Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jia, F.; Gao, Y.; Wang, H. Drug Delivery System Fabricated by Microfluidics. Encyclopedia. Available online: https://encyclopedia.pub/entry/33308 (accessed on 07 February 2026).
Jia F, Gao Y, Wang H. Drug Delivery System Fabricated by Microfluidics. Encyclopedia. Available at: https://encyclopedia.pub/entry/33308. Accessed February 07, 2026.
Jia, Fuhao, Yanbing Gao, Hai Wang. "Drug Delivery System Fabricated by Microfluidics" Encyclopedia, https://encyclopedia.pub/entry/33308 (accessed February 07, 2026).
Jia, F., Gao, Y., & Wang, H. (2022, November 07). Drug Delivery System Fabricated by Microfluidics. In Encyclopedia. https://encyclopedia.pub/entry/33308
Jia, Fuhao, et al. "Drug Delivery System Fabricated by Microfluidics." Encyclopedia. Web. 07 November, 2022.
Copy Citation
Traditional drug therapy faces challenges such as drug distribution throughout the body, rapid degradation and excretion, and extensive adverse reactions. In contrast, micro/nanoparticles can controllably deliver drugs to target sites to improve drug efficacy. Unlike traditional large-scale synthetic systems, microfluidics allows manipulation of fluids at the microscale and shows great potential in drug delivery and precision medicine. Well-designed microfluidic devices have been used to fabricate multifunctional drug carriers using stimuli-responsive materials.
microfluidics
drug delivery
micro/nanoparticles
1. Introduction
Microfluidic technology is a highly interdisciplinary science and technology, involving engineering, physics, chemistry, microfabrication, and other disciplines [1][2][3]. Microfluidics can process or manipulate tiny amounts (10−9 L to 10−8 L) of fluid within microchannels, ranging in size from tens to hundreds of micrometers. Since the heat and mass transfer processes at the microscale are scale-dependent, the fluids in microfluidic chips exhibit specific properties that are different from those at the macroscale [4]. In short, surface tension and capillary forces dominate the device, and the effects of gravity and inertial forces are negligible. The interface factors such as diffusion, surface tension and viscosity become the main factors affecting the fluid behavior [3]. In addition, the high surface area to volume ratio ensures thermal homogeneity and rapid heat transfer. These fundamental properties give rise to a wide range of advantages, including minimal use of reagents, low energy consumption, fast reaction rates, and massively parallel processes [5]. Thus, microfluidic devices are widely used in industrial and academic research.
Currently, both small drugs and macromolecular drugs require the appropriate drug delivery strategies [6]. For example, traditional small molecule drugs tend to have low solubility, which limits their bioavailability. Furthermore, the in vivo stability of protein drugs after administration is compromised by proteases, temperature and pH. Nucleic acid drugs need to be delivered into the cytoplasm to be effective. To address these challenges, it is necessary to develop appropriate drug delivery systems. Proper drug delivery allows not only on-demand delivery of active drug to target tissues or cells, but also proper control of pharmacokinetics (e.g., in vivo distribution, half-life, maximum concentration in serum, etc.) [7]. Although pharmacokinetics can be modulated by controlling the number and frequency of dosing, many disease treatments require frequent long-term dosing, which compromises patients’ quality of life. A variety of controlled release systems, including injectable hydrogels, polymer implants and micro/nanoparticles, have been used to improve drug delivery behaviors [8]. Drugs within a delivery system have multiple release modes, including drug diffusion, degradation of the delivery material, and external stimuli. Macroscopic delivery systems such as drug-eluting stents require surgical implantation and may induce fibrocystic reaction [9]. In addition, the macroscopic delivery system has a singular drug release pattern. Compared with macroscopic delivery systems, micro/nanoparticle delivery systems offer many advantages in protecting protein and nucleic acid drugs from degradation, controlling drug release profiles, enhancing small molecule solubility of hydrophobic drugs, and tunable tissue targeting [10].
2. Microfluidic Device
Researchers first introduce the material and processing technology of microfluidic devices before describing their applications. The foremost step for microfluidic applications is the selection of the appropriate materials. Depending on the application, factors to consider in material selection include durability, clarity, biocompatibility, chemical compatibility with application reagents, temperature, pressure, and surface modification [11]. A variety of materials have been developed to fabricate microfluidic chips, including silicon, glass, polymer, and paper [12][13]. Silicon is used to fabricate microfluidic devices due to its thermal stability, chemical stability, solvent resistance, and high thermal conductivity (Figure 1a) [13][14]. However, the opacity of silicon limits its application in optical detection. Furthermore, silicon material is very fragile, making it difficult to introduce valves or pumps on the device. An alternative material could be glass due to its chemical inertness, thermal resistance, electrical insulation, biocompatibility and easy functionalization of surfaces [13][15]. Glass capillaries can be assembled to form microfluidic devices to fabricate micro/nanoparticles (Figure 1b) [16]. A typical setup consists of three coaxially assembled basic modules: an injection tube, a transition tube, and a collection tube. Although the cost of glass is low, the process of fabricating chips from glass is time-consuming and labor-intensive [17][18][19]. In addition, the extremely impermeable properties of glass and silicon limit on-chip cell culture [20].
Alternatively, polymeric materials have been extensively explored to fabricate microfluidic devices. Compared to silicon and glass, polymeric materials have the advantages of lower cost, simple processing technology, high transparency and high thermal resistance [21]. A representative material is polydimethylsiloxane (PDMS), an optically transparent soft elastomer (Figure 1c) [22]. PDMS is relatively inexpensive, easy to mold, and possesses properties such as light transmission, air permeability, biocompatibility, natural hydrophobicity, and high elasticity [23][24]. Therefore, it is suitable for long-term cell culture, cell screening and biochemical assays. At the same time, high flow rates lead to microchannel expansion [25]. PDMS may adsorb organic solvents that cause deformation of the microchannels. In addition to PDMS, polymethyl methacrylate, polyvinyl chloride, fluoropolymers, and cyclic olefin polymers have also been employed for microfluidic device fabrication [26][27][28][29].
Paper-based microfluidic devices represent a new type of microfluidic system developed in recent years, with the advantages of low cost, reproducibility, easy fabrication and handling, portability, and easy integration with other devices [30]. The flow of fluids in paper is primarily controlled by adhesion and cohesion forces that create capillary action in the cellulose matrix. Therefore, the fluid can be precisely directed by hydrophobic modification of certain regions in the matrix (Figure 1d) [31]. The inherent micro/nanostructure of the paper matrix can be used to simulate the cellular microenvironment, such as oxygen/nutrient gradients, shear forces, etc. [32]. Thus, paper can serve as an attractive 3D scaffold to culture cells.
