| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisabet Roca-Millan | + 2281 word(s) | 2281 | 2020-12-02 08:51:02 | | | |
| 2 | Rita Xu | -823 word(s) | 1458 | 2020-12-03 02:32:49 | | |
Video Upload Options
Titanium membranes used as barrier element in guided bone regeneration procedures. These membranes prevent the penetration of epithelial cells and fibroblasts and allow access to the bone defect of osteogenic and stem cells originating from the native bone. They have different formats, from a titanium sheet to be formed intraoperatively to a custom-made occlusive barrier from a previous computed tomography scan of the bone defect.
1. Introduction
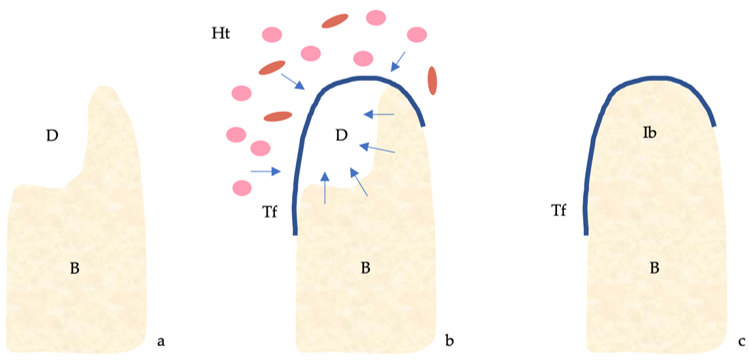
The four classical principles of guided bone regeneration (GBR) are primary closure, angiogenesis, space maintenance and blood clot stability [1] (Figure 1).
Based on these concepts, different techniques and a wide variety of biomaterials have been developed with the aim of achieving greater predictability, lower risk of complications, lower morbidity and shorter operative time in this type of treatment, which is becoming more and more common [2][3]. Research on new biomaterials for bone regeneration is advancing rapidly; even recently they have been manufactured by combining biopolymers and natural nanoparticles [4][5]. Ideally, the proposed method should provide a solution to the four precepts [2][3].
On the other hand, the properties that an ideal biomaterial should fulfill are osteogenesis, osteoconduction and osteoinduction. Therefore, autologous bone is considered the gold standard [2]. However, the great resorption, the unlimited availability, the morbidity and the longer surgical time represent inconveniences in its use. That is why a combination of several biomaterials is generally used in GBR procedures [2][3][6].
Figure 1. Guided bone regeneration (GBR) mechanism. (a) Bone defect. (b) The titanium barrier prevents the penetration of epithelial cells and fibroblasts and allows access to the defect of osteogenic and stem cells originating from the native bone. (c) Regeneration of the bone defect. Abbreviations: B, bone; D, defect; Ht, healing tissue; Ib, immature bone; Tf, titanium foil.
Since clot formation is the first and essential step in bone healing [7][8], in the last years, there have been numerous studies that focus on the use of blood concentrates (platelet-rich plasma (PRP), platelet-rich fibrin (PRF) and platelet-rich growth factor (PRGF)) in these surgical procedures [9][10][11].
However, due to the presence of erythrocytes, blood has a greater capacity to generate thrombin and activate platelets than these concentrates [12]. Likewise, the lower porosity and density of the fibrin layer present in the complete clot facilitate cell migration [13]. However, to benefit from its properties, it would be necessary to at least maintain the space and stabilize the clot [14][15][16].
To comply with these two principles, there are different types of membranes or barrier elements, such as titanium-reinforced polytetrafluoroethylene membranes, perforated titanium meshes and titanium foils [17][18]. The main drawback of the first two is the high exposure rate, associated with a high failure rate [3][6][18]. However, titanium barriers tolerate prolonged exposure to the oral environment, with good hygiene and the use of antiseptics to avoid bacterial colonization being essential [19][20][21].
Several studies published in recent years defend the use of these barrier elements in the regeneration of large maxillary atrophies [20][21][22][23][24], in post-extraction socket reconstruction [19][25][26], in the regeneration of periodontal defects [27] and even simultaneously with implant placement [28].
The concept of using these barriers is to take advantage of the properties of the blood clot, which is why, in some studies, they are used without biomaterial filling [21][24]; although, in other publications, these membranes have been used in combination with PRF [19], allograft [20][22][23][29], xenograft [25][26][30], mixed autograft and allograft [31] or even tricalcium β-phosphate [28].
