
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shahid Bashir | + 4805 word(s) | 4805 | 2020-11-26 10:17:46 | | | |
| 2 | Rita Xu | -1715 word(s) | 3090 | 2020-12-03 04:36:57 | | |
Video Upload Options
Hydrogels are three-dimensional crosslinked porous networks and can be synthesized from natural polymers, synthetic polymers, polymerizable synthetic monomers, and combination of natural and synthetic polymers. Synthesis of hydrogels involves physical, chemical and hybrid bonding. The bonding is formed via different routes such as solution casting, solution mixing, bulk polymerization, free radical mechanism, radiation method, and interpenetrating network formation. The synthesized hydrogels have significant properties such as mechanical strength, flexibility, biocompatibility, biodegradability, swellability, and stimuli sensitivity. Furthermore, owing to the smart and aqueous medium, robust mechanical strength, adhesiveness, stretchability, strain sensitivity, and self-healability, hydrogels can be potentially used in biomedical, electrochemical, sensors, contact lens, and soft robotic applications.
1. Introduction of Hydrogels
The first reported hydrogel can be traced back to 1960, when Wichterle and Lim synthesized poly (2-hydroxethyl methacrylate) (PHEMA) and utilized it in the contact lens industry with the ability of imbibing moisture while asserting its network structure, demonstrating the modern hydrogel [1][2]. Hydrogels, with their peculiar structure of three-dimensional crosslinked polymer meshwork, have the tendency to absorb considerable amounts of water within their interstices and keep bonding it while maintaining the network structure in the swollen state. The demonstration of such phenomena in hydrogels is due to the availability of polar hydrophilic moieties, for example, SO3H, OH, NH2, COOH, CONH2, etc., along the polymer network as branched groups. The tendency of water absorption in hydrogels is due to the swelling character, which is monitored by the hydrophilicity of attached groups, swelling media, and crosslinked bonding strength. Crosslinking controls water absorption as well as helping in maintaining the network structure in the swollen state [3][4][5][6]. The crosslinkers play a prime role for secondary interactions with biological tissues along with the participation of hydrophilic groups for water uptake [7].
Due to the distinctive characteristic properties, such as biodegradability, biocompatibility, hydrophilicity, superabsorbancy, viscoelasticity, softness, and fluffiness, hydrogels play a prime role in biomedical applications. Apart from that, hydrogels also respond to various stimuli, such as temperature, electric field, magnetic field, biological molecules, and ionic strength [8][9]. Many hydrogels have the ability to increase the dwelling period of drugs due to the mucoadhesive and bioadhesive characteristics which promote them as suitable nominees as drug carriers [10].
Recently, hydrogels attained striking attention for energy storage and conversion applications owing to their semi-solid phase and inherent flexibility. Traditional energy storage and conversion devices suffer from heavy weight, mechanical rigidity, and unsustainability. Additionally, there is another issue of device leakage because commercially available devices contain liquid electrolytes. Liquid electrolytes are expensive and harmful to human beings due to their organic nature. Therefore, hydrogel electrolytes have been introduced into these devices. Hydrogel electrolytes are semi-solid, biocompatible, biodegradable, cost-effective, and environmentally friendly. These characteristics, along with the inherent flexibility, are indispensable towards practical applications in energy storage devices, especially supercapacitors [11]. Electrochemical devices using hydrogels become flexible, stretchable, and elastic and can work under stretching, bending, folding, and twisting. These characteristics are seriously devastating over traditional electrochemical devices. Furthermore, hydrogels are self-healable, which is a critical element for wearable and portable electronic devices. Self-healability or damage management ability makes the devices ideal for smart and light-weight electronics [12][13].
2. Classification of Hydrogels
Hydrogels can be classified based on different factors and their classification is summed up briefly in Figure 1. Classification of hydrogels depends on the materials (polymers) involved, the source of the polymers, the crosslinking method, their response to stimuli, and their ionic charge. Polymers involved in the hydrogels are natural, synthetic, or combination of natural and synthetic polymers. These polymers can form hydrogels as homopolymer hydrogels, copolymer hydrogels, block copolymer hydrogels, terpolymers, and so on. Moreover, hydrogels are prepared by crosslinking polymers and the crosslinking can be physical, chemical, or both, simultaneously. Crosslinking is formed is numerous ways as well, such as simple mixing, solution casting, bulk polymerization, free radical polymerization, UV and gamma irradiation, and interpenetrating network formation method. Hydrogels can also be classified, based on ionic charge, as cationic, anionic, and neutral hydrogels. The charge on the overall network depends on the charge on the polymer.
