
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katia Avina Padilla | + 2912 word(s) | 2912 | 2019-03-02 07:31:14 | | | |
| 2 | Peter Tang | -3 word(s) | 2909 | 2020-10-30 03:34:59 | | | | |
| 3 | Katia Avina Padilla | Meta information modification | 2909 | 2020-10-30 03:39:44 | | |
Video Upload Options
Mitochondrial porins or voltage-dependent anionic channels (VDAC) are nonspecific channels that determine the permeability of the mitochondrial outer membrane (MEM). Its molecular mass oscillates around 28 to 36 kDa. These channels represent the most abundant proteins of the MEM. They are freely permeable to hydrophilic solutes and the limit of exclusion of metabolites is 3 to 6 kDa. These channels are the main route for the diffusion of ions such as pyruvate and ATP, among others. It has been proposed that the transport of these metabolites regulates the cellular energy supply. This review focus on some of the main aspects of the VDAC porins and its relevance in some physiological processes related with cancer and neurodegeneration diseases.
1. Properties and Structures of Mitochondrial Porins
Mitochondrial porins or voltage-dependent anionic channels (VDAC) are nonspecific channels that determine the permeability of the outer mitochondrial membrane (OMM). Its molecular mass oscillates around 28 to 36 kDa. These channels represent the most abundant proteins of the OMM. They are freely permeable to hydrophilic solutes and the limit of exclusion of metabolites is 3 to 6 kDa. These channels are the main route for the diffusion of ions such as pyruvate and ATP, among others. It has been proposed that these metabolites transport regulates the cellular energy supply[1]. These channels remain open at low voltages of ± 10 mV; VDAC has a diameter of 20-30 Ǻ, according to electron microscopy analysis, with a conductance of 3.6 to 4.5 nS, mainly showing fluxes of negatively charged metabolites, such as succinate, malate, citrate and adenine nucleotides. Voltage application in liposomes with VDAC of ± 20 mV, reduces the permeability of the pore, conductance is very low, converting the open pore to a partially closed state, and its selectivity changes from anionic to cationic. The relevance of this process is not known, but it reflects the sensitivity to voltage in the mitochondrial porin[2].
However, it has been suggested that the pore is not a stable transverse structure in the outer or inner membrane, it remains open only in the inner membrane, for example when the structure is not assembled to the adenine nucleotide transporter (ANC), on the opposite, the porin is closed when it is assembled forming the VDAC / ANC complex[3]. Mitochondrial porins are encoded in the nucleus, they are synthesized in the cytoplasmic ribosomes, from where they are transferred to the mitochondria[4].
The gene encoding the VDAC protein has conserved regions in number and position of introns. As mentioned, the conserved sequences of amino acids are few, maybe due the beta barrel structure that tolerates amino acid variations without altering the selectivity, function and secondary structure of the protein. It has been proposed that the beta barrel is important when controlling the movement of different metabolites in the MEM through the anions flow, allowing an adequate potential to readjust cellular homeostasis, which can be interrupted under environmental stress[5] .By secondary structure predictions, common structures are suggested, which are functionally similar. VDAC forms the beta barrel through transmembrane domains with antiparallel beta sheet structures. Attached to the amino terminus, residues form a small a-helix barrel with an amphipathic character, where two possible phosphorylation motifs have been identified: one for protein kinase C (PKC) and another for casein kinase II (CK2). The PKC motif is found in all reported VDACs, while the second is found mainly in plants[6].