2. Discussion
According to the results obtained in the present systematic review, the horizontal bone gain was between 2.3 and 9 mm [22][25][26][29][30], and the vertical between 4.5 and 7.3 mm [22][26][31]. These last values without taking into account the randomized clinical trial in which alveolar ridge preservation of well-conserved post-extraction sockets was performed, in which the mean vertical gain was greater than 8 mm [32].
In a recent RCT comparing vertical bone gain by using d-PTFE titanium-reinforced membranes or titanium meshes, a gain of 4.2 ± 1.0 mm (range 2.7–5.8) and 4.1 ± 1.0 mm (range 2.6–6.3) was obtained, respectively [33]. Likewise, a meta-analysis obtained similar results, with a mean vertical bone gain of 4.42 mm by using non-resorbable membranes (d-PTFE and e-PTFE), of 4.26 mm by using titanium meshes covered by resorbable membranes and of 5.2 mm by using titanium meshes alone [34].
Based on these data, it appears that the use of titanium foils is predictable in terms of the amount of bone gain, regardless of the filling material, and the gain may be even higher than with the use of other commonly used non-resorbable membranes or meshes.
With regard to horizontal bone gain, the values obtained in the different studies analyzed are very heterogeneous and do not seem to be related to the filling material used either. Other studies in which horizontal regeneration procedures were performed with collagen membranes and particulate grafts obtained average bone gains of 2.27 ± 1.68 mm [35], 5.68 ± 1.42 mm [36] and 5.03 ± 2.15 mm [37]. Thus, it seems that, in terms of horizontal bone gain, titanium barriers are comparable to collagen membranes, the most widely used in horizontal ridge augmentation procedures.
Based on the included articles, there is no evidence to believe that a filling material is better than another or even blood clot, in combination with occlusive titanium barriers. Furthermore, this type of membrane could be useful in the regeneration of defects of different types, from contained defects such as a post-extraction socket to a combined vertical and horizontal defect such as a posterior mandibular atrophy.
Regarding complications, it appears that titanium foils are prone to exposure, as is the case of titanium meshes and non-resorbable membranes with titanium reinforcement. The mean exposure rate in the present work was 23.81% (range 0–50%) [19][22][24][30][31][38][39][40][41][32][42], and the mean infection rate was 1.19% (range 0–11.9%) [19][22][25][26][28][29][30][31][32][41]. If these results are compared with those of other studies, it can be observed that the rate of postoperative complications of titanium foils is slightly higher than that of GBR procedures with other types of membranes. In an RCT in which the rate of complications in vertical ridge augmentation was evaluated through the use of titanium meshes covered with collagen membranes and the use of non-resorbable membranes with titanium reinforcement, a postoperative complication rate (exposure and infection) of 21.1% and 15% was obtained, respectively [33]. A meta-analysis obtained an intra- and postoperative complications rate of 21% for titanium meshes covered with resorbable membranes, of 6.9% for non-resorbable membranes and of 20% for titanium meshes [34].
In different studies on the use of native collagen membranes in horizontal bone regeneration, a percentage of complications of 3.2% [36] and 0% [37] was recorded. In a recent meta-analysis, an exposure rate of 28.62% for crosslinked membranes and of 20.74 for non-crosslinked membranes was obtained [35].
The postoperative complication rate of titanium barriers was higher than that of native collagen membranes and non-resorbable titanium-reinforced membranes, and similar to that of crosslinked collagen membranes and titanium meshes.
It must be taken into account that the complication rate obtained in this systematic review is surely lower than the real one in the included studies, since, in some articles, patients were excluded due to membrane displacement [41] or very premature exposure [27], not taking into account these cases in the complication rate reported. Furthermore, in two of the studies, some exposed membranes are associated with graft failure, but it is not specified whether it is due to graft infection [24][42].
On the one hand, some of the included articles defend that the exposure of the titanium foil does not influence the success of the GBR [19][22][32][41], even if one of them sustains that the very early exposure favors the increase in width of the attached gingiva, unlike what happens with a later exposure [22]. On the other hand, two studies support that early exposure (before 14 days) has a worse prognosis than late exposure, with very poor bone gain [24][42].
From the results obtained, it appears that the survival rate of implants placed in regenerated bone is similar to that of implants placed in native bone [43].