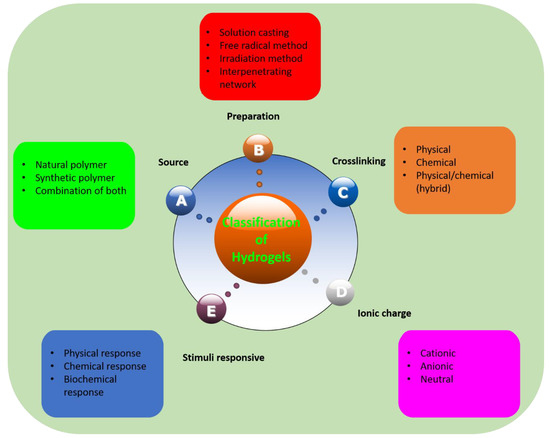
Figure 1. Classification of hydrogels.
3. Composition of Hydrogels
Crosslinked polymers are known as hydrogels regardless of whether they are synthetic grafted polymer derivatives or natural and/or a combination of both. Hydrogels comprising of naturally occurring polymers are called natural hydrogels, owing to the characteristic of non-toxicity, and are exuberantly marketed at cheap prices. Natural polymers can be classified into various categories depending upon their chemical structure. These belong to a variety of classes based on their chemical structure: (i) polysaccharides (chitin, chitosan, cellulose, starch, gums, alginate, and carrageenan), (ii) biological polymers (nucleic acid and DNA), (iii) polyamides (collagen), (iv) polyphenols (lignin), (v) organic polyesters, (vi) inorganic polyesters (polyphosphazene), and (vii) polyanhydrides (poly sebacic acid) [14]. Hydrogels formed from naturally occurring polymers, especially polysaccharides and proteins, are similar to extracellular matrix due to their natural origin and can easily be identified by the cells and, hence, appear to be biocompatible [15]. Nevertheless, hydrogels synthesized from natural polymers, specifically chitosan and other polysaccharides, are delicate. In order to improve their mechanical properties, natural polymers are crosslinked, grafted with monomers, or blended with synthetic polymers [16]. For example, synthetic polymer poly (vinyl alcohol) is used as a blending agent to increase the mechanical strength and flexibility of natural polymers [17].
Synthetic hydrogels contain synthetic polymers which offer more flexibility to tune the mechanical properties of the hydrogel. The most commonly used synthetic polymers are polycaprolactone [18], poly (vinyl pyrrolidone) (PVP) [19], poly (lactic acid) (PLA) [20], poly (ethylene glycol) (PEG) [21], and poly (vinyl alcohol) (PVA) [22]. PEG is water-soluble, biocompatible, and biodegradable and is especially important as it can conjugate with peptides, proteins, and some other drugs. PEG is also utilized to graft with some natural polymers to induce typical properties required for specific application. Bhattarai et al. synthesized PEG-graft-chitosan as injectable thermo-responsive hydrogels that are liquid at low temperatures but solidify at room temperature [23].
4. Natural Polymer Hydrogels
Hydrogels can be categorized as natural, synthetic, or a combination of both. Hydrogels obtained from natural polymers are classified as natural polymer hydrogels. These natural polymers include polysaccharides, polynucleotides, and polypeptides. The natural polymers can be obtained from diverse natural sources and are classified as neutral, cationic, and anionic in nature. These polymers are easy to access, abundant, inexpensive, non-toxic, and biodegradable and possess other attractive biological properties. There has always been a great interest in the relationship of structure and function, especially in natural biologically active compounds. The advances in structural and functional substances over the past few decades made an increasing number of developments in materials to be used in biomedical technology. Natural polymers have well-defined, larger structures formed by covalently bonded monomeric units. Natural polymer hydrogels can be used for numerous biomedical applications, such as controlled and targeted release of drugs, tissue engineering, and wound healing [24][25][26].
4.1. Polysaccharide Hydrogels
Polysaccharides are a distinctive class of naturally occurring polymers that acquire a massive variety of structural characteristics. Polysaccharides can be used as renewable biomaterials due to their exceptional biological properties. They are formed from long-chain carbohydrate molecules of repeated monomeric units held by glycosidic bonds. These act as promising biomaterials and carry special physiological functions and biological activities and are helpful for a variety of applications. Naturally available polysaccharides, such as cellulose, starch, chitin, chitosan, carrageenan, alginates, dextran, pullulan, and pectin, are studied extensively for industrial, medical, pharmaceutical, and tissue engineering applications [27].
There has been great interest in the growth and development of polysaccharide hydrogels during the past few years for biomedical applications. Hydrogels loaded with drugs can sustain their levels in blood and can be controlled subcutaneously, orally, and/or intramuscularly [28][29]. Although synthetically-developed biocompatible and biodegradable polymer hydrogels are also useful for biomedical applications, polysaccharides remain smart and attractive due to their abundant availability, non-toxicity, good biocompatibility and biodegradability, ease of modification and preparation, low cost, and renewable physico-chemical properties and, hence, have been used by mankind in different forms [30].