As mentioned above, there are a variety of materials used to fabricate microfluidic devices. It is also essential to select the appropriate processing method according to the material properties and product requirements. Researchers highlight several widely used fabrication techniques. Wet and dry etching techniques are commonly used in silicon and glass processing technologies (Figure 1e) [33]. Wet etching is well-regarded for its fast etch speed. However, hydrofluoric acid is mostly used as an etchant, which is highly corrosive and harmful to the environment. In addition, wet etched channels tend to exhibit an isotropic distribution. In contrast, dry etching (also known as reactive ion beam etching) utilizes an ion beam to bombard a substrate, creating anisotropic, dimensionally accurate channels. However, dry etching is slow and expensive to process. Micromachining, including mechanical cutting, abrasive jet processing, and the ultrasonic processing method, has also been used to process silicon and glass, but the precision and productivity of micromachining are relatively modest.
Soft lithography (also known as replica molding) is also one of the most commonly used microfluidic chip fabrication techniques [34]. Traditional fabrication processes for PDMS microfluidics devices include photomask design, photoresist spin coating, optical exposure, development and formation of PDMS replicas, and sealing (Figure 1f) [35]. In brief, to obtain the desired device, the first process is to design a patterned photomask. The photoresist is then spin coated onto the silicon wafer substrate. Then, the coated silicon wafer is heated to remove the solvent from the photoresist. A photomask is placed on top of the photoresist and both are exposed to UV light. After removing non-crosslinked areas, PDMS is poured to form the rubbery layer. Then, two peeled PDMS or PDMS and glass are sealed after oxygen plasma treatment. Finally, the device is connected to the pipeline for liquid flow. Soft lithography technology has high processing accuracy and small differences in the geometry of different devices, which can improve the repeatability of experiments and increase the credibility of results [36]. The limitations of soft lithography include pattern distortion during method replication, and the process requires a dedicated clean room.
Due to its unique properties, 3D printing has emerged as one of the alternative manufacturing methods for fabricating microfluidic devices (Figure 1g) [37]. There are several conventional 3D printing techniques, including inkjet 3D printing, fused deposition modeling, stereolithography, and two-photon polymerization techniques [38]. Three-dimensional printing can fabricate microfluidic chips with complex three-dimensional geometries and permits the rapid adjustment of the functions of the device by optimizing the design. In addition, it allows high throughput fabrication of microfluidic devices [39]. Importantly, as technology advances, the accuracy of 3D printing continues to improve and is approaching the accuracy of soft lithography. The majority of 3D printing materials are based on acrylates and acrylonitrile butadiene styrene, which can lead to undesired biotoxicity [40][41]. The compatibility of 3D printing materials with solvents remains a challenge. For example, some materials undergo swelling in aqueous environments.
In summary, each material and processing technology has its own challenges and limitations. It is critical to select the appropriate materials and fabrication techniques to fabricate microfluidic devices based on the desired properties and potential application.
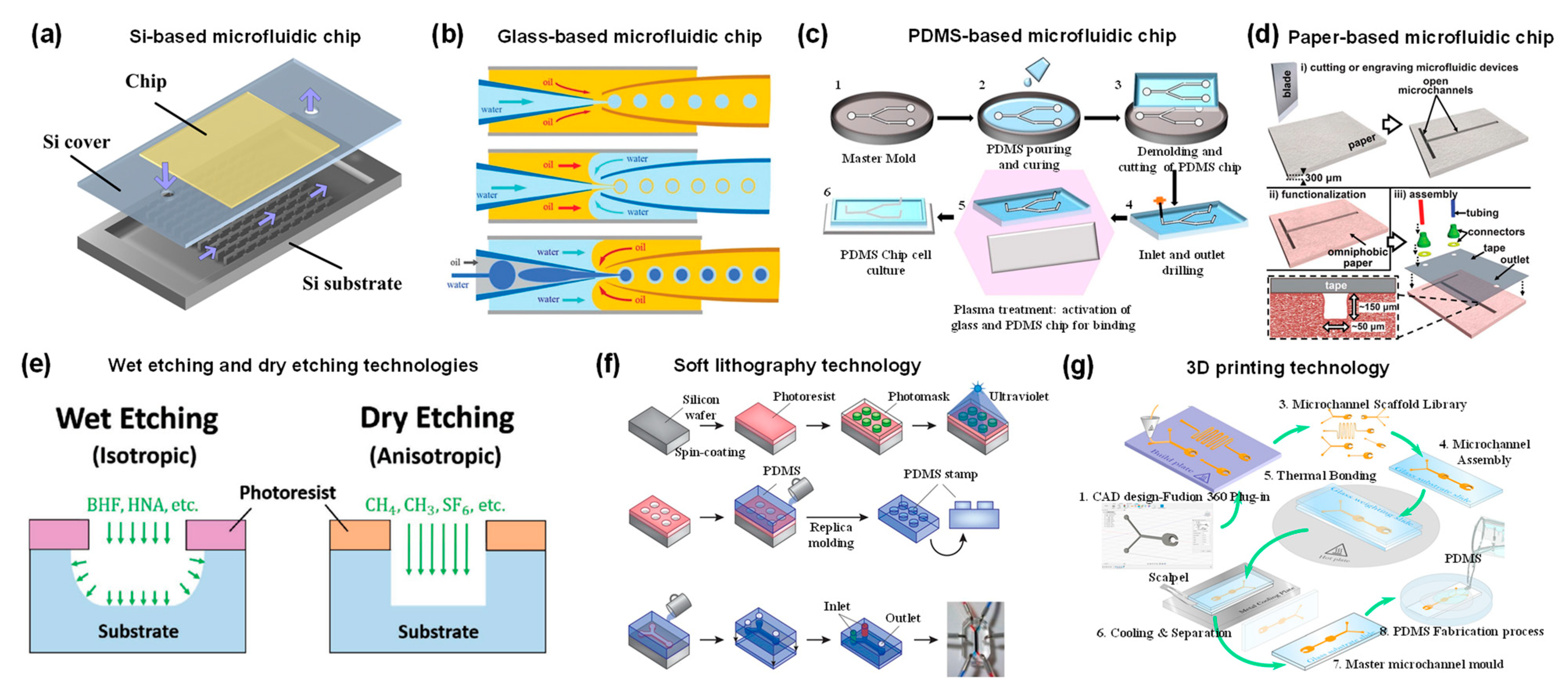
Figure 1. (a) Schematic diagram of silicon-based microfluidic device. Reproduced with permission [16]. Copyright 2015, Multidisciplinary Digital Publishing Institute. (b) Schematic illustration of the coaxial capillary microfluidic devices. Reproduced with permission [16]. Copyright 2018, Royal Society of Chemistry. (c) Fabrication process of PDMS devices by replica molding method. Reproduced with permission [22]. Copyright 2020, Multidisciplinary Digital Publishing Institute. (d) Schematic diagram of paper-based microfluidic device fabrication. Reproduced with permission [31]. Copyright 2013, Royal Society of Chemistry. (e) Schematic diagram of anisotropic wet etching and anisotropic dry etching techniques for processing glass. Reproduced with permission [33]. Copyright 2018, Wiley. (f) Schematic illustration of a typical PDMS microfluidic device fabricated by soft lithography. Reproduced with permission [35]. Copyright 2014, Springer Nature. (g) Schematic illustration of the 3D printing technique to fabricate microfluidic molds. Reproduced with permission [37]. Copyright 2021, Public Library of Science.
