Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nisha Garg | -- | 2244 | 2022-11-07 14:56:43 | | | |
| 2 | Beatrix Zheng | + 3 word(s) | 2247 | 2022-11-08 06:53:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Avalos-Borges, E.E.; Rios, L.E.; Jiménez-Coello, M.; Ortega-Pacheco, A.; Garg, N.J. Trypanosoma cruzi Congenital Transmission. Encyclopedia. Available online: https://encyclopedia.pub/entry/33357 (accessed on 07 February 2026).
Avalos-Borges EE, Rios LE, Jiménez-Coello M, Ortega-Pacheco A, Garg NJ. Trypanosoma cruzi Congenital Transmission. Encyclopedia. Available at: https://encyclopedia.pub/entry/33357. Accessed February 07, 2026.
Avalos-Borges, Eduardo E., Lizette E. Rios, Matilde Jiménez-Coello, Antonio Ortega-Pacheco, Nisha J. Garg. "Trypanosoma cruzi Congenital Transmission" Encyclopedia, https://encyclopedia.pub/entry/33357 (accessed February 07, 2026).
Avalos-Borges, E.E., Rios, L.E., Jiménez-Coello, M., Ortega-Pacheco, A., & Garg, N.J. (2022, November 07). Trypanosoma cruzi Congenital Transmission. In Encyclopedia. https://encyclopedia.pub/entry/33357
Avalos-Borges, Eduardo E., et al. "Trypanosoma cruzi Congenital Transmission." Encyclopedia. Web. 07 November, 2022.
Copy Citation
Chagas disease, initiated by the etiological agent Trypanosoma cruzi, is an endemic infection in the American continent. Although vectorial transmission of T. cruzi is recognized as the main mode of infection, other routes such as congenital and blood transfusion are also documented as important methods of transmission. T. cruzi maternal–fetal transmission has been recorded in humans and examined by some investigators in naturally and experimentally infected mammals.
Trypanosoma cruzi
Chagas disease
congenital transmission
1. Introduction
The Trypanosoma cruzi (discovered by Chagas in 1909) (Tc) pathogen is the causative agent of Chagas disease (CD), also known as American Trypanosomiasis (AT) [1]. T. cruzi is a flagellate parasite of the phylum Euglenozoa, class Kinetoplastida, family Trypanosomatidae, and genus Trypanosoma, and it is known to infect humans and various species of wild and domestic mammals [1]. Trypanosomes are unicellular organisms with a single nucleus located in the center of the body. The infectious trypomastigote form of T. cruzi is typically spindle-shaped with an undulating membrane along its axis and a DNA-rich mitochondrial organelle called kinetoplast located in the anterior part of the parasite [2]. A series of microtubules originating near the kinetoplast make up the basal body that extends along the undulating membrane to the opposite end of the parasite. The flagellar tubules are surrounded by a membrane, and together they form the flagellar pocket [3]. Trypanosomes contain many of the same organelles as noted in their mammalian host, but also have organelles, e.g., glycosome, acidocalcisome, cytoplasmic vacuole, and reservosome, that are unique to these parasites [3][4].
Patients exposed to T. cruzi encounter a high parasitic load in circulation and various tissues for 1–2 months, after which it is significantly controlled by the adaptive immune response, and then intermittent low level of parasites are observed during the indeterminate phase of the disease [5]. Progression to clinically symptomatic chronic disease phase in ~30–40% of the infected patients is presented with the development of cardiomyopathy, though other morbidities including gastroenterological and neurological disorders are also noted in Chagas patients. Chagas disease can ultimately cause death due to heart failure [6].
T. cruzi populations survive in a wide ecological range and exhibit a highly diversified genetic profile. By analyzing a variety of molecular markers, T. cruzi clones have been categorized into seven discrete typing units (DTUs) referred as TcI-TcVI and Tcbat [7][8][9][10]. The parasite isolates belonging to these DTUs differ in geographical distribution and epidemiological association, as well as in virulence, pathogenicity, and tissue tropism in the host [11][12][13]. In Mexico and Central America, T. cruzi isolates of TcI DTU are found with the highest prevalence [14], though mixed infections with TcII, TcV, and TcVI are also noted in Mexico and Central America [15]. T. cruzi isolates of TcI and TcII DTUs with some cases of TcIV, TcV, and TcVI DTUs are noted in Brazil [16] and Argentina [11][17]. TcI-TcVI DTUs have been reported in large number of vectors species and mammals, Tcbat is transmitted from infected bats, and all DTUs can cause Chagas disease in humans and animals, though some evidence indicates an association between DTUs and clinical outcomes [18]. Readers interested in more details of geographic distribution of T. cruzi lineages in mammalian and vectorial hosts are referred to recent review articles [9][19].