4.2. Synthetic Polymer Hydrogels
Synthetic polymers are attractive for the synthesis of synthetic polymer hydrogels as they have highly controllable physical and chemical properties than natural polymers. Synthetic polymers can be produced with long-chain structures and high molecular weight. Unfortunately, synthetic polymer hydrogels have lower biological activity than natural hydrogels. Synthetic polymer hydrogels can be synthesized via numerous ways, employing polymerizable vinyl monomers or chemical crosslinking of polymers. Synthetic polymers used in the synthesis of hydrogels are poly (vinyl alcohol) (PVA), poly (ethylene glycol) (PEG), poly (ethylene oxide) (PEO), poly (2-hydroxyethyl methacrylate) (PHEMA), poly (acrylic acid) (PAA), and poly (acrylamide) (PAAm), etc. Some of synthetic polymer hydrogels are discussed in the sections below.
4.3. Hydrogels Based on Poly (Acrylic Acid) Derivatives
Acrylic acid-based hydrogels are pH-sensitive due to the availability of anionic carboxyl groups and show higher swelling at basic pH but low swelling at acidic pH compared to hydrogels containing nonionic or neutral pendant groups. The high swelling at basic pH is owing to the electrostatic repulsion of carboxylate ions formed at basic pH because of deprotonation of carboxylic groups. Acrylic acid-based hydrogels have been extensively prepared and exploited in numerous disciplines, specifically in the biomedical field, due to their excellent adhesive characteristics. Poly (acrylic acid) and glycidyl methacrylate dextran copolymer hydrogels were synthesized using UV irradiation. Synthesized hydrogels demonstrated pH-sensitive swelling in the presence of dextranase at pH 7.4 [31]. Lee et al. reported glycerol crosslinked poly (acrylic acid) hydrogel by polymerizing acrylic acid in the presence of benzoyl peroxide and novozym 435. This hydrogel showed 100% higher swelling in neutral, acidic, and basic pH [32]. Furthermore, poly (acrylic acid) hydrogel was synthesized by free radical co-polymerization using N, N′-methylenebisacrylamide as a crosslinking agent [33]. Nho et al. synthesized poly (acrylic acid) hydrogels by irradiating the acrylic acid solution using an electron beam of maximum 75 kGy, and physical properties, such as swelling ratio, mucoadhesion, and gel contents, were investigated [34].
Methacrylic acid is a derivative of acrylic acid and it also has significant attraction in the biomedical field. Kou et al. synthesized poly (2-hydroxyethyl methacrylate-co-methacrylic acid) hydrogel slabs and cylinders using free radical polymerization. Hydrogel slabs were synthesized using redox couple ammonium persulfate and sodium metabisulfite as an initiator while using tetraethyleneglycol dimethacrylate (TEGDMA) as a crosslinker. Hydrogel cylinders were synthesized using 2, 2′-azobisisobutyronitrile (AIBN) as an initiator and TEGDMA as a crosslinking agent. Furthermore, phenylpropanolamine drug release was studied at different pH values via a desorption method [35]. In another study, Park et al. synthesized pH-sensitive poly (vinyl alcohol-co-acrylic acid) and poly (vinyl alcohol-co-methacrylic acid) hydrogels via grafting of acrylic acid and methacrylic acid on PVA hydrogels in two steps using gamma irradiation. Initially, PVA hydrogels were synthesized using gamma rays of 50 kGy and then acrylic acid and methacrylic acid were grafted onto PVA hydrogels with an irradiation of 5–20 kGy. Grafted hydrogels exhibit pH-sensitive swelling properties and pH-controlled insulin release behavior [36]. Moreover, an interpenetrating network (IPN) of methacrylic acid and PVA hydrogels was prepared using glutaraldehyde as a crosslinking agent via the water-in-oil emulsion method. The synthesized hydrogels showed a pH-sensitive swelling property and release of ibuprofen [37]. Khan and his research group recently reported a poly (acrylic acid) hydrogel using ammonium persulfate and N, N′-methylenebisacrylamide as an initiator and a crosslinking agent, respectively. The synthesis mechanism of the hydrogel is presented in Figure 2 [38].
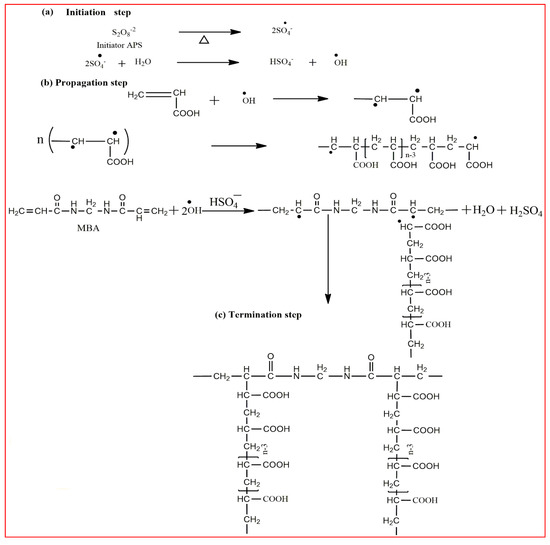
Figure 2. The reaction mechanism of the poly (acrylic acid) hydrogel [38].