3. Drug Delivery System Fabricated by Microfluidics
Compared with a traditional drug delivery system, micro/nanoparticles can improve stability, solubility, and circulation time in vivo [42]. In order to improve the therapeutic efficacy and reduce the side effects of drugs, different kinds of micro/nanocarriers have been developed using biocompatible materials, including microgels, microcapsules, nanoliposomes, nanomicelles, and nanoemulsions [43][44]. However, conventional synthetic methods fail to obtain micro/nanocarriers with uniform size, shape and composition. Microfluidic devices offer an alternative option to address this problem. In general, the fabrication of microparticles is mainly based on the manipulation of immiscible fluids on droplet microfluidics to generate monodisperse droplets. The fabrication of nanoparticles is mainly based on the rapid mixing of multiphase fluids in microfluidics mixer. This section summarizes the preparation of micro/nanoparticles based on droplet microfluidics, centrifugal microfluidics and microfluidic mixers.
3.1. Micro/Nanogels for Drug Delivery
Hydrogels have high water content, tunable chemical and physical structure, excellent mechanical properties, and biocompatibility [45]. Micro/nanogels are three-dimensional crosslinked polymer particles with the features of hydrogels and colloidal particles [46]. Typical materials used to prepare hydrogels include natural polymers (such as alginates, chitosan, hyaluronic acid, etc.) and synthetic polymers (such as polyvinyl alcohol, polyethylene glycol, polyacrylamide, polyhydroxyethylmethacrylate, etc.). The crosslinking methods of hydrogels include ionic crosslinking, covalent crosslinking, UV curing, photoinitiation, electrostatic complexation, interfacial assembly, etc. [47]. Due to the advantages of tunable size, large surface area, abundant internal macromolecular network, and good biocompatibility, micro/nanogels can be candidates for clinical therapeutic drug delivery [48]. In addition, micro/nanogels can effectively protect the encapsulated drugs from the external environment and transport the drugs to specific tissues and/or cells through surface modification.
Alginate hydrogels have been widely used as drug carriers due to their excellent biocompatibility. It is well known that a method available for alginate hydrogels is to crosslink alginate with divalent cations such as Ca2+ and Ba2+ [49]. For example, Cai et al. reported a concentration-controlled microfluidic chip to synthesize DOX-encapsulated alginate nanogels (CaA@Dox) [50]. As shown in Figure 2a, alginate and Ca2+ ions diffused into the central channel driven by a concentration gradient, forming alginate nanogels. The diameter of alginate microgels is positively related to the ratio of channel lengths and flow rate. With the increase of pH value, the carboxyl group is continuously dissociated, the hydrophilicity of sodium alginate increases, and the molecular chain is elongated. Therefore, sodium alginate has significant pH sensitivity. Sodium alginate microgels can respond to the acidic microenvironment of tumors.
In addition to microfluidic chips based on a diffusion mixing mode, centrifugal microfluidic chips are also applied to generate high-throughput centrifugal droplets [51][52]. Kim et al. utilized photo-crosslinkable methacrylate-modified hyaluronic acid microgels using a portable centrifugal microfluidic chip [53]. In the initial state, aqueous solution and oil were injected into the inlet and outlet chambers of the microchip, respectively. Due to the different specific gravity and immiscibility of oil and water, droplets were generated at the oil–water interface under the action of centrifugal force (Figure 2b). Parallel microchannels at the oil–water interface were designed to restrict the droplet size.
By exploiting the environmental sensitivity of polymer networks, hydrogels can be easily designed as stimuli-responsive carriers [54][55]. Depending on the source, stimuli can be divided into physiological environmental stimuli (i.e., tumor acidic microenvironment, inflammation or enzymes at the lesion site) and external stimuli (i.e., temperature, light or magnetic field) [56]. Poly(N-isopropylacrylamide) (PNIPAM) is a representative thermosensitive polymer [57]. PNIPAM shrinks and changes from hydrophilic state to hydrophobic state when the temperature is higher than its lower critical solution temperature (LCST) [58]. Luo et al. fabricated photosensitive microgels using PNIPAM polymers and polypyrrole (PPy) nanoparticles via a co-flow microfluidic chip (Figure 2c) [59]. PPy nanoparticles have excellent photothermal conversion properties. After the first irradiation cycle, PNIPAM microgels containing PPy nanoparticles released 45% of FITC-albumin, which was significantly higher than the control group. Among the various stimulus responses, enzyme-triggered drug release is an attractive approach [56]. Enzymatic reactions provide higher selectivity and specificity due to the specificity of the enzymes. Enzymes play an integral role in physiological processes and are involved in all biological processes. Malignant diseases or lesions lead to elevated expression of specific enzymes in certain tissues. Several natural polymers such as fibrin, collagen, gelatin, and hyaluronic acid have been used to prepare enzyme-responsive hydrogels. Hyaluronidase is overexpressed in aggressive malignancies or secreted by pathogenic bacteria at the site of infection. Busatto et al. synthesized an oil-in-water nanoemulsion to encapsulate hydrophobic drug progesterone [60]. The nanoemulsion and hyaluronic acid precursor solution were mixed in a microfluidic chip to form an oil-in-water-in-oil nanostructure (Figure 2d). Ultimately, hyaluronic acid precursors were crosslinked under ultraviolet light and formed microgels. When exposed to a concentration of 100 UI/mL of hyaluronidase, the hyaluronic acid microgels sustained drug release over two days. Another typical degradative enzyme is matrix metalloproteinase. Previous study showed that PEG microgels were prepared by using a matrix protease-degradable crosslinked peptide and dithiothreitol as the crosslinking agent [61]. The degradation rate of microgels in matrix metalloproteinase can be regulated by changing the ratio of crosslinking peptide to a dithiothreitol crosslinking agent.
