, M.; Ikram, M.; Ullah, M.W.; Israr, M.; Subhan, F.; Kim, Y.; Jang, J.H.; Yoon, S.; Park, J.K. Three-dimensionally microporous and highly biocompatible bacterial cellulose–gelatin composite scaffolds for tissue engineering applications. RSC Advances 2016, 6, 110840-110849, doi:10.1039/C6RA18847H.
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials 2017, 7, 257, doi:10.3390/nano7090257.
- Torres, F.; Commeaux, S.; Troncoso, O. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864-878.
- Liyaskina, E.; Revin, V.; Paramonova, E.; Nazarkina, M.; Pestov, N.; Revina, N.; Kolesnikova, S. Nanomaterials from bacterial cellulose for antimicrobial wound dressing. J. Phys. Conf. Ser. 2016, 784, 012034.
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358-3393.
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of bacterial cellulose production and application. Agric. Agric. Sci. Proc. 2014, 2, 113-119.
- Pandele, A.M.; Comanici, F.E.; Carp, C.A.; Miculescu, F.; Voicu, S.I.; Thakur, V.K.; Serban, B.C. Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599-605, doi:https://doi.org/10.1016/j.vacuum.2017.05.008.
- Pandele, A.M.; Neacsu, P.; Cimpean, A.; Staras, A.I.; Miculescu, F.; Iordache, A.; Voicu, S.I.; Thakur, V.K.; Toader, O.D. Cellulose acetate membranes functionalized with resveratrol by covalent immobilization for improved osseointegration. Appli. Surf. Sci. 2018, 438, 2-13, doi:https://doi.org/10.1016/j.apsusc.2017.11.102.
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T.J.M. Synthesis and characterization of pla-micro-structured hydroxyapatite composite films. Materials 2020, 13, 274.
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent Advances in Solvents for the Dissolution, Shaping and Derivatization of Cellulose: Quaternary Ammonium Electrolytes and their Solutions in Water and Molecular Solvents. Molecules 2018, 23, doi:10.3390/molecules23030511.
- Miyamoto, T.; Takahashi, S.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125-133, doi:10.1002/jbm.820230110.
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65-83, doi:10.1007/s40089-015-0176-1.
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236-250, doi:10.1016/j.biomaterials.2017.10.004.
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mat. Sci. Eng.: C 2017, 77, 1363-1375, doi:https://doi.org/10.1016/j.msec.2017.03.196.
- Kumar, A.; Rao, K.M.; Han, S.S. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydr. Polym. 2018, 193, 228-238, doi:https://doi.org/10.1016/j.carbpol.2018.04.004.
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opinion Coll. Interf. Sci. 2015, 20, 367-382, doi:https://doi.org/10.1016/j.cocis.2015.11.004.
- Lin, J.; Chen, X.; Huang, P. Graphene-based nanomaterials for bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242-254, doi:https://doi.org/10.1016/j.addr.2016.05.013.
- Syama, S.; Mohanan, P.V. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546-555, doi:10.1016/j.ijbiomac.2016.01.116.
- Azarniya, A.; Eslahi, N.; Mahmoudi, N.; Simchi, A. Effect of graphene oxide nanosheets on the physico-mechanical properties of chitosan/bacterial cellulose nanofibrous composites. Compos. Part A: Appl. Sci. Manuf. 2016, 85, 113-122, doi:https://doi.org/10.1016/j.compositesa.2016.03.011.
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y., et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mat. Sci. Eng.: C 2020, 109, 110560, doi:https://doi.org/10.1016/j.msec.2019.110560.
- Foo, M.E.; Gopinath, S.C.B. Feasibility of graphene in biomedical applications. Biomed. Pharmacother. 2017, 94, 354-361, doi:https://doi.org/10.1016/j.biopha.2017.07.122.
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424, doi:10.3389/fchem.2020.00424.
- Ramezani, M.; Alibolandi, M.; Nejabat, M.; Charbgoo, F.; Taghdisi, S.M.; Abnous, K. Graphene-Based Hybrid Nanomaterials for Biomedical Applications. In Biomedical Applications of Graphene and 2D Nanomaterials, Nurunnabi, M., McCarthy, J.R., Eds. Elsevier, 2019, Chapter 6, pp. 119-141.
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564.
- Voicu, S.; Pandele, M.; Vasile, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomater. Bios. 2013, 8, 1389-1394.
- Erdal, N.B.; Adolfsson, K.H.; Pettersson, T.; Hakkarainen, M. Green Strategy to Reduced Nanographene Oxide through Microwave Assisted Transformation of Cellulose. ACS Sustain. Chem. Eng. 2018, 6, 1246-1255, doi:10.1021/acssuschemeng.7b03566.
- Erdal, N.B.; Hakkarainen, M. Construction of Bioactive and Reinforced Bioresorbable Nanocomposites by Reduced Nano-Graphene Oxide Carbon Dots. Biomacromol. 2018, 19, 1074-1081, doi:10.1021/acs.biomac.8b00207.
- B. Erdal, N.; Yao, J.G.; Hakkarainen, M. Cellulose-Derived Nanographene Oxide Surface-Functionalized Three-Dimensional Scaffolds with Drug Delivery Capability. Biomacromol. 2019, 20, 738-749, doi:10.1021/acs.biomac.8b01421.
- Yadav, A.; Erdal, N.B.; Hakkarainen, M.; Nandan, B.; Srivastava, R.K. Cellulose-Derived Nanographene Oxide Reinforced Macroporous Scaffolds of High Internal Phase Emulsion-Templated Cross-Linked Poly(ε-caprolactone). Biomacromol. 2020, 21, 589-596, doi:10.1021/acs.biomac.9b01330.