4.4. Hydrogels Based on Poly (Acrylamide) Derivatives
Many acrylamide hydrogels have been prepared and utilized in numerous fields. Poly (acrylamide) hydrogels are hydrophilic, neutral, and possess significant valuable physical and chemical properties for potential applications as a biomaterial, for the immobilization of cells and biocatalysts, in drug delivery systems, for heavy metal ions absorption, and as bio-separators. Saraydin et al. synthesized poly (acrylamide) hydrogels using the chemical free radical initiation and gamma ray irradiation (0.72 kGy hr−1) method in the presence of three different crosslinking agents. The synthesized gels displayed different swelling ratios, ranging from 255 to 1450%, depending upon the synthesis protocol, crosslinking agent, and concentration [39]. Poly (acrylamide) hydrogels were also synthesized using vinyl hybrid silica nanoparticles (VSNPs) as a crosslinking agent. Firstly, VSPNs were prepared using vinyl-triethoxysilane via the sol-gel process. The purpose of VSNPs’ introduction was to promote dynamic crosslinking of the poly (acrylamide) network. VSNPs served as crosslinking agents as well as stress buffers to dissipate energy. Preparation of vinyl hybrid silica nanoparticles (VSNPs) from vinyl-triethoxysilane nanoparticles, followed by the synthesis of the VSNP-PAM hydrogel, is demonstrated below in Figure 3 [11].
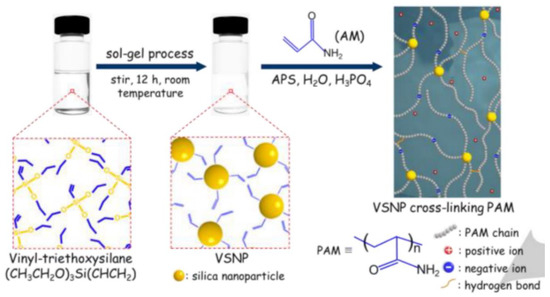
Figure 3. Preparation of vinyl hybrid silica nanoparticles (VSNPs) from vinyl-triethoxysilane nanoparticles followed by the synthesis of VSNP-PAM from VSNPs (crosslinking agent), ammonium persulfate (APS, initiator), acrylamide (AM, main monomer), and phosphoric acid (proton source) [11].
Xu et al. reported poly (acrylamide) hydrogel membranes grafted over cellulose nanofibers (CNFs) crosslinked through N, N′-methylenebisacrylamide via free radical mechanism. The grafting was owing to the interconnection of amide group of poly (acrylamide) and hydroxyl and carboxyl groups present on the surface of CNFs (Figure 4) [40].
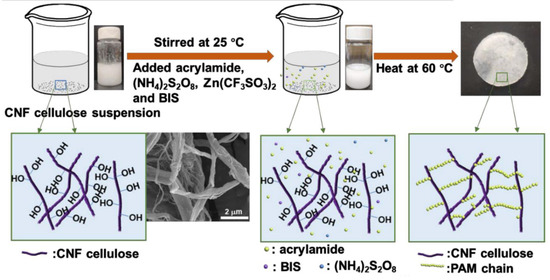
Figure 4. Schematic of the synthesis route to form solid-state electrolytes by grafting PAM on cellulose nanofibers (CNFs) via a facile free radical polymerization approach [40].