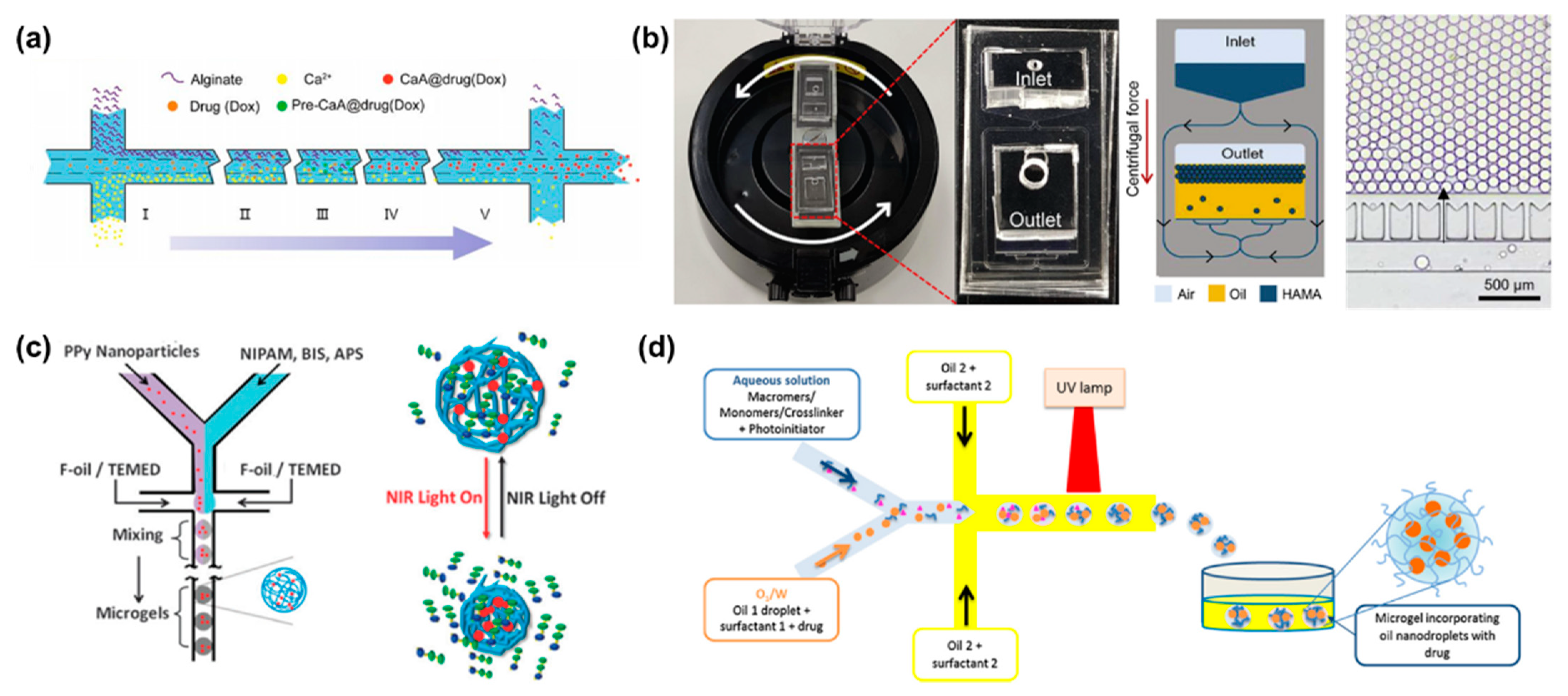
Figure 2. (a) Schematic illustration of microfluidic diffusion mixing mode, where alginate and Ca2+ ions diffuse into the intermediate channel and mix to form nanoparticles. Reproduced with permission [50]. Copyright 2019, Switzerland. (b) Photograph of a portable centrifugal microfluidic system assembled on a commercial centrifuge. Schematic diagram of microdroplet formation during centrifugation, and optical micrographs of microdroplets prepared in the microfluidic device. Reproduced with permission [53]. Copyright 2020, Elsevier. (c) Schematic illustration of PPy nanoparticles mixed with PNIPAM and served as photosensitive drug carriers fabricated with droplet microfluidics. Reproduced with permission [59]. Copyright 2013, Royal Society of Chemistry. (d) Schematic illustration of the preparation of biodegradable HA microgels with O/W/O as a template. Reproduced with permission [60]. Copyright 2017, Elsevier.
3.2. Microcapsules for Drug Delivery
In contrast to micro/nanogels, microcapsules have a core-shell structure where the core can be solid, liquid or gas [62][63]. Microcapsules do not exhibit sudden volume changes in the same way as micro/nanogels, due to shell support [64]. The high versatility of the shell materials allows the production of microcapsules with versatile functionality, such as enhanced retention, controlled release, and stimulus responsiveness [65][66]. The well-designed microcapsule shells not only protect encapsulated drugs, but also rupture and release the internal drugs in response to external stimuli, including pH, temperature, osmotic pressure, electric fields and stress [67].
Stimuli-responsive microcapsules have been widely applied in drug delivery systems. Kim et al. prepared microcapsules based on water/oil/water (W/O/W) double emulsion droplets, showing molecular polarity and temperature-dependent permeability [68]. The intermediate oil phase contains the photocurable monomer and the phase change material dodecanol (Figure 3a). Under UV light, dodecanol remained liquid and occupied the microcapsule space without polymerization. Very different from physical voids, the permeability of microcapsules depends on molecular size and molecular polarity [69]. In this case, low polarity molecules can be dissolved in water and transferred to the other side of the shell by transferring within dodecanol. However, the transport of highly polar molecules is inhibited. Similar to other phase-change materials, dodecanol melts at temperature above its melting point. Therefore, dodecanol freezes reversibly when the temperature is below the melting point. The release of ICG from microcapsules was minimal at 4 °C for 72 h, while the in vitro release process accelerated rapidly at 37 °C. The smart design of the shell material endows the microcapsules with polarity-dependent and temperature-dependent drug release patterns, providing a smart drug release strategy.
Electric fields can also be used to control the release of active substances in microcapsules by methods including electroporation and iontophoresis [70][71]. Depending on the location of the device, drug release near the skin can be triggered by a voltage generated by an external conductive skin patch. Alternatively, a minimally invasive implantable device controlled by radio frequency can be used to control drug release [72]. The microcapsules prepared by electro-responsive materials rupture was attributed to interface deformation caused by maximum well interface stresses at the droplet/dielectric interface induced by the applied alternating electric field (Figure 3b). During the interfacial deformation process, the fragile parts of the microcapsules were prone to defects, leading to rupture and eventually the release of internal drugs. Due to the competition between separation pressure and electrocompression, there is a critical voltage that causes microcapsules to rupture. The threshold voltages increased with the applied field frequency. In addition, high conductivity facilitated drug release at low voltage and was not limited by ion types. This capability can be extended to dual core droplets for drug co-delivery.