The World Health Organization estimates that approximately 6–7 million people are affected by Chagas disease worldwide, and nearly 100 million people live in the endemic areas with high risk of exposure to T. cruzi infection in the Latin American countries [20][21]. The main form of transmission is by contact with feces of infected triatomine insects. Infection occurs when T. cruzi-infected triatomines feed on the host, contaminated feces from the insect is excreted, and parasites gain access to the blood stream of the host via skin puncture or mucous membranes [22]. Parasite transmission may also occur by oral ingestion of infected bugs [23], transfusion of infected blood [24], and congenital transmission from infected mothers [25]. After the vector control programs implemented in 1980–1990s were highly successful in controlling the acute transmission of T. cruzi from infected bugs in South America, the non-vectorial transmission pathways have emerged as major issues of public health concern [24]. In fact, congenital transmission has led to the globalization of Chagas disease in non-endemic countries [26][27][28][29]. It is projected that internationally over two million women of reproductive age are infected with T. cruzi, and 1–10% of fetuses carried by infected mothers are born with CD [30][31][32].
In this research, the researchers summarized the current knowledge regarding T. cruzi congenital transmission in humans and animal models with an aim to point out the similarities and dissimilarities of placental and maternal factors between animals and humans. It is the researchers' hope that the current research will serve as an essential source of knowledge for understanding the pathophysiology of congenital transmission and permit the reader to make an informed decision when using experimental models of T. cruzi infection for studying the pathomechanisms of congenital transmission or examining the efficacy of new diagnostic tests or therapies to prevent congenital CD.
2. Diagnosis and Burden of Congenital Transmission of T. cruzi in Humans
Women, irrespective of being in the acute or chronic phases of CD, can congenitally transmit the parasite to the fetus and newborn [33]. Yet, there is significant evidence that a high rate of transmission occurs when pregnant women are acutely infected and/or develop reactivated acute infection due to immunosuppression [34]. Indeed, ~53% of women who became infected during pregnancy exhibited prenatal or perinatal transmission of T. cruzi to their fetuses and newborns [34][35][36][37]. Likewise, women infected with T. cruzi prior to pregnancy who became exposed to HIV or were treated with immunosuppressive drugs during gestation transmitted T. cruzi with high frequency to their newborns [38][39]. Besides, high parasite burden in umbilical cord of infants is associated with the severity of congenital CD [31][40]. In Mexico, studies indicate that an estimated seroprevalence of 2.21% (95% CI 1.46–2.96) would result in 50,675 births from T. cruzi-infected pregnant women and ~3193 cases of vertical transmission and infected neonates per year [41]. It is of note that infected mothers transmitted the same DTUs of T. cruzi to their newborns as were predominantly identified in the local population [17][42][43]. When pregnant women were exposed to mixed or multiclonal infections, predominance of different clones in the mother and their newborns has been noted [42][44][45]. Overall, the current literature allows the researchers to surmise that (a) all DTUs can potentially be transmitted via congenital route, (b) natural selection of the transmitted parasite may occur during pregnancy, and (c) diagnostic screening of pregnant women and newborns and identification of parasite lineage would inform the timely treatment of newborns and restrict T. cruzi transmission.
The criteria for the occurrence of congenital transmission in humans are that the mother is T. cruzi-seropositive, parasites are detectable in the peripheral blood of the newborn, and anti-T. cruzi antibodies are made in the newborn after passive immunity acquired during lactation has disappeared (if vector and blood transfusion infection have also been ruled out) [37][46]. Microscopic observation of parasite in fresh blood smears offers the simplest approach to diagnosis of T. cruzi infection in newborns [47]. When there is a low number of blood parasites, concentration methods such as Strout or micro Strout test are useful [48][49]. Another form of T. cruzi detection is micro hematocrit, which may be more convenient for parasite detection because of its simplicity, low cost, and capacity to detect 40 parasites/mL with 97.4% sensitivity [50]. However, parasite identification with micro hematocrit requires ~30 min per sample evaluation by trained personnel, and even then, this method identifies only 40–60% of the congenital transmission in newborns [46][51]. Indirect parasite detection methods such as hemoculture or xenodiagnosis, although sensitive, have the disadvantage in that they require several weeks for positive T. cruzi identification [48][52].
In infants of nine months age or older, serological detection of anti-T. cruzi antibodies by indirect hemagglutination assay (IHA) [53], indirect immunofluorescence (IIF) assay [54][55], or enzyme-linked immunosorbent assay (ELISA) [52][53] can be employed. Serology is routinely applied for diagnosis of T. cruzi infection in clinical laboratories in Latin America [48][52][56]; however, difficulty in following up the newborns is a major limitation of this method in diagnosing and treating the infected infants in a timely manner.