Hina et al. synthesized novel self-healable poly (acrylamide) clay crosslinked hydrogels through the free radical approach [41]. Poly (acrylamide) hydrogels are weak in hydrolytic stability and mechanical strength. Efforts have been made to ameliorate their properties by co-polymerization with other monomers [39]. Poly (acrylamide-co-acrylic acid) hydrogel has been prepared by free radical polymerization. A rheological study of the hydrogels exhibited an increase in the mechanical strength of copolymer hydrogels [42]. In another study, poly (acrylamide-co-acrylic acid)/poly (acrylamide) super porous and IPN hydrogels were prepared by pre-polymerization, synchronous polymerization, and the frothing process. The swelling property was observed to be high while low in compressive strength. Furthermore, the IPN hydrogels showed a decrease in water absorption but an increase in water retention and compression strength [43]. However, poly (alkylacrylamide) hydrogels are thermosensitive, although most hydrogels show negative temperature sensitivity. These hydrogels have a lower critical solution temperature (LCST) and show contraction upon heating above the lower critical solution temperature (LCST) [44]. Poly (N-isopropylacrylamide) (PNIPAM) is the most important thermo-sensitive polymer and its hydrogels have been extensively studied in numerous fields. Matzelle et al. prepared PNIPAM and poly (acrylamide) hydrogels to analyze the effect of temperature on elastic parameters. The elastic properties of the PNIPAM hydrogel were temperature-dependent while the poly (acrylamide) hydrogel had a very slight response to changes in temperature [45]. PNIPAM copolymer hydrogels have been extensively observed as biomaterials for the pulsatile delivery of thrombolytic and antithrombotic streptokinase and heparin [46]. Fathi et al. prepared dual thermo- and pH-sensitive hydrogels of poly (N-isopropylacrylamide-co-itaconic acid) and chitosan. The hydrogels were engineered by blending poly (N-isopropylacrylamide-co-itaconic acid) with chitosan and glycerophosphate was added as an ionic crosslinking agent [47]. Temperature-sensitive hydrogels of poly (N-isopropylacrylamide) are significant in drug delivery and the method of crosslinking plays a role in defining the characteristics of hydrogels. Mesoporous silica nanoparticles (MSNs) improve the network, morphology, and pore size, and drug-loading/release properties. The synthesis mechanism of poly (N-isopropylacrylamide) (PNIPAM) hydrogel using mesoporous silica nanoparticles is presented in Figure 5 [48].
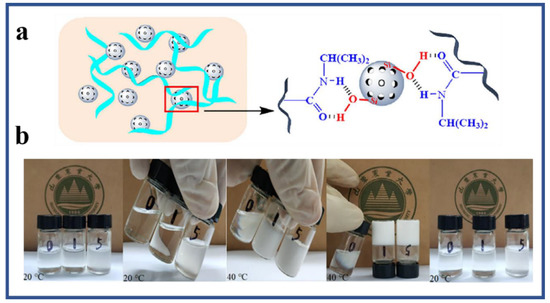
Figure 5. (a) Synthesis mechanism of poly (N-isopropylacrylamide)/mesoporous silica nanoparticle (PNIPAM/MSN) composite hydrogels; (b) digital photographs showing the PNIPAM/MSN-0, PNIPAM/MSN-1, and PNIPAM/MSN-5 hydrogels at 20 °C and 40 °C [48].
Panayiotou et al. prepared poly (N, N′-diethyl acrylamide) (PNDEAM) and poly (N-isopropylacrylamide) hydrogels through free radical polymerization and compared their swelling–deswelling–reswelling, insulin storage, and controlled release ability. The results revealed the stronger dependency of swelling of the PNDEAM hydrogel on the crosslinking agent, slower reswelling kinetics, and faster insulin release compared to the PNIPAM hydrogel [49].
4.5. Natural Polymers in Combination with Synthetic Polymer Hydrogels
Naturally occurring polymers exhibit a weak mechanical strength; therefore, due to this drawback, their applications are limited in biomedical areas. However, synthetic polymers take precedence in this challenge over the naturally occurring ones and are being exploited in various applications due to their facile synthesis protocol, cost effectiveness, and tailor-made properties, suitable for particular application. In addition to that, synthetic polymers show excellent mechanical strength [50][51]. Nevertheless, synthetic polymers are not environmentally friendly because the produced solid waste material could not be degrading biologically.
In chitosan-based hydrogel formulations, formaldehyde is used as a crosslinking agent which releases the drug at a pH equal to the pH of stomach [52]. Chen and his co-workers prepared semi-interpenetrating networks (semi-IPNs) of chitosan-graft-poly (methacrylic acid) via free radical mechanism using formaldehyde as a crosslinker and 2, 2′-azo bis-isobutyronitrile as an initiator [53]. Chitosan-graft-poly (acrylic acid-co-hydroxyethyl methacrylate) hydrogel membranes were synthesized using cerium ammonium nitrate chemical initiator. The grafting of copolymers on chitosan was further characterized using different techniques [54]. Agnihotri et al. fabricated semi-IPN chitosan-poly (acrylamide-co-ethylene oxide) hydrogel microspheres. In the first step, poly (acrylamide-co-ethylene oxide) was prepared by free radical mechanism and embedded in the glutaraldehyde (GA) crosslinked chitosan hydrogel. These hydrogel microspheres were used to encapsulate and deliver anti-cancer drugs [55]. On the other hand, a novel pH-sensitive IPN of acrylamide-grafted-PVA crosslinked with chitosan using glutaraldehyde was used to deliver antibiotic drugs. The hydrogel demonstrated prolonged release of the drug up