Preparation of temperature-responsive or pH-responsive microcapsules usually requires the addition of functional materials to the precursor solution, such as temperature-sensitive or pH-sensitive materials. However, osmolarity-triggered drug release avoids the addition of additional precursors [73]. Zhang synthesized osmotic pressure-responsive microcapsules with non-uniform shell thickness, which can be controlled by changing the flow rate ratio of the mesophase to the inner phase [74]. The thickness of the microcapsule shell varies widely, from 40 μm in the thickest part to 600 nm in the thinnest part (Figure 3c). As a result, non-uniform microcapsules are more prone to rupture from the thinnest areas than uniform microcapsules. It is also fascinating to impart multiple stimulation responses to single microcapsules. Multifunctional responsive microcapsules with a n-hexane layer were prepared with capillary microfluidic [69]. By inducing oil layer instability, hydrophilic drugs encapsulated in microcapsules were released on demand (Figure 3d). Ethanol solution, mechanical stress and osmotic pressure can all cause the oil layer rupture. However, the microencapsulated shell layer remained intact.
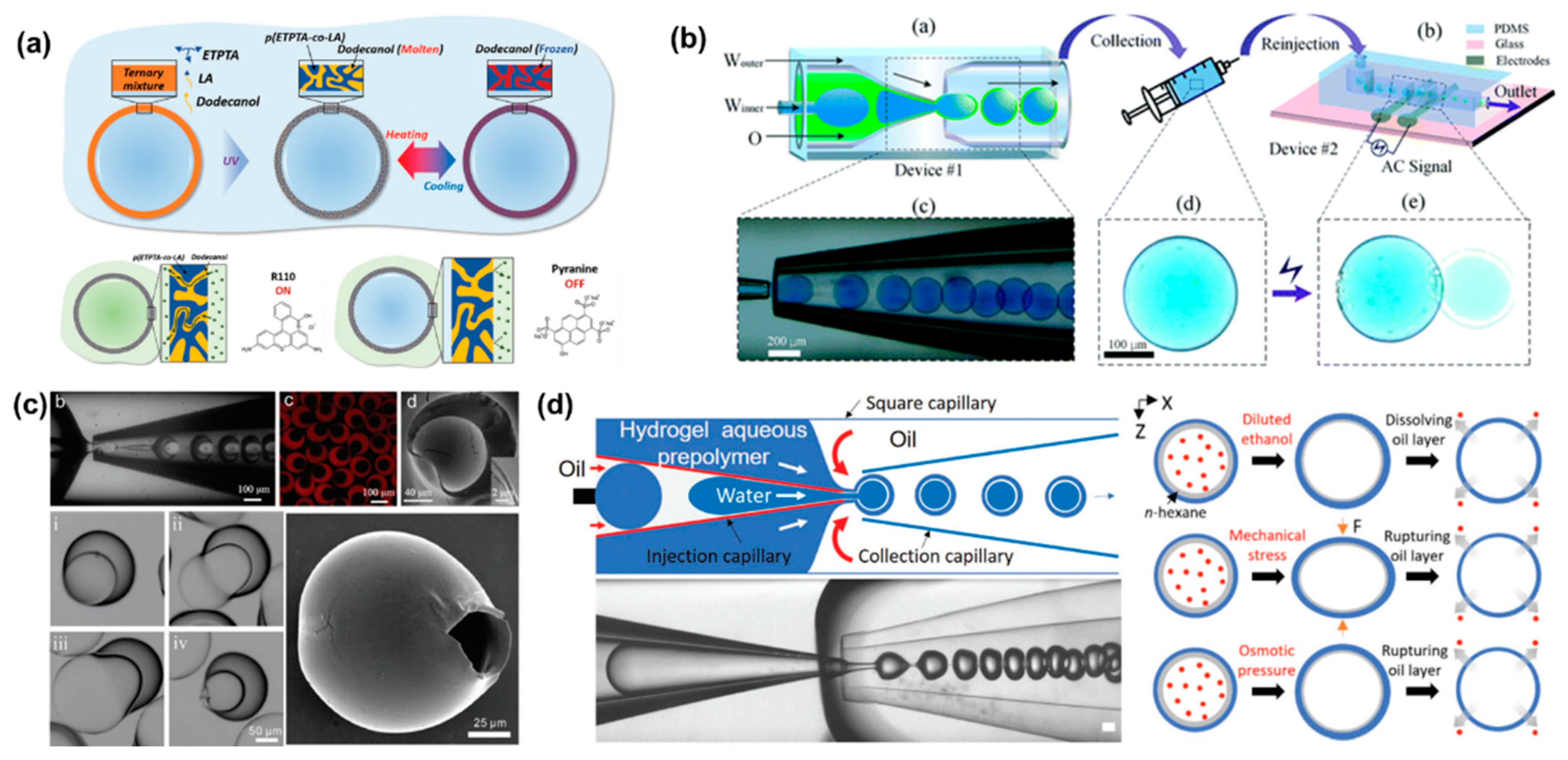
Figure 3. (a) Schematic illustration of microcapsule formation by double emulsion droplet templates and reversible phase change of dodecanol in shell voids during heating and cooling. Reproduced with permission [68]. Copyright 2019, Wiley. (b) Schematic illustration of co-flow capillary microfluidics chip for encapsulation and controlled release of drugs. The applied electric field can control the release of the internal drug from the microcapsule. Reproduced with permission [70]. Copyright 2018, Royal Society of Chemistry. (c) The optical images of capillary microfluidic chip preparation of inhomogeneous microcapsules. Confocal images, SEM images and optical images of inhomogeneous microcapsules. The optical images showed that the thinnest part of the microcapsule shell starts to swell and the volume increased until it ruptures. Reproduced with permission [74]. Copyright 2019, Wiley. (d) Schematic and optical image of microfluidic chip to produce microencapsulated cargos with a thin oil layer, where different external stimuli including dissolution, mechanical pressure and osmotic pressure induce destabilization of the interstitial oil layer to release the cargo. Reproduced with permission [69]. Copyright 2021, Wiley.
For malignant diseases, multidrug codelivery strategies tend to have a significant efficacy. Encouraging progress has been made in the design of stimuli-responsive microcapsule shells based on intrinsic properties of materials. Programmable drug release can be combined with multiple stimulation sequences to achieve a combination of different release modalities, such as burst release and sustained release. Yang et al. designed a multi-stimulus-responsive microcapsule structure consisting of a pH-responsive chitosan hydrogel shell and an oil core containing curcumin and drug-loaded poly(lactic acid-ethanolic acid copolymer) (PLGA) nanoparticles (Figure 4a) [75]. In pH solution of 1.5, the microcapsules ruptured rapidly within 60 s, which corresponds to the gastric environment. Since PLGA is semipermeable, the drug-loaded PLGA nanoparticles achieved sustained release in a neutral environment, with only 63.6% of curcumin released from the nanoparticles in 28 days. The combination of burst release and sustained release provides an effective treatment for acute diseases, with burst release alleviating the acute symptoms and sustained release maintaining the therapeutic effects. More complex Trojan-like microcapsules were prepared in a O1/W2/O3/W4/O5 quadruple microemulsion as templates for complex programmed release [76]. Functional shell materials were incorporated into the W2 and W4 phases to create capsule-in-capsule structures (Figure 4b). Chitosan, poly (ethylene glycol) diacrylate (PEGDA), and PNIPAM constituted three Trojan-like stimulus-responsive microcapsules, including CS@CS microcapsules, PEGDA@CS microcapsules, CS@PNIPAM microcapsules. The pH and temperature can stimulate the chitosan shell and the PNIPAM shell, respectively, resulting in shell rupture. Meanwhile, PEGDA has no temperature or pH response, so the encapsulated drug can be released sustainably. In general, microcapsules containing two stimuli-responsive hydrogel shells are able to provide a flexible trigger mechanism for multifunctional programmed sequential release behavior.