Figure 1. Alignment of representative porin sequences. Amino acid sequence of plants (O. sativa VDAC1, Osat1), of protists (P.sojae, Psoj), in animals (H. sapiens VDAC1, Hsap1), and in fungi (N. crassa, Ncra). The identical amino acids are designated with (I) and the amino acids conserved with (:). The helical a region represented by the blue line, gray residues marked in the different sequences are b structures. The GLK motifs (glycine, leucine, lysine) are framed in residues 87-89 (Young et al., 2007)
The beta barrel formed in VDAC consists of a hydrophilic interior with exterior hydrophobic character. The polar residue is around 46 to 50%. It has been reported that in the VDAC1 isoform of amino acids positively charged (Arg and Lys), it is located in the internal part as in the loops that form the length of 2 to 10 residues; In addition, 2 hydrophobic L150 and V143 amino acids were detected in the inner part of the barrel b. It was demonstrated that the amino terminal segment Helix with amphipathic character, of 23 residues, is also located in the internal part and that the deletion of these residues leads to the loss of anchoring of the porin to the mitochondrial outer membrane. It is believed that the segment can not apply external conditions. Using nuclear magnetic resonance (NMR), the three-dimensional structure of human VDAC1 was determined. In it, the binding site for Bcl-XL of the Bcl2 family was revealed; this is attached to the lateral part of thread 17 and 18 to basic residues[7]. 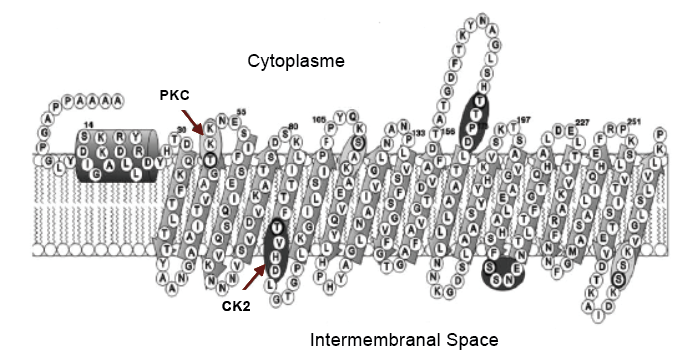
Figure 2. Secondary structure of VDAC2 of O. sativa. Showing the small a-helix segment of the amino terminal, the phosphorylation sites for protein kinase C (PKC) and another for casein kinase II (CK2). With antiparallel lamina structures b, along the mitochondrial outer membrane (Al Bitar et al., 2003) .
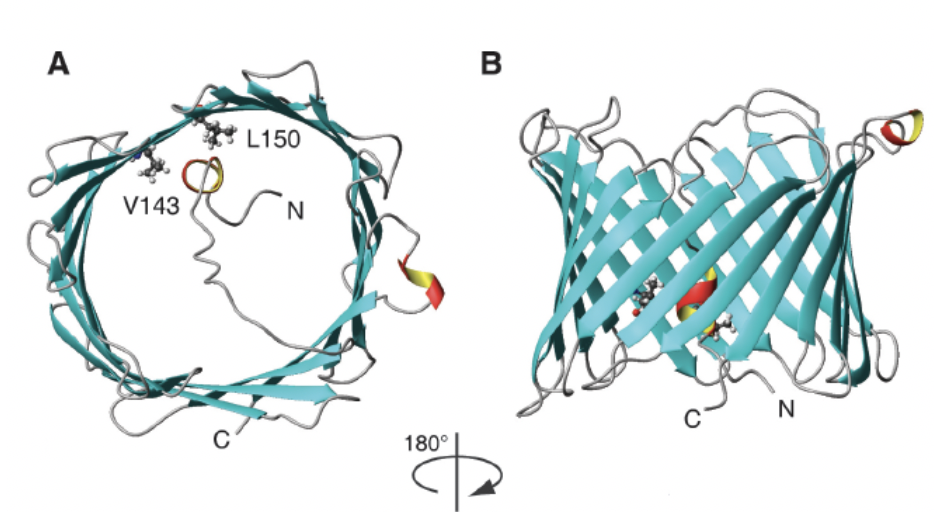
Figure 3. Human VDAC1 structure obtained by NMR. A) VDAC1, taken from the top, with the hydrophobic amino acids valine 123 (V123), leucine 150 (L150) and the amino end of 23 residues inwards. B) Barrel b of VDAC1 with antiparallel structures (Hiller et al., 2008).
2. Permeability of the mitochondrial membrane mediated by VDAC in cell death
Recently, the role of mitochondria in cell death and apoptosis has received considerable attention. The increase in the permeability of the mitochondrial membrane (PMM) is key in these processes. When the cellular homeostasis is broken, the apoptotic level is promoted by several members of the proapoptotic Bcl-2 family such as Bax, Bak, Bid and stimuli such as the increase of the Ca + level in the cytosol which promote the opening of the permeable mitochondrial transition pore (PTPm) contributing to the subcellular relocation of proteins normally found in mitochondria such as cytochrome c, AIF (apoptosis induction factor) and adenylate kinase to the cytosolic extracellular compartment. The increase in the level of Ca + in the cytosol induces mitochondrial permeability, causing swelling and rupture of the MEM. Cytochrome c in turn induces caspase activation, which further induces protease activation factor-one (APAF-1) and the apoptosis-inducing factor AIF, which generates DNA fragmentation and chromatin condensation. Finally, the generation of reactive oxygen species (ROS) is increased, and oxidative phosphorylation stops by the inhibition of the V complex. The activation of genes in response to stress intervenes in the bioenergetic crisis scenario that progressively allows cell death[8].