This review is based on the scant scientific literature published on the matter so far, and, for the moment, it is the only existing systematic review, so the results obtained cannot be compared and cannot be given much value. Other limitations are the heterogeneity of the included studies, the small sample size of some of them and the lack of information regarding bone gain or membrane removal. For these reasons, a quantitative analysis could not be performed.
3. Conclusions
Based on the data presented above, titanium membranes in GBR should be considered as an incipient technique, versatile in terms of the type of bone defect to regenerate, in which there is still no evidence of the need of filling material and which is the most appropriate, that can better tolerate exposure than titanium meshes and titanium-reinforced non-resorbable membranes and that can be tailored to the patient’s bone defect.
More randomized clinical trials comparing occlusive titanium barriers and other types of membranes are necessary to obtain more robust data that allow us to reach solid conclusions regarding the predictability and complications rate associated with the use of titanium foils, and how to manage complications when they occur.
References
- Wang, H-L; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17.
- McAllister, B.S.; Hahgighat, K. Bone augmentation techniques. J Periodontol. 2007, 78, 277-96.
- Benic, G.I.; Hämmerle, C.H.F. Horizontal bone augmentation by means of guided bone regeneration. Periodontol 2000. 2014, 66, 13-40.
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr Polym. 2020, 245,
- Cavallaro, C.; Lazzara, G.; Fakhrullin, R. Mesoporous inorganic nanoscale particles for drug adsorption and controlled release. Ther Deliv. 2018, 9, 287-301.
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131-47.
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003, 30, 809-19.
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in dog. J Clin Periodontol. 2005, 32, 212-8.
- Srinivas, B.; Das, P.; Rana, M.M.; Qureshi, A.Q.; Vaidya, K.C.; Raziuddin, S.J.A. Wound healing and bone regeneration in postextraction sockets with and without platelet-rich fibrin. Ann Maxillofac Surg. 2018, 8, 28-34.
- Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet-rich plasma for bone regeneration. Materials (Basel). 2017, 10,
- Batas, L.; Tsalikis, L.; Stavropoulos, A. PRGF as adjunct to DBB in maxillary sinus floor augmentation: histological results of a pilot Split-mouth study. Int J Implant Dent. 2019, 5, 14.
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009, 102, 1169-75.
- Thor, A.; Rasmusson, L.; Wennerberg, A.; Thomsen, P.; Hirsch, J-M.; Nilsson, B.; et al. The role of whole blood in thrombin generation in contact with various titanium surfaces. 2007, 28, 966-74.
- Polimeni, G.; Koo, K-T.; Qahash, M.; Xiropaidis, A.V.; Albandar, J.M.; Wikesjö, M.E. Prognostic factors for alveolar regeneration: effect of a space-providing biomaterial on guided tissue regeneration. J Clin Periodontol. 2004, 31, 725-9.
- Jovanovic, S.S.; Nevins, M. Bone formation utilizing titanium-reinforced barrier membranes. Int J Periodontics Restorative Dent. 1995, 15, 56-69.
- Simion, M.; Trisi, P.; Piattelli, A. Vertical risge augmentation using a membrane technique associated with osseointegrated implants. Int J Periodontics Restorative Dent. 1994, 14, 496-511.
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci. 2017, 125, 315-37.
- Caballé-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Peñarrocha, M.; Herández-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J Clin Exp Dent. 2018, 10, e477-83.
- Kfir, E.; Kfir, V.; Kaluski, E. Immediate bone augmentation after infected tooth extraction using titanium membranes. J Oral Implantol. 2007, 33, 133-8.
- Bassi, M.A.; Andrisani, C.; Lico, S.; Ormanier, Z.; Ottria, L.; Gargari, M. Guided bone regeneration via a performed titanium foil: clinical, histological and histomorphometric outcome of a case series. Oral Implantol (Rome). 2016, 9, 164-74.
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin Oral Implants Res. 2003, 14, 63-71.
- Bassi, M.A.; Andrisani, C.; López, M.A.; Gaudio, R.M.; Lombardo, L.; Lauritano, D.; et al. Guided bone regeneration in distal mandibular atrophy by means of a performed titanium foil: a case series. J Biol Regul Homeost Agents. 2016, 30, 61-8.
- Bassi, M.A.; Andrisani, C.; López, M.A.; Gaudio, R.M.; Lombardo, L.; Carinci, F. Guided bone regeneration by means of a preformed titanium foil: a case of severe atrophy of edentulous posterior mandible. J Biol Homeost Agents. 2016, 30, 35-41.