to 10 h [56]. Stimuli sensitive chitosan grafted poly (acrylic acid), poly (hydroxy propyl methacrylate), PVA, and gelatin hydrogels were prepared using gamma rays. [57]. Lejardi et al. reported hydrogel blends of chitosan and modified PVA-graft-glycolic acid by mixing chitosan solution with modified PVA under constant agitation. These hydrogels showed enhanced rheological properties [58]. A pH-responsive semi-IPN of N-carboxyethyl chitosan and PHEMA hydrogels were synthesized through photo polymerization. This hydrogel exhibited good mechanical properties and sustained release of 5-fluorouracil [59]. Hydrogel films of pectin-grafted acrylamide crosslinked with glutaraldehyde were also reported elsewhere. This formulation showed better film forming, gelling, and mechanical properties compared to pure pectin [60]. Cellulose-supported synthetic polymerizable monomer hydrogels were prepared using chemical, photo, and gamma ray initiation. Some disadvantages of these techniques are the cost and non-availability of required equipment and difficulties in homopolymerization [61]. Interpenetrating network of guar gum and PVA were reported using glutaraldehyde as a crosslinking agent. These IPN hydrogels were used in drug delivery systems [62]. In another study, grafted copolymers of guar gum and acrylamide hydrogel microspheres were synthesized using GA as a crosslinking agent. These hydrogels were further analyzed and tested as an anti-hypertensive drug delivery [63]. Hydrogel microparticles of hydroxyethyl starch-grafted- PHEMA were synthesized by free radical polymerization and protein release was studied. The results showed that protein release was dependent upon hydrogel network density and the size of the entrapped proteins [64]. Other hydrogel formulations, including alginate, carrageenan, alginic acid, gum arabic, and xanthan gum modified with synthetic polymers as well as synthetic polymerizable monomers through different methods, have been reported. In our work, we synthesized hydrogels comprised of natural and synthetic polymers using different crosslinking agents for anti-cancer, anti-asthma, and anti-inflammatory drug delivery at various pH levels [65].
References
- Ghorpade, S.; Dias, R.J.; Mali, K.K.; Mulla, S.I. Citric acid crosslinked carboxymethylcellulose-polyvinyl alcohol hydrogel films for extended release of water soluble basic drugs. J. Drug Deliv. Sci. Technol. 2019, 52, 421–430.
- Dimatteo, ; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184.
- Abad, V.; Relleve, L.S.; Aranilla, C.T.; Dela Rosa, A.M. Properties of radiation synthesized PVP-kappa carrageenan hydrogel blends. Radiat. Phys. Chem. 2003, 68, 901–908, doi:10.1016/S0969-806X(03)00164-6.
- Ali, W.; Zaidi, S.A.R. Synthesis of copolymeric acrylamide/potassium acrylate hydrogels blended with poly (vinyl alcohol): Effect of crosslinking and the amount of poly (vinyl alcohol) on swelling behavior. J. Appl. Polym. Sci. 2005, 98, 1927–1931.
- Bhattarai, ; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99, doi:10.1016/j.addr.2009.07.019.
- Ahmed, M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121, doi:10.1016/j.jare.2013.07.006.
- Nie, ; Pei, B.; Wang, Z.; Hu, Q. Construction of ordered structure in polysaccharide hydrogel: A review. Carbohydr. Polym. 2019, 205, 225–235.
- Siepmann, ; Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011.
- Miyata, ; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98.
- Nezhad-Mokhtari, ; Ghorbani, M.; Roshangar, L.; Rad, J.S. A review on the construction of hydrogel scaffolds by various chemically techniques for tissue engineering. Eur. Polym. J. 2019, 117, 64–76.
- Huang, ; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An intrinsically stretchable and compressible supercapacitor containing a polyacrylamide hydrogel electrolyte. Angew. Chem. Int. Ed. 2017, 56, 9141–9145.
- Wang, ; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel electrolytes for flexible aqueous energy storage devices. Adv. Funct. Mater. 2018, 28, 1804560.
- Huang, ; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte. Nat. Commun. 2015, 6, 10310.
- Sharma, ; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747
- Mahinroosta, ; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55.
- Islam, ; Yasin, T.; Bano, I.; Riaz, M. Controlled release of aspirin from pH‐sensitive chitosan/poly (vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2012, 124, 4184–4192.
- Kirschner, M.; Anseth, K.S. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013, 61, 931–944, doi:10.1016/j.actamat.2012.10.037.
- Vijayavenkataraman, ; Vialli, N.; Fuh, J.Y.; Lu, W.F. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprinting 2019, 5, 229.
- Bonelli, ; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly (vinyl alcohol)/poly (vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348.
- Munim, A.; Raza, Z.A. Poly (lactic acid) based hydrogels: Formation, characteristics and biomedical applications. J. Porous Mater. 2019, 26, 881–901.
- Atta, ; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245.
- Rodríguez‐Rodríguez, ; García‐Carvajal, Z.; Jiménez‐Palomar, I.; Jiménez‐Avalos, J.; Espinosa‐Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136, 47149.