The application of conventional polymerase chain reaction (PCR) for positive or negative diagnosis of congenital T. cruzi infection has increased in recent years [48][56][57]. When used at birth, PCR test provides higher sensitivity and specificity compared with other parasitological identification methods [37][58]. Indeed, accuracy of PCR test in diagnosis of congenital T. cruzi infection has been demonstrated in several studies [56][59][60][61]. A variation of PCR is the quantitative PCR (qPCR), which allows enumerating the parasite burden [62]. The qPCR assay based on T. cruzi satellite DNA and kinetoplast DNA can detect 0.85 and 0.43 parasite equivalents per mL blood, respectively, making it a highly sensitive approach [63]. The main drawback of this technique is the requirement for specific laboratory instrument and well-qualified employees who maintain rigorous quality control. As an alternative, loop-mediated isothermal amplification (LAMP) can be performed using a heat-block at a constant temperature of 60–65 °C and it does not require specialized PCR equipment [64][65]. LAMP has been successfully implemented to amplify T. cruzi DNA with a similar sensitivity as was noted with qPCR [64][66]. One caveat in implementation of molecular assays in the field is that trained personnel are needed to accurately identify infected newborns and avoid false-positives due to contamination of maternal parasite DNA. Readers are directed to a recent review discussing the efficacy and implementation of old and new diagnostic tests and an ideal algorithm for diagnosis of congenital transmission of T. cruzi in infants [25].
3. Characteristics and Classification of the Mammalian Placental Barrier
The placenta is a temporary organ formed during pregnancy. It primarily functions in anchoring the fetus to the uterine wall and mediating the immune tolerance to avoid immunological rejection of the fetus while also maintaining the anti-infectious capacity [67][68][69]. Placenta is also required for the transfer of nutrients such as amino acids, lipids, and glucose to the fetus, and the exchange of oxygen, carbon dioxide, and fetal waste excretion [70].
The two forms of placenta classification are based on gross shape and histological structure (Figure 1). Gross morphology based placental classification describes whether maternal–fetal exchange occurs on all of the available surface of the chorionic sac or if it is restricted to specific zones [71][72]. Accordingly, four types of placentas categorized based on gross shape are shown in Figure 1A. These include (1) diffuse placenta, which appears over the complete surface of the uterine luminal epithelium with the formation of folds and villi, and it is seen in horses and pigs; (2) cotyledonary placenta, which consists of numerous spot-like placental regions of the endometrium known as caruncles or cotyledons with smooth and avascular intervening areas in the chorion, and it is seen in ruminants; (3) zonary placenta, which shows an intimate interdigitating contact zone that forms a strap or girdle around the chorionic sac, and it is seen in carnivores such as dogs, cats, bats, seals and bears; and (4) discoid/bidiscoid placenta, which may contain a single (discoid) or double (bidiscoid) disc, wherein maternal–placental interaction occurs in a roughly circular area, and it is seen in humans, rodents, rabbits, and primates [71][73].