- Molly, L.; Quirynen, M.; Michiels, K.; van Steenberghe, D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: a retrospective clinical assessment. Clin Oral Implants Res. 2006, 17, 481-7.
- Maeda, D.; Lima, F.; Meza, J.; Ciotti, D.L.; Mizutani, F.S.; Doyle, H.; et al. Alveolar ridge regeneration of damaged extraction sockets using bovine-derived bone graft in association with a titanium foil: prospective case series. J Int Acad Periodontol. 2020, 22, 109-16.
- Perret, F.; Romano, F.; Ferrarotti, F.; Aimetti, M. Occlusive titanium barrier for immediate bone augmentation of severely resorbed alveolar sockets with secondary soft tissue healing: a 2-year case series. Int J Periodontics Restorative Dent. 2019, 39, 97-105.
- Toygar, H.U.; Guzeldemir, E.; Cilasun, U.; Akkor, D.; Arpak, N. Long-term clinical evaluation and SEM analysis of the e-PTFE and titanium membranes in guided tissue regeneration. J Biomed Mater Res B Appl Biomater. 2009, 91, 772-9.
- Engelke, W.; Deccó, O.; Cura, A.C.; Borie, E.; Beltrán, V. Rigid occlusive titanium barriers for alveolar bone augmentation: two reports with 24-month follow-up. Int J Clin Exp Med. 2014, 7, 1160-5.
- Beltrán, V.; Engelke, W.; Fuentes, R.; Decco, O.; Prieto, R.; Wilckens, M.; et al. Bone augmentation with occlusive barriers and cortical particulate allograft in transverse maxillary defects: a pilot study. Int J Morphol. 2014, 32, 364-8.
- Beltrán, V.; Matthijs, A.; Borie, E.; Fuentes, R.; Valdivia-Gandur, I.; Engelke, W. Bone healing in transverse maxillary defects with different surgical procedures using anorganic bovine bone in humans. Int. J. Morphol. 2013, 31, 75–81.
- Gaggl, A.; Schultes, G. Titanium foil-guided tissue regeneration in the treatment of periimplant bone defects. Implant Dent. 1999, 8, 368–375.
- Pinho, M.N.; Roriz, V.L.M.; Novaes, A.-B.; Taba, M.; Grisi, M.F.M.; de Souza, S.L.S.; Palioto, D.B. Titanium membranes in prevention of alveolar collapse after tooth extraction. Implant Dent. 2006, 15, 53–61.
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meses and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832.
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339.
- Wessing, B.; Lettner, S.; Zechner, W. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int. J. Oral Maxillodac. Implants 2018, 33, 87–100.
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal risge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 25 patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307.
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Baldoni, E.; Pisano, M.; Tallarico, M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulate xenograft and autologous bone: A 3-year after final loading prospective clinical study. Clin. Implant Dent. Relat. Res. 2019, 21, 669–677.
- Toygar, H.U.; Guzeldemir, E.; Cilasun, U.; Akkor, D.; Arpak, N. Long-term clinical evaluation and SEM analysis of the e-PTFE and titanium membranes in guided tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 772–779.
- Engelke, W.; Deccó, O.; Cura, A.C.; Borie, E.; Beltrán, V. Rigid occlusive titanium barriers for alveolar bone augmentation: Two reports with 24-month follow-up. Int. J. Clin. Exp. Med. 2014, 7, 1160–1165.
- Beltrán, V.; Engelke, W.; Fuentes, R.; Decco, O.; Prieto, R.; Wilckens, M.; Borie, E.; Beltran, V.; Engelke, W.; Fuentes, R.; et al. Bone augmentation with occlusive barriers and cortical particulate allograft in transverse maxillary defects: A pilot study. Int. J. Morphol. 2014, 32, 364–368.
- Khanna, R.; Khanna, R.; Pardhe, N.D.; Srivastava, N.; Bajpai, M.; Gupta, S. Pute titanium membrane (Ultra-Ti®) in the treatment of periodontal osseous defects: A split-mouth comparative study. J. Clin. Diagn. Res. 2016, 10, ZC47–ZC51.
- Watzinger, F.; Luksch, J.; Millesi, W.; Schopper, C.; Neugebauer, J.; Moser, D.; Ewers, R. Guided bone regeneration with titanium membranes: A clinical study. Br. J. Oral Maxillofac. Surg. 2000, 38, 312–315.
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.C.; Barboza, E.S.P. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388.