Furthermore, monodisperse multiple emulsions can contain several smaller droplets inside [77][78]. A two-stage co-flow microfluidic device was constructed by a sequential and coaxial nested assembly of multiple glass capillaries (Figure 4c). The uniform droplets generated in the first capillary were encapsulated into larger droplets by the second capillary, forming the drop-in-drop structure [78]. The number, proportion and size of the innermost droplets can be precisely controlled by adjusting the capillary size and fluid flow rate. Higher-order multiple emulsions, such as triple, quadruple, and quintuple emulsions, can be further controllably produced by more complex nested structures.
The conventional preparation method of microcapsules is usually to make bulk emulsion first, and then form the shell of the capsule through interfacial polymerization, precipitation or coagulation. With the bulk emulsification method, it is difficult to control the size, shape and structure of the microcapsules, which greatly limits their potential clinical application. In contrast, microfluidic devices provide a new route for the construction and synthesis of microcapsules by generating emulsions. The structure of microcapsule can be precisely controlled by multiple emulsions through T-junction, flow-focusing, or co-flowing microfluidic devices. In addition, by using a microfluidic device, the drug encapsulation efficiency of the microcapsules can be greatly improved.
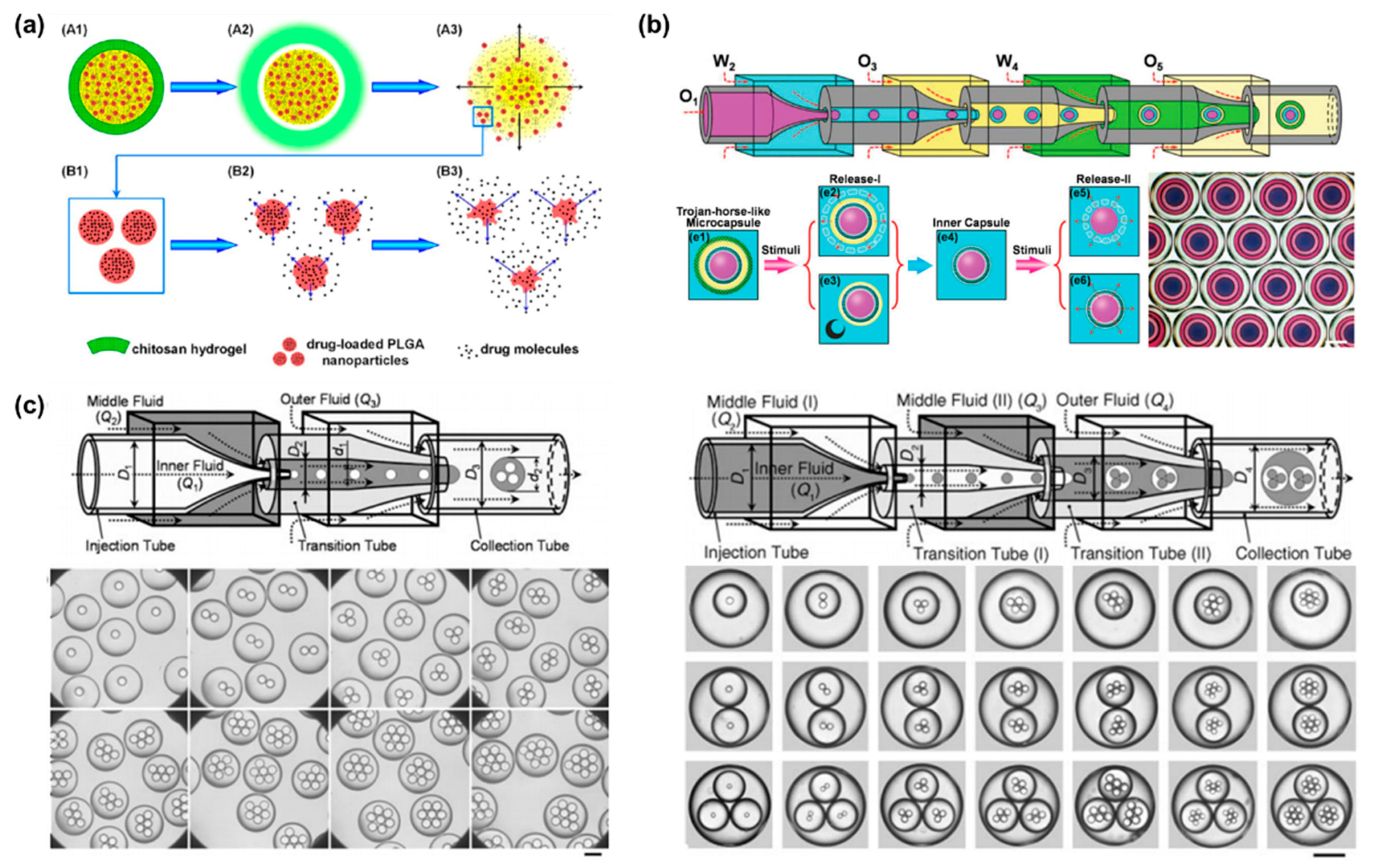
Figure 4. (a) Schematic illustration of programmed sequential drug release from multi-stimulus-responsive microcapsules. Acid triggered the decomposition of chitosan resulting in release of free drug and drug-loaded PLGA nanoparticles. Then PLGA degradation achieved sustained release. Reproduced with permission [75]. Copyright 2016, American Chemical Society. (b) Schematic illustration of a microfluidic chip prepared by sequential emulsification of Trojan-horse-like microcapsules with a capsular-in-capsule structure. Two-stage sequential release of microcapsules were triggered by different stimuli. Reproduced with permission [76]. Copyright 2018, Wiley. (c) Schematic illustration of capillary microfluidics chip for precisely controlled generation of monodisperse double and triple emulsions and optical images of monodisperse double and triple emulsions containing controlled numbers of droplets. Reproduced with permission [78]. Copyright 2007, Wiley.
References
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040.
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406.