VDAC, is part of the channel known as PTPm. This channel opening allows the flow of solutes of a molecular mass less than 1.5 kDa. The exact molecular structure of PTPm has not been characterized, but it is believed to be a macromolecular protein complex10. PTPm includes cyclophilin D (Cyp D), located in the mitochondrial matrix and the adenine nucleotide translocator (ANT) of the internal membrane[9].
It has been postulated that the interaction of VDAC with pro and anti-apoptotic members of the Bcl-2 family including Bax, Bid, Biol, Bcl-XL regulates cytochrome c levels involving the opening or closing of VDAC. Also it has been proposed, that under physiological conditions, VDAC exists in a reduced conductance state sufficient to ensure the metabolites change between the mitochondria and the cytosol and that, after the induction of apoptosis, VDAC increases the conductance favoring cell death[10]. It is not clear whether the binding of HK to VDAC causes the closure of PTPm and inhibits the mitochondrial permeable transition (MPT). It has been shown that the displacement of HK II from the mitochondria promotes the translocation of Bax and the increase of cytochrome c, while the overexpression of HK II causes opposite effects[11]. It has been shown that HK bound to mitochondria inhibits apoptosis and that this protective effect is independent of Bax and Bak[12].
Caspases and proteins of the Bcl-2 family are absent in plants. Cell death in plants shows some characteristics similar to those of apoptotic cells of animals such as the mitochondrial permeable transition, cytochrome c in the cytosol, proteases, nuclear condensation and DNA fragmentation. Mitochondrial proteins such as cytochrome c, VDAC and ANT are conserved in plants and animals[13]. The diameter of VDAC is too small to allow the passage of cytochrome c, so it is essential to increase the diameter, to allow its passage. The opening of the barrel structure de of VDAC, can not form a mega-pore. Recently, the existence of VDAC monomers, dimers, trimers, tetramers and hexamers on the surface of potato MEM has been confirmed by atomic force microscopy. The organization of VDAC oligomers possibly allows the formation of the mega-pore, in the hexameric state consisting of six circular depressions in the form of a hexagon. The hexameric organization could represent the ideal platform for the binding of hexokinase tetramers[14]. It has been demonstrated that the dimeric form and the increase in the assembly of VDAC to form oligomers induces the apoptosis events, this was analyzed computationally by means of structural data predictions.
3. Cellular Metabolism
3.1. Mitochondrial metabolic channeling
A special case is observed in mitochondria, due to the flow of the internal ATP to the outside or cytoplasm where it is used as a substrate by the HK, which is interacting with the mitochondrial porin. The binding of HK to VDAC involves the hydrophobic amino terminus of HK, which can form an α-helix that has been proposed to be inserted into the membrane or inside the VDAC pore. The ATP produced inside the mitochondria by oxidative phosphorylation is preferentially channeled towards the HK-II bound to VDAC. This produces 6P glucose which is used mainly to form lactate under aerobic conditions ("Warburg effect"). In cancer cells the interaction of HK II with VDAC is not interrupted; It is known that methyl jasmonate interrupts the interaction, so this compound has been proposed as a medical alternative in the treatment of cancer [15].
4. VDAC: Disease Relevance
VDAC as a target for anticancer drugs:
a) Hexokinase isoforms in mammals: properties, cellular localization and metabolic function
In mammals it has been observed that hexokinase II binds to VDAC. This binding is accompanied by the formation of tetramers of HK 21, with hexokinase having preferential access to ATP synthesized in the internal membrane by the synasome. In tumor cells, the binding of hexokinase to VDAC contributes to the survival and growth of cancer cells, whereas in normal cells such binding prevents apoptosis [16]. The amino terminal domain of HK is required for binding to the carboxyl terminus of VDAC by a hydrophobic interaction[17].
Hexokinases isoforms differ in their cellular location, as well as in their regulatory and catalytic properties. This could be an important factor in determining the dispersion of glucose metabolism in mammalian cells or tissues. Hexokinase is considered a glycolytic enzyme, it phosphorylates glucose during metabolism. Four different hexokinase isoenzymes are known in mammals; 100 kDa isoenzymes type I, II, III; it is believed that these isoenzymes evolved from a gene that encoded a 50 kDa ancestor which doubled and fused. These isoenzymes show a repeated internal sequence, the carboxyl and amino motives have similar sequences. The 50 kDa ancestral gene, sensitive to glucose 6-phosphate when duplicated and fused should give a product more susceptible to this inhibition. This characteristic only correlates to the isoenzyme type II , where the catalytic function resides in half of the amino and carboxyl. However, in isoenzymes type I and III, the catalytic function resides only in the middle of the carboxyl end after its duplication and fusion. Possibly the amino moieties underwent mutations, differing to function not as a catalytic domain but as a regulatory one 24. Susceptibility