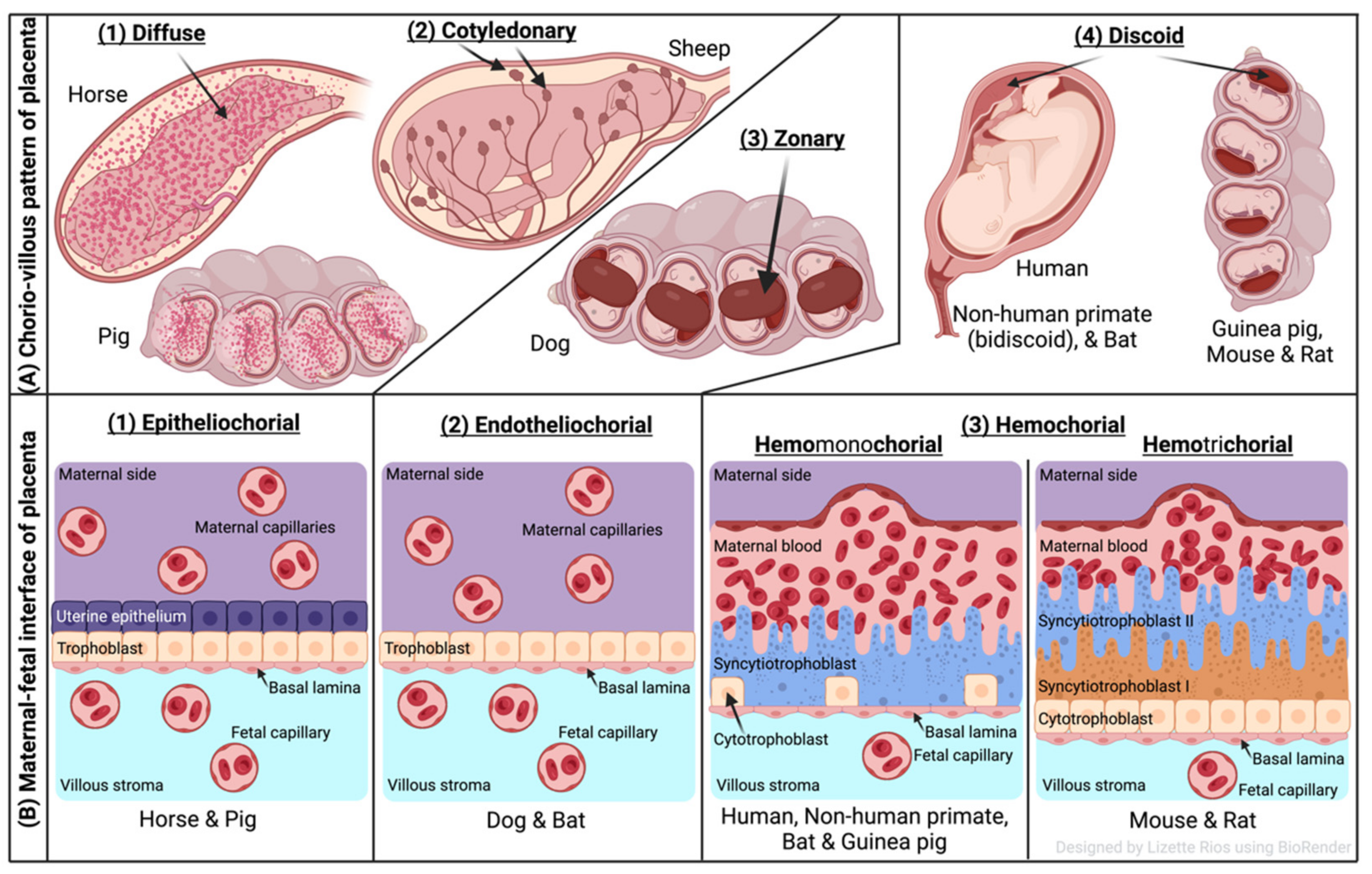
Figure 1. Comparative features of mammalian placenta. (A) Chorio-villous patterns of placenta. There are four types of placentas that are recognized: (1) diffuse: occurs over the entire surface of the uterine luminal epithelium with formation of folds/villi (seen in horses and pigs); (2) cotyledonary: characterized by many spot-like placental regions of the endometrium known as caruncles or cotyledons with smooth and poor vascularized intervening areas in the chorion (seen in ruminants); (3) zonary: placenta shows an intimate interdigitating contact zone that forms a belt or girdle around the chorionic sac (seen in carnivores such as dogs); (4) discoid/bidiscoid: characterized by a single (discoid) or double disc (bidiscoid), and mother–product interaction is confined to a roughly circular area (seen in humans, rodents, bats, and primates). (B) Maternal–fetal interface of placenta. This classification of placentas depends on the cell layers between the tissues of the mother and the fetus. (1) Epitheliochorial placenta (seen in horse, pig, and ruminants) is the least intimate because the interaction between the maternal blood and the fetal tissue is limited by a layer of uterine epithelial cells and a layer of trophoblast cells. (2) Endotheliochorial type (seen in dogs and cats) is the second more invasive barrier, where the uterine epithelium in degraded after implantation, leaving to the trophoblast adjacent only to the maternal endothelium. (3) Hemochorial barrier (hemomonochorial in primates and hemotrichorial in rodents) is the most intimate interface. The hemochorial epithelial and endothelial cells of the mother are degraded, leaving the trophoblast cells (syncytiotrophoblast and cytotrophoblast) in direct contact with the maternal blood.
Three main types of placental classification were proposed by Grosser based on histological structure (Figure 1B) [74]. This classification recognizes the histologic relationship of the chorion and uterine wall, and therefore, it is more apt and informative in describing the placental function [71][72]. The epitheliochorial type of placenta (seen in horses, pigs, and ruminants) is most superficial or least intimate because the maternal blood—fetal tissue interactions are limited by layers of uterine epithelial cells and fetal trophoblast cells only. In endotheliochorial placenta, withdrawal of maternal uterine epithelium and connective tissue after implantation informs the maternal endometrial contact with fetal trophoblasts. The endotheliochorial type of placenta is seen in four major clades of eutherian mammals, including carnivores, dogs, and cats. Lastly, the hemochorial placenta is considered most invasive, as in this case maternal hemochorial epithelial and endothelial cells are degraded, and thereby maternal blood is in open exchange with fetal trophoblast cells (syncytiotrophoblast and cytrotrophoblast). The hemomonochorial, hemodichorial, and hemotrichorial placentas consist of one, two, and three trophoblast layers, and are noted in primates, rabbits, and rodents, respectively [73][75].