- Bhattarai, ; Matsen, F.A.; Zhang, M. PEG-Grafted Chitosan as an Injectable Thermoreversible Hydrogel. Macromol. Biosci. 2005, 5, 107–111, doi:10.1002/mabi.200400140.
- Iresha, ; Kobayashi, T. Smart Polysaccharide Hydrogels in Drug Delivery and Release. In Advanced Biopolymeric Systems for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 135–149.
- Hu, ; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual‐Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, 1800069.
- Kwiecień, ; Kwiecień, M. Application of polysaccharide-based hydrogels as probiotic delivery systems. Gels 2018, 4, 47.
- Gholamali, Stimuli-responsive polysaccharide hydrogels for biomedical applications: A review. Regen. Eng. Transl. Med. 2019, 1–24, doi:10.1007/s40883-019-00134-1.
- Piyakulawat, ; Praphairaksit, N.; Chantarasiri, N.; Muangsin, N. Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. Aaps Pharmscitech 2007, 8, 120.
- Reis, V.; Guilherme, M.R.; Moia, T.A.; Mattoso, L.H.; Muniz, E.C.; Tambourgi, E.B. Synthesis and characterization of a starch‐modified hydrogel as potential carrier for drug delivery system. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2567–2574.
- Berger, ; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34.
- Kim, -S.; Oh, I.-J. Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly (acrylic acid). Arch. Pharmacal Res. 2005, 28, 983–987.
- Lee, L.S.; Ordeñez, G.; Chakraborty, S. Novel glycerol cross-linked poly (acrylic acid) hydrogel for encapsulation and release of benzocaine. Philipp. Sci. Lett. 2011, 4, 81–87.
- Amin, C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo-and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473.
- Nho, -C.; Park, J.-S.; Lim, Y.-M. Preparation of poly (acrylic acid) hydrogel by radiation crosslinking and its application for mucoadhesives. Polymers 2014, 6, 890–898.
- Kou, H.; Amidon, G.L.; Lee, P.I. pH-Dependent Swelling and Solute Diffusion Characteristics of Poly (Hydroxyethyl Methacrylate–CO–Methacrylie Acid) Hydrogels. Pharm. Res. 1988, 5, 592–597.
- Park, E.; Nho, Y.C.; Lim, Y.M.; Kim, H.I. Preparation of pH‐sensitive poly (vinyl alcohol‐g‐methacrylic acid) and poly (vinyl alcohol‐g‐acrylic acid) hydrogels by gamma ray irradiation and their insulin release behavior. J. Appl. Polym. Sci. 2004, 91, 636–643.
- Mundargi, ; Patil, S.; Kulkarni, P.; Mallikarjuna, N.; Aminabhavi, T. Sequential interpenetrating polymer network hydrogel microspheres of poly (methacrylic acid) and poly (vinyl alcohol) for oral controlled drug delivery to intestine. J. Microencapsul. 2008, 25, 228–240.
- Khan, ; Bashir, S.; Hina, M.; Subramaniam, R.T.; Kasi, R.; Lahiri, I. Effect of Salt Concentration on Poly (acrylic acid) Hydrogel Electrolytes and Their Applications in Supercapacitor. J. Electrochem. Soc. 2020, doi:10.1149/1945-7111/ab992a.
- Saraydın, ; Karadag, E.; Işıkver, Y.; Şahiner, N.; Güven, O. The influence of preparation methods on the swelling and network properties of acrylamide hydrogels with crosslinkers. J. Macromol. Sci. Part A 2004, 41, 419–431.
- Xu, ; Liu, C.; Wu, Q.; Xie, W.; Kim, W.-Y.; Lee, S.-Y.; Gwon, J. A stretchable solid-state zinc ion battery based on a cellulose nanofiber–polyacrylamide hydrogel electrolyte and a Mg0.23V2O5·1.0H2O cathode. J. Mater. Chem. A 2020, 8, 18327–18337.
- Hina, ; Bashir, S.; Kamran, K.; Ramesh, S.; Ramesh, K. Synthesis and Characterization of Self-healable Poly (acrylamide) Hydrogel Electrolytes and Their Application in Fabrication of Aqueous Supercapacitors. Polymer 2020, 210, 123020.
- Nesrinne, ; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly (acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2013, doi:10.1016/j.arabjc.2013.11.027, doi:10.1016/j.arabjc.2013.11.027.
- Ao, ; Huang, M.; Wu, J.; Lin, J.; Tang, Q.; Sun, H. Synthesis and properties of poly (acrylamide‐co‐acrylic acid)/polyacrylamide superporous IPN hydrogels. Polym. Adv. Technol. 2009, 20, 1044–1049.
- Hirotsu, ; Hirokawa, Y.; Tanaka, T. Volume‐phase transitions of ionized N‐isopropylacrylamide gels. J. Chem. Phys. 1987, 87, 1392–1395.