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv, Rev. 2016, 105, 145-162, doi:https://doi.org/10.1016/j.addr.2016.08.009.
- Ignat, S.R.; Lazăr, A.D.; Şelaru, A.; Samoilă, I.; Vlăsceanu, G.M.; Ioniţă, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile Biomaterial Platform Enriched with Graphene Oxide and Carbon Nanotubes for Multiple Tissue Engineering Applications. Int. J. Mol. Sci. 2019, 20(16), 3868, doi:10.3390/ijms20163868.
- Xie, H.; Cao, T.; Franco-Obregón, A.; Rosa, V. Graphene-Induced Osteogenic Differentiation Is Mediated by the Integrin/FAK Axis. Int. J. Mol. Sci. 2019, 20, 574, doi:10.3390/ijms20030574.
- Mukherjee, S.; Sriram, P.; Barui, A.K.; Nethi, S.K.; Veeriah, V.; Chatterjee, S.; Suresh, K.I.; Patra, C.R. Graphene Oxides Show Angiogenic Properties. Adv. Healthc. Mat. 2015, 4, 1722-1732, doi:10.1002/adhm.201500155.
- Chakraborty, S.; Ponrasu, T.; Chandel, S.; Dixit, M.; Muthuvijayan, V. Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. R. Soc. Open Sci. 2018, 5, 172017-172017, doi:10.1098/rsos.172017.
- Li, J.; Liu, X.; Tomaskovic-Crook, E.; Crook, J.M.; Wallace, G.G. Smart graphene-cellulose paper for 2D or 3D “origami-inspired” human stem cell support and differentiation. Coll. Surf. B: Biointerf. 2019, 176, 87-95, doi:https://doi.org/10.1016/j.colsurfb.2018.12.040.
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mat. Sci. Eng. C 2019, 98, 1277-1293, doi:https://doi.org/10.1016/j.msec.2019.01.064.
- Terzopoulou, Z.; Kyzas, G.Z.; Bikiaris, D.N. Recent Advances in Nanocomposite Materials of Graphene Derivatives with Polysaccharides. Materials 2015, 8, 652-683, doi:10.3390/ma8020652.
- Pal, N.; Dubey, P.; Gopinath, P.; Pal, K. Combined effect of cellulose nanocrystal and reduced graphene oxide into poly-lactic acid matrix nanocomposite as a scaffold and its anti-bacterial activity. Int. J. Biol. Macromol. 2017, 95, 94-105, doi:https://doi.org/10.1016/j.ijbiomac.2016.11.041.
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Reduced graphene oxide and PEG-grafted TEMPO-oxidized cellulose nanocrystal reinforced poly-lactic acid nanocomposite film for biomedical application. Mat. Sci. Eng.: C 2019, 104, 109956, doi:https://doi.org/10.1016/j.msec.2019.109956.
- Neibolts, N.; Platnieks, O.; Gaidukovs, S.; Barkane, A.; Thakur, V.K.; Filipova, I.; Mihai, G.; Zelca, Z.; Yamaguchi, K.; Enachescu, M. Needle-free electrospinning of nanofibrillated cellulose and graphene nanoplatelets based sustainable poly (butylene succinate) nanofibers. Mater. Today Chem. 2020, 17, 100301, doi:https://doi.org/10.1016/j.mtchem.2020.100301.
- Patel, D.K.; Seo, Y.-R.; Dutta, S.D.; Lim, K.-T. Enhanced osteogenesis of mesenchymal stem cells on electrospun cellulose nanocrystals/poly(ε-caprolactone) nanofibers on graphene oxide substrates. RSC Adv. 2019, 9, 36040-36049, doi:10.1039/C9RA06260B.
- Pandele, A.M.; Serbanescu, O.S.; Voicu, S.I. Polysulfone Composite Membranes with Carbonaceous Structure. Synthesis and Applications. Coatings 2020, 10, 609, doi.org/10.3390/coatings10070609.
- Serbanescu, O.; Pandele, A.; Miculescu, F.; Voicu, Ş.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468, doi:10.3390/coatings10050468.
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface modified cellulose acetate membranes for the reactive retention of tetracycline. Sep. Purif. Technol. 2020, 249, 117145, doi:https://doi.org/10.1016/j.seppur.2020.117145.
- Corobea, M.C.; Muhulet, O.; Miculescu, F.; Antoniac, I.V.; Vuluga, Z.; Florea, D.; Vuluga, D.M.; Butnaru, M.; Ivanov, D.; Voicu, S.I. Novel nanocomposite membranes from cellulose acetate and clay‐silica nanowires. 2016, 27, 1586-1595.
- Ionita, M.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Iovu, H.J.P.f.A.T. Fabrication of cellulose triacetate/graphene oxide porous membrane. Polym. Adv. Tech. 2016, 27, 350-357.
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B: Eng. 2015, 72, 108-115.
- Ioniță, M.; Crică, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50-61.
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B: Eng. 2017, 121, 34-57.
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154-186.
- Rusen, E.; Mocanu, A.; Nistor, L.C.; Dinescu, A.; Călinescu, I.; Mustăţea, G.; Voicu, Ş.I.; Andronescu, C.; Diacon, A. Design of Antimicrobial Membrane Based on Polymer Colloids/Multiwall Carbon Nanotubes Hybrid Material with Silver Nanoparticles. ACS Appl. Mater. Interf. 2014, 6, 17384-17393, doi:10.1021/am505024p.
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233, doi:10.1007/s00339-020-3408-9.