References
- Coura, J.R.; Dias, J.C. Epidemiology, control, and surveillance of Chagas disease: 100 years after its discovery. Mem Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 31–40.
- De Souza, W. Basic cell biology of Trypanosoma cruzi. Curr. Pharm. Des. 2002, 8, 269–285.
- Gonçalves, C.S.; Ávila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors 2018, 11, 83.
- Girard-Dias, W.; Alcantara, C.L.; Cunha-e-Silva, N.; de Souza, W.; Miranda, K. On the ultrastructural organization of Trypanosoma cruzi using cryopreparation methods and electron tomography. Histochem. Cell Biol. 2012, 138, 821–831.
- D’Ávila, D.A.; Galvão, L.M.C.; Sousa, G.R.; Britto, C.; Moreira, O.C.; Chiari, E. Monitoring the parasite load in chronic Chagas disease patients: Comparison between blood culture and quantitative real time PCR. PLoS ONE 2018, 13, e0208133.
- Meymandi, S.; Hernandez, S.; Park, S.; Sanchez, D.R.; Forsyth, C. Treatment of Chagas disease in the United States. Curr. Treat. Options Infect. Dis. 2018, 10, 373–388.
- Guhl, F.; Ramirez, J.D. Trypanosoma cruzi diversity i: Towards the need of genetic subdivision? Acta Trop. 2011, 119, 1–4.
- Ramirez, J.D.; Hernandez, C. Trypanosoma cruzi ii: Towards the need of genetic subdivision? part ii. Acta Trop 2018, 184, 53–58.
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360.
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52.
- Cura, C.I.; Lucero, R.H.; Bisio, M.; Oshiro, E.; Formichelli, L.B.; Burgos, J.M.; Lejona, S.; Bruses, B.L.; Hernandez, D.O.; Severini, G.V.; et al. Trypanosoma cruzi discrete typing units in Chagas disease patients from endemic and non-endemic regions of Argentina. Parasitology 2012, 139, 516–521.
- Virreira, M.; Alonso-Vega, C.; Solano, M.; Jijena, J.; Brutus, L.; Bustamante, Z.; Truyens, C.; Schneider, D.; Torrico, F.; Carlier, Y.; et al. Congenital Chagas disease in Bolivia is not associated with DNA polymorphism of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2006, 75, 871–879.
- Del Puerto, R.; Nishizawa, J.E.; Kikuchi, M.; Iihoshi, N.; Roca, Y.; Avilas, C.; Gianella, A.; Lora, J.; Velarde, F.U.; Renjel, L.A.; et al. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Negl. Trop. Dis. 2010, 4, e687.
- Dorn, P.L.; McClure, A.G.; Gallaspy, M.D.; Waleckx, E.; Woods, A.S.; Monroy, M.C.; Stevens, L. The diversity of the Chagas parasite, Trypanosoma cruzi, infecting the main central American vector, Triatoma dimidiata, from Mexico to Colombia. PLoS Negl. Trop. Dis. 2017, 11, e0005878.
- Villanueva-Lizama, L.; Teh-Poot, C.; Majeau, A.; Herrera, C.; Dumonteil, E. Molecular genotyping of Trypanosoma cruzi by next-generation sequencing of the mini-exon gene reveals infections with multiple parasite discrete typing units in chagasic patients from Yucatan, Mexico. J. Infect. Dis. 2019, 219, 1980–1988.
- Abolis, N.G.; Araujo, S.M.; Toledo, M.J.; Fernandez, M.A.; Gomes, M.L. Trypanosoma cruzi I-III in southern Brazil causing individual and mixed infections in humans, sylvatic reservoirs and triatomines. Acta Trop. 2011, 120, 167–172.
- Corrales, R.M.; Mora, M.C.; Negrette, O.S.; Diosque, P.; Lacunza, D.; Virreira, M.; Breniere, S.F.; Basombrio, M.A. Congenital Chagas disease involves Trypanosoma cruzi sub-lineage IId in the northwestern province of Salta, Argentina. Infect. Genet. Evol. 2009, 9, 278–282.
- Messenger, L.A.; Miles, M.A.; Bern, C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert. Rev. Anti-Infect. Ther. 2015, 13, 995–1029.
- Izeta-Alberdi, A.; Ibarra-Cerdeña, C.N.; Moo-Llanes, D.A.; Ramsey, J.M. Geographical, landscape and host associations of Trypanosoma cruzi DTUs and lineages. Parasites Vectors 2016, 9, 631.
- World Health Organization. Chagas Disease: Control and Elimination; UNDP: New York, NY, USA; World Bank: Washington, DC, USA; WHO: Geneva, Switzerland, 2010; Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf (accessed on 4 March 2022).