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189.
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182.
- Wang, L.; Li, P.C.H. Microfluidic DNA microarray analysis: A review. Anal. Chim. Acta 2011, 687, 12–27.
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022, 7, 406–420.
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370.
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883.
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967.
- Kearney, C.J.; Mooney, D.J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 2013, 12, 1004–1017.
- Sticker, D.; Geczy, R.; Häfeli, U.O.; Kutter, J.P. Thiol–Ene Based Polymers as Versatile Materials for Microfluidic Devices for Life Sciences Applications. ACS Appl. Mater. Interfaces 2020, 12, 10080–10095.
- Shakeri, A.; Jarad, N.A.; Leung, A.; Soleymani, L.; Didar, T.F. Biofunctionalization of Glass- and Paper-Based Microfluidic Devices: A Review. Adv. Mater. Interfaces 2019, 6, 1900940.
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168.
- Wang, G.-L.; Yang, D.-W.; Wang, Y.; Niu, D.; Zhao, X.-L.; Ding, G.-F. Heat Transfer and Friction Characteristics of the Microfluidic Heat Sink with Variously-Shaped Ribs for Chip Cooling. Sensors 2015, 15, 9547–9562.
- Ofner, A.; Moore, D.G.; Rühs, P.A.; Schwendimann, P.; Eggersdorfer, M.; Amstad, E.; Weitz, D.A.; Studart, A.R. High-Throughput Step Emulsification for the Production of Functional Materials Using a Glass Microfluidic Device. Macromol. Chem. Phys. 2017, 218, 1600472.
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683.
- Funano, S.-i.; Ota, N.; Tanaka, Y. A simple and reversible glass–glass bonding method to construct a microfluidic device and its application for cell recovery. Lab Chip 2021, 21, 2244–2254.
- Nabavi, S.A.; Vladisavljević, G.T.; Gu, S.; Ekanem, E.E. Double emulsion production in glass capillary microfluidic device: Parametric investigation of droplet generation behaviour. Chem. Eng. Sci. 2015, 130, 183–196.
- Leister, N.; Vladisavljević, G.T.; Karbstein, H.P. Novel glass capillary microfluidic devices for the flexible and simple production of multi-cored double emulsions. J. Colloid Interface Sci. 2022, 611, 451–461.
- Yu, Y.; Shang, L.; Guo, J.; Wang, J.; Zhao, Y. Design of capillary microfluidics for spinning cell-laden microfibers. Nat. Protoc. 2018, 13, 2557–2579.
- Ter Schiphorst, J.; Saez, J.; Diamond, D.; Benito-Lopez, F.; Schenning, A.P.H.J. Light-responsive polymers for microfluidic applications. Lab Chip 2018, 18, 699–709.
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182.
- Rhyou, J.; Youn, J.; Eom, S.; Kim, D.S. Facile Fabrication of Electrospun Nanofiber Membrane-Integrated PDMS Microfluidic Chip via Silver Nanowires-Uncured PDMS Adhesive Layer. ACS Macro Lett. 2021, 10, 965–970.
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174.
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958.
- Wu, N.; Zhu, Y.; Brown, S.; Oakeshott, J.; Peat, T.S.; Surjadi, R.; Easton, C.; Leech, P.W.; Sexton, B.A. A PMMA microfluidic droplet platform for in vitroprotein expression using crude E. coli S30 extract. Lab Chip 2009, 9, 3391–3398.
- Voicu, D.; Lestari, G.; Wang, Y.; DeBono, M.; Seo, M.; Cho, S.; Kumacheva, E. Thermoplastic microfluidic devices for targeted chemical and biological applications. RSC Adv. 2017, 7, 2884–2889.
- Riche, C.T.; Zhang, C.; Gupta, M.; Malmstadt, N. Fluoropolymer surface coatings to control droplets in microfluidic devices. Lab Chip 2014, 14, 1834–1841.
- Alsharhan, A.T.; Acevedo, R.; Warren, R.; Sochol, R.D. 3D microfluidics via cyclic olefin polymer-based in situ direct laser writing. Lab Chip 2019, 19, 2799–2810.
- Lepowsky, E.; Ghaderinezhad, F.; Knowlton, S.; Tasoglu, S. Paper-based assays for urine analysis. Biomicrofluidics 2017, 11, 051501.
- Glavan, A.C.; Martinez, R.V.; Maxwell, E.J.; Subramaniam, A.B.; Nunes, R.M.D.; Soh, S.; Whitesides, G.M. Rapid fabrication of pressure-driven open-channel microfluidic devices in omniphobic RF paper. Lab Chip 2013, 13, 2922–2930.
- Akyazi, T.; Tudor, A.; Diamond, D.; Basabe-Desmonts, L.; Florea, L.; Benito-Lopez, F. Driving flows in microfluidic paper-based analytical devices with a cholinium based poly(ionic liquid) hydrogel. Sens. Actuators B Chem. 2018, 261, 372–378.
- Anbari, A.; Chien, H.-T.; Datta, S.S.; Deng, W.; Weitz, D.A.; Fan, J. Microfluidic Model Porous Media: Fabrication and Applications. Small 2018, 14, 1703575.
- Nady, E.; Nagy, G.; Huszánk, R. Improvement in mixing efficiency of microfluidic passive mixers functionalized by microstructures created with proton beam lithography. Chem. Eng. Sci. 2022, 247, 117006.
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772.
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502.
- Felton, H.; Hughes, R.; Diaz-Gaxiola, A. Negligible-cost microfluidic device fabrication using 3D-printed interconnecting channel scaffolds. PLoS ONE 2021, 16, e0245206.
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013.
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012.
- Monia Kabandana, G.K.; Zhang, T.; Chen, C. Emerging 3D printing technologies and methodologies for microfluidic development. Anal. Methods 2022, 14, 2885–2906.
- Chan, H.N.; Tan, M.J.A.; Wu, H. Point-of-care testing: Applications of 3D printing. Lab Chip 2017, 17, 2713–2739.
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Batalov, I.; Stevens, K.R.; DeForest, C.A. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc. Natl. Acad. Sci. USA 2021, 118, e2014194118.
- Wang, Y.; Guo, L.; Dong, S.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019, 266, 1–20.
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275.
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43.
- Mao, A.S.; Shin, J.-W.; Utech, S.; Wang, H.; Uzun, O.; Li, W.; Cooper, M.; Hu, Y.; Zhang, L.; Weitz, D.A.; et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat. Mater. 2017, 16, 236–243.
- Cai, S.; Shi, H.; Li, G.; Xue, Q.; Zhao, L.; Wang, F.; Hu, B. 3D-Printed Concentration-Controlled Microfluidic Chip with Diffusion Mixing Pattern for the Synthesis of Alginate Drug Delivery Microgels. Nanomaterials 2019, 9, 1451.