- Matzelle, ; Geuskens, G.; Kruse, N. Elastic properties of poly (N-isopropylacrylamide) and poly (acrylamide) hydrogels studied by scanning force microscopy. Macromolecules 2003, 36, 2926–2931.
- Brazel, S.; Peppas, N.A. Pulsatile local delivery of thrombolytic and antithrombotic agents using poly (N-isopropylacrylamide-co-methacrylic acid) hydrogels. J. Control. Release 1996, 39, 57–64.
- Fathi, ; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly (N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964.
- Zhang, ; Wang, S.; Waterhouse, G.I.; Zhang, Q.; Li, L. Poly (N‐isopropylacrylamide)/mesoporous silica thermosensitive composite hydrogels for drug loading and release. J. Appl. Polym. Sci. 2020, 137, 48391.
- Panayiotou, ; Freitag, R. Synthesis and characterisation of stimuli-responsive poly (N, N′-diethylacrylamide) hydrogels. Polymer 2005, 46, 615–621.
- Sohail, ; Minhas, M.U.; Khan, S.; Hussain, Z.; de Matas, M.; Shah, S.A.; Khan, S.; Kousar, M.; Ullah, K. Natural and synthetic polymer-based smart biomaterials for management of ulcerative colitis: A review of recent developments and future prospects. Drug Deliv. Transl. Res. 2019, 9, 595–614.
- Bashir, ; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Synthesis, characterization, properties of N-succinyl chitosan-g-poly (methacrylic acid) hydrogels and in vitro release of theophylline. Polymer 2016, 92, 36–49.
- Chen, -C.; Wu, Y.-C.; Mi, F.-L.; Lin, Y.-H.; Yu, L.-C.; Sung, H.-W. A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300.
- Chen, ; Liu, M.; Jin, S.; Chen, Y. Synthesis and swelling properties of pH‐sensitive hydrogels based on chitosan and poly (methacrylic acid) semi‐interpenetrating polymer network. J. Appl. Polym. Sci. 2005, 98, 1720–1726.
- Dos Santos, ; Coelho, J.; Ferreira, P.; Pinto, I.; Lorenzetti, S.G.; Ferreira, E.; Higa, O.Z.; Gil, M. Synthesis and characterization of membranes obtained by graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto chitosan. Int. J. Pharm. 2006, 310, 37–45.
- Agnihotri, A.; Aminabhavi, T.M. Novel interpenetrating network chitosan-poly (ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int. J. Pharm. 2006, 324, 103–115.
- Rao, K.; Naidu, B.V.K.; Subha, M.; Sairam, M.; Aminabhavi, T. Novel chitosan-based pH-sensitive interpenetrating network microgels for the controlled release of cefadroxil. Carbohydr. Polym. 2006, 66, 333–344.
- Sokker, ; Ghaffar, A.A.; Gad, Y.; Aly, A. Synthesis and characterization of hydrogels based on grafted chitosan for the controlled drug release. Carbohydr. Polym. 2009, 75, 222–229.
- Lejardi, ; Hernández, R.; Criado, M.; Santos, J.I.; Etxeberria, A.; Sarasua, J.; Mijangos, C. Novel hydrogels of chitosan and poly (vinyl alcohol)-g-glycolic acid copolymer with enhanced rheological properties. Carbohydr. Polym. 2014, 103, 267–273.
- Zhou, ; Yang, D.; Ma, G.; Tan, H.; Jin, Y.; Nie, J. A pH‐sensitive water‐soluble N‐carboxyethyl chitosan/poly (hydroxyethyl methacrylate) hydrogel as a potential drug sustained release matrix prepared by photopolymerization technique. Polym. Adv. Technol. 2008, 19, 1133–1141.
- Sutar, B.; Mishra, R.K.; Pal, K.; Banthia, A.K. Development of pH sensitive polyacrylamide grafted pectin hydrogel for controlled drug delivery system. J. Mater. Sci. Mater. Med. 2008, 19, 2247–2253.
- Karlsson, ; Gatenholm, P. Preparation and characterization of cellulose-supported HEMA hydrogels. Polymer 1997, 38, 4727–4731.
- Soppimath, S.; Kulkarni, A.R.; Aminabhavi, T.M. Controlled release of antihypertensive drug from the interpenetrating network poly (vinyl alcohol)–guar gum hydrogel microspheres. J. Biomater. Sci. Polym. Ed. 2000, 11, 27–43.
- Soppirnath, S.; Aminabhavi, T.M. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur. J. Pharm. Biopharm. 2002, 53, 87–98.
- Schwoerer, D.; Harling, S.; Scheibe, K.; Menzel, H.; Daniels, R. Influence of degree of substitution of HES–HEMA on the release of incorporated drug models from corresponding hydrogels. Eur. J. Pharm. Biopharm. 2009, 73, 351–356.
- Li, ; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.