- World Health Organization. Chagas Disease (also Known as American Trypanosomiasis): Key Facts; UNDP: New York, NY, USA; World Bank: Washington, DC, USA; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 7 April 2022).
- Martinez-Ibarra, J.A.; Grant-Guillen, Y.; Morales-Corona, Z.Y.; Haro-Rodriguez, S.; Ventura-Rodriguez, L.V.; Nogueda-Torres, B.; Bustos-Saldana, R. Importance of species of triatominae (heteroptera: Reduviidae) in risk of transmission of Trypanosoma cruzi in western Mexico. J. Med. Entomol. 2008, 45, 476–482.
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852.
- Moncayo, A. Chagas disease: Current epidemiological trends after the interruption of vectorial and transfusional transmission in the southern cone countries. Mem. Inst. Oswaldo Cruz 2003, 98, 577–591.
- Rios, L.; Campos, E.E.; Menon, R.; Zago, M.P.; Garg, N.J. Epidemiology and pathogenesis of maternal-fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165591.
- Garraud, O.; Andreu, G.; Elghouzzi, M.H.; Laperche, S.; Lefrere, J.J. Measures to prevent transfusion-associated protozoal infections in non-endemic countries. Travel Med. Infect. Dis. 2007, 5, 110–112.
- Schmunis, G.A. Epidemiology of Chagas disease in non-endemic countries: The role of international migration. Mem. Inst. Oswaldo Cruz 2007, 102 (Suppl. 1), 75–85.
- Gascon, J.; Bern, C.; Pinazo, M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010, 115, 22–27.
- Rodari, P.; Angheben, A.; Gennati, G.; Trezzi, L.; Bargiggia, G.; Maino, M.; Ruggeri, M.; Rampello, S.; Soavi, L.; Rizzi, M. Congenital Chagas disease in a non-endemic area: Results from a control programme in Bergamo province, northern Italy. Travel Med. Infect. Dis. 2018, 25, 31–34.
- Oliveira, I.; Torrico, F.; Munoz, J.; Gascon, J. Congenital transmission of Chagas disease: A clinical approach. Expert. Rev. Anti-Infect. Ther. 2010, 8, 945–956.
- Carlier, Y.; Sosa-Estani, S.; Luquetti, A.O.; Buekens, P. Congenital Chagas disease: An update. Mem. Inst. Oswaldo Cruz 2015, 110, 363–368.
- Carlier, Y.; Truyens, C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta, and fetuses. Acta Trop. 2015, 151, 103–115.
- Paricio-Talayero, J.M.; Benlloch-Muncharaz, M.J.; Ignacio Collar-del-Castillo, J.; Rubio-Soriano, A.; Serrat-Pérez, C.; Magraner-Egea, J.; Landa-Rivera, L.; Sánchez-Palomares, M.; Beseler-Soto, B.; Santos-Serrano, L.; et al. Vigilancia epidemiológica de la transmisión vertical de la enfermedad de chagas en tres maternidades de la comunidad valenciana. Enferm. Infecc. Microbiol. Clínica 2008, 26, 609–613.
- Cevallos, A.M.; Hernández, R. Chagas disease: Pregnancy and congenital transmission. Biomed. Res. Int. 2014, 2014, 401864.
- Buekens, P.; Cafferata, M.L.; Alger, J.; Althabe, F.; Belizan, J.M.; Bustamante, N.; Carlier, Y.; Ciganda, A.; Del Cid, J.H.; Dumonteil, E.; et al. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: An observational prospective study. Am. J. Trop. Med. Hyg. 2018, 98, 478–485.
- Moya, P.; Basso, B.; Moretti, E. Congenital Chagas disease in Cordoba, Argentina: Epidemiological, clinical, diagnostic, and therapeutic aspects. Experience of 30 years of follow up. Rev. Soc. Bras. Med. Trop. 2005, 38 (Suppl. 2), 33–40.
- Bustos, P.L.; Milduberger, N.; Volta, B.J.; Perrone, A.E.; Laucella, S.A.; Bua, J. Trypanosoma cruzi infection at the maternal-fetal interface: Implications of parasite load in the congenital transmission and challenges in the diagnosis of infected newborns. Front. Microbiol. 2019, 10, 1250.
- Scapellato, P.G.; Bottaro, E.G.; Rodriguez-Brieschke, M.T. Mother-child transmission of Chagas disease: Could coinfection with human immunodeficiency virus increase the risk? Rev. Soc. Bras. Med. Trop. 2009, 42, 107–109.
- Agosti, M.R.; Ercoli, P.; Dolcini, G.; Andreani, G.; Peralta, L.M.; Ayala, S.G. Two cases of mother-to-child transmission of HIV and Trypanosoma cruzi in Argentina-clinical key. Braz. J. Infect. Dis. 2012, 16, 398–399.