- Maeda, K.; Onoe, H.; Takinoue, M.; Takeuchi, S. Controlled Synthesis of 3D Multi-Compartmental Particles with Centrifuge-Based Microdroplet Formation from a Multi-Barrelled Capillary. Adv. Mater. 2012, 24, 1340–1346.
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229.
- Kim, S.; Yim, S.-G.; Chandrasekharan, A.; Seong, K.-Y.; Lee, T.W.; Kim, B.; Kim, K.; Choi, S.; Yang, S.Y. On-site fabrication of injectable 131I-labeled microgels for local radiotherapy. J. Control. Release 2020, 322, 337–345.
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178.
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941.
- Shigemitsu, H.; Kubota, R.; Nakamura, K.; Matsuzaki, T.; Minami, S.; Aoyama, T.; Urayama, K.; Hamachi, I. Protein-responsive protein release of supramolecular/polymer hydrogel composite integrating enzyme activation systems. Nat. Commun. 2020, 11, 3859.
- Sun, J.; Rijpkema, S.J.; Luan, J.; Zhang, S.; Wilson, D.A. Generating biomembrane-like local curvature in polymersomes via dynamic polymer insertion. Nat. Commun. 2021, 12, 2235.
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012.
- Luo, R.-C.; Ranjan, S.; Zhang, Y.; Chen, C.-H. Near-infrared photothermal activation of microgels incorporating polypyrrole nanotransducers through droplet microfluidics. Chem. Commun. 2013, 49, 7887–7889.
- Busatto, C.A.; Labie, H.; Lapeyre, V.; Auzely-Velty, R.; Perro, A.; Casis, N.; Luna, J.; Estenoz, D.A.; Ravaine, V. Oil-in-microgel strategy for enzymatic-triggered release of hydrophobic drugs. J. Colloid Interface Sci. 2017, 493, 356–364.
- Foster, G.A.; Headen, D.M.; González-García, C.; Salmerón-Sánchez, M.; Shirwan, H.; García, A.J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 2017, 113, 170–175.
- Vericella, J.J.; Baker, S.E.; Stolaroff, J.K.; Duoss, E.B.; Hardin, J.O.; Lewicki, J.; Glogowski, E.; Floyd, W.C.; Valdez, C.A.; Smith, W.L.; et al. Encapsulated liquid sorbents for carbon dioxide capture. Nat. Commun. 2015, 6, 6124.
- Song, Y.; Jeong, Y.; Kwon, T.; Lee, D.; Oh, D.Y.; Park, T.-J.; Kim, J.; Kim, J.; Kwon, S. Liquid-capped encoded microcapsules for multiplex assays. Lab Chip 2017, 17, 429–437.
- Keidel, R.; Ghavami, A.; Lugo, D.M.; Lotze, G.; Virtanen, O.; Beumers, P.; Pedersen, J.S.; Bardow, A.; Winkler, R.G.; Richtering, W. Time-resolved structural evolution during the collapse of responsive hydrogels: The microgel-to-particle transition. Sci. Adv. 2018, 4, eaao7086.
- Zheng, Y.; Yu, Z.; Parker, R.M.; Wu, Y.; Abell, C.; Scherman, O.A. Interfacial assembly of dendritic microcapsules with host–guest chemistry. Nat. Commun. 2014, 5, 5772.
- Lee, H.; Choi, C.-H.; Abbaspourrad, A.; Wesner, C.; Caggioni, M.; Zhu, T.; Weitz, D.A. Encapsulation and Enhanced Retention of Fragrance in Polymer Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 4007–4013.
- Kim, S.-H.; Park, J.-G.; Choi, T.M.; Manoharan, V.N.; Weitz, D.A. Osmotic-pressure-controlled concentration of colloidal particles in thin-shelled capsules. Nat. Commun. 2014, 5, 3068.
- Kim, J.-W.; Lee, S.S.; Park, J.; Ku, M.; Yang, J.; Kim, S.-H. Smart Microcapsules with Molecular Polarity- and Temperature-Dependent Permeability. Small 2019, 15, 1900434.
- Jeong, H.-S.; Kim, E.; Nam, C.; Choi, Y.; Lee, Y.-J.; Weitz, D.A.; Lee, H.; Choi, C.-H. Hydrogel Microcapsules with a Thin Oil Layer: Smart Triggered Release via Diverse Stimuli. Adv. Funct. Mater. 2021, 31, 2009553.
- Jia, Y.; Ren, Y.; Hou, L.; Liu, W.; Jiang, T.; Deng, X.; Tao, Y.; Jiang, H. Electrically controlled rapid release of actives encapsulated in double-emulsion droplets. Lab Chip 2018, 18, 1121–1129.
- Mirvakili, S.M.; Langer, R. Wireless on-demand drug delivery. Nat. Electron. 2021, 4, 464–477.
- Neumann, S.E.; Chamberlayne, C.F.; Zare, R.N. Electrically controlled drug release using pH-sensitive polymer films. Nanoscale 2018, 10, 10087–10093.
- Lee, S.; Lee, T.Y.; Kim, D.J.; Kim, B.; Kim, S.-H. Osmotic-Stress-Mediated Control of Membrane Permeability of Polymeric Microcapsules. Chem. Mater. 2018, 30, 7211–7220.
- Zhang, W.; Qu, L.; Pei, H.; Qin, Z.; Didier, J.; Wu, Z.; Bobe, F.; Ingber, D.E.; Weitz, D.A. Controllable Fabrication of Inhomogeneous Microcapsules for Triggered Release by Osmotic Pressure. Small 2019, 15, 1903087.
- Yang, X.-L.; Ju, X.-J.; Mu, X.-T.; Wang, W.; Xie, R.; Liu, Z.; Chu, L.-Y. Core–Shell Chitosan Microcapsules for Programmed Sequential Drug Release. ACS Appl. Mater. Interfaces 2016, 8, 10524–10534.
- Mou, C.-L.; Wang, W.; Li, Z.-L.; Ju, X.-J.; Xie, R.; Deng, N.-N.; Wei, J.; Liu, Z.; Chu, L.-Y. Trojan-Horse-Like Stimuli-Responsive Microcapsules. Adv. Sci. 2018, 5, 1700960.
- Okushima, S.; Nisisako, T.; Torii, T.; Higuchi, T. Controlled Production of Monodisperse Double Emulsions by Two-Step Droplet Breakup in Microfluidic Devices. Langmuir 2004, 20, 9905–9908.
- Chu, L.-Y.; Utada, A.S.; Shah, R.K.; Kim, J.-W.; Weitz, D.A. Controllable Monodisperse Multiple Emulsions. Angew. Chem. Int. Ed. 2007, 46, 8970–8974.
More
Information
Subjects:
Engineering, Manufacturing
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
08 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