- Carlier, Y.; Truyens, C.; Deloron, P.; Peyron, F. Congenital parasitic infections: A review. Acta Trop. 2012, 121, 55–70.
- Arnal, A.; Waleckx, E.; Rico-Chavez, O.; Herrera, C.; Dumonteil, E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0006859.
- Ortiz, S.; Zulantay, I.; Solari, A.; Bisio, M.; Schijman, A.; Carlier, Y.; Apt, W. Presence of Trypanosoma cruzi in pregnant women and typing of lineages in congenital cases. Acta Trop. 2012, 124, 243–246.
- Herrera, C.; Truyens, C.; Dumonteil, E.; Alger, J.; Sosa-Estani, S.; Cafferata, M.L.; Gibbons, L.; Ciganda, A.; Matute, M.L.; Zuniga, C.; et al. Phylogenetic analysis of Trypanosoma cruzi from pregnant women and newborns from Argentina, Honduras, and Mexico suggests an association of parasite haplotypes with congenital transmission of the parasite. J. Mol. Diagn. 2019, 21, 1095–1105.
- Llewellyn, M.S.; Messenger, L.A.; Luquetti, A.O.; Garcia, L.; Torrico, F.; Tavares, S.B.; Cheaib, B.; Derome, N.; Delepine, M.; Baulard, C.; et al. Deep sequencing of the Trypanosoma cruzi gp63 surface proteases reveals diversity and diversifying selection among chronic and congenital Chagas disease patients. PLoS Negl. Trop. Dis. 2015, 9, e0003458.
- Burgos, J.M.; Altcheh, J.; Petrucelli, N.; Bisio, M.; Levin, M.J.; Freilij, H.; Schijman, A.G. Molecular diagnosis and treatment monitoring of congenital transmission of Trypanosoma cruzi to twins of a triplet delivery. Diagn. Microbiol. Infect. Dis. 2009, 65, 58–61.
- Messenger, L.A.; Bern, C. Congenital Chagas disease: Current diagnostics, limitations, and future perspectives. Curr. Opin. Infect. Dis. 2018, 31, 415–421.
- Gomes, Y.M.; Lorena, V.M.; Luquetti, A.O. Diagnosis of Chagas disease: What has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem. Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 115–121.
- Mora, M.C.; Sanchez Negrette, O.; Marco, D.; Barrio, A.; Ciaccio, M.; Segura, M.A.; Basombrio, M.A. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. J. Parasitol. 2005, 91, 1468–1473.
- Strout, R.G. A method for concentrating hemoflagellates. J. Parasitol. 1962, 48, 100.
- Feilij, H.; Muller, L.; Gonzalez Cappa, S.M. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J. Clin. Microbiol. 1983, 18, 327–330.
- De Rissio, A.M.; Riarte, A.R.; Garcia, M.M.; Esteva, M.I.; Quaglino, M.; Ruiz, A.M. Congenital Trypanosoma cruzi infection. Efficacy of its monitoring in an urban reference health center in a non-endemic area of Argentina. Am. J. Trop. Med. Hyg 2010, 82, 838–845.
- Balouz, V.; Aguero, F.; Buscaglia, C.A. Chagas disease diagnostic applications: Present knowledge and future steps. Adv. Parasitol. 2017, 97, 1–45.
- Eguez, K.E.; Alonso-Padilla, J.; Teran, C.; Chipana, Z.; Garcia, W.; Torrico, F.; Gascon, J.; Lozano-Beltran, D.F.; Pinazo, M.J. Rapid diagnostic tests duo as alternative to conventional serological assays for conclusive Chagas disease diagnosis. PLoS Negl. Trop. Dis. 2017, 11, e0005501.
- Taibi, A.; Plumas-Marty, B.; Guevara-Espinoza, A.; Schöneck, R.; Pessoa, H.; Loyens, M.; Piras, R.; Aguirre, T.; Gras-Masse, H.; Bossus, M. Trypanosoma cruzi: Immunity-induced in mice and rats by trypomastigote excretory-secretory antigens and identification of a peptide sequence containing a T cell epitope with protective activity. J. Immunol. 1993, 151, 2676–2689.
- Pereiro, A.C. Guidelines for the diagnosis and treatment of Chagas disease. Lancet 2019, 393, 1486–1487.
- Bua, J.; Volta, B.J.; Perrone, A.E.; Scollo, K.; Velazquez, E.B.; Ruiz, A.M.; De Rissio, A.M.; Cardoni, R.L. How to improve the early diagnosis of Trypanosoma cruzi infection: Relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Negl. Trop. Dis. 2013, 7, e2476.
- Picado, A.; Cruz, I.; Redard-Jacot, M.; Schijman, A.G.; Torrico, F.; Sosa-Estani, S.; Katz, Z.; Ndung’u, J.M. The burden of congenital Chagas disease and implementation of molecular diagnostic tools in Latin America. BMJ Glob. Health 2018, 3, e001069.
- Torrico, F.; Alonso-Vega, C.; Suarez, E.; Rodriguez, P.; Torrico, M.C.; Dramaix, M.; Truyens, C.; Carlier, Y. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am. J. Trop. Med. Hyg. 2004, 70, 201–209.
- Schijman, A.G.; Bisio, M.; Orellana, L.; Sued, M.; Duffy, T.; Mejia Jaramillo, A.M.; Cura, C.; Auter, F.; Veron, V.; Qvarnstrom, Y.; et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Trop. Dis. 2011, 5, e931.
- Saavedra, M.; Zulantay, I.; Apt, W.; Martinez, G.; Rojas, A.; Rodriguez, J. Chronic Chagas disease: PCR-xenodiagnosis without previous microscopic observation is a useful tool to detect viable Trypanosoma cruzi. Biol. Res. 2013, 46, 295–298.
- Murcia, L.; Simon, M.; Carrilero, B.; Roig, M.; Segovia, M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J. Infect. Dis. 2017, 215, 1452–1458.
- Torres-Vargas, J.; Jiménez-Coello, M.; Guzmán-Marín, E.; Acosta-Viana, K.Y.; Yadon, Z.E.; Gutiérrez-Blanco, E.; Guillermo-Cordero, J.L.; Garg, N.J.; Ortega-Pacheco, A. Quantitative and histological assessment of maternal-fetal transmission of Trypanosoma cruzi in guinea pigs: An experimental model of congenital Chagas disease. PLoS Negl. Trop. Dis. 2018, 12, e0006222.
- Cura, C.I.; Ramirez, J.C.; Rodriguez, M.; Lopez-Albizu, C.; Irazu, L.; Scollo, K.; Sosa-Estani, S. Comparative study and analytical verification of PCR methods for the diagnosis of congenital Chagas disease. J. Mol. Diagn. 2017, 19, 673–681.
- Besuschio, S.A.; Llano Murcia, M.; Benatar, A.F.; Monnerat, S.; Cruz, I.; Picado, A.; Curto, M.L.A.; Kubota, Y.; Wehrendt, D.P.; Pavia, P.; et al. Analytical sensitivity and specificity of a loop-mediated isothermal amplification (lamp) kit prototype for detection of Trypanosoma cruzi DNA in human blood samples. PLoS Negl. Trop. Dis. 2017, 11, e0005779.
- Rivero, R.; Bisio, M.; Velazquez, E.B.; Esteva, M.I.; Scollo, K.; Gonzalez, N.L.; Altcheh, J.; Ruiz, A.M. Rapid detection of Trypanosoma cruzi by colorimetric loop-mediated isothermal amplification (lamp): A potential novel tool for the detection of congenital Chagas infection. Diagn. Microbiol. Infect. Dis. 2017, 89, 26–28.
- Jimenez-Coello, M.; Shelite, T.; Castellanos-Gonzalez, A.; Saldarriaga, O.; Rivero, R.; Ortega-Pacheco, A.; Acevedo-Arcique, C.; Amaya-Guardia, K.; Garg, N.; Melby, P.; et al. Efficacy of recombinase polymerase amplification to diagnose Trypanosoma cruzi infection in dogs with cardiac alterations from an endemic area of Mexico. Vector Borne Zoonotic Dis. 2018, 18, 417–423.
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433.
- Zhang, S.; Regnault, T.R.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental adaptations in growth restriction. Nutrients 2015, 7, 360–389.
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017, 21, 561–567.
- Heerema-McKenney, A. Defense and infection of the human placenta. APMIS 2018, 126, 570–588.
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18.
- Miglino, M.A.; Ambrosio, C.E.; dos Santos Martins, D.; Wenceslau, C.V.; Pfarrer, C.; Leiser, R. The carnivore pregnancy: The development of the embryo and fetal membranes. Theriogenology 2006, 66, 1699–1702.
- Gundling, W.E., Jr.; Wildman, D.E. A review of inter- and intra-specific variation in the eutherian placenta. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140072.
- Grosser, O. Vergleichende Anatomie und Entwicklungsgeschichte der Eihäute und der Placenta, Mit Besonderer Berücksichtigung des Menschen; Hardpress: Miami, FL, USA, 1909.
- Liempi, A.; Castillo, C.; Medina, L.; Galanti, N.; Maya, J.D.; Parraguez, V.H.; Kemmerling, U. Comparative ex vivo infection with Trypanosoma cruzi and Toxoplasma gondii of human, canine and ovine placenta: Analysis of tissue damage and infection efficiency. Parasitol. Int. 2020, 76, 102065.
More
Information
Subjects:
Parasitology; Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
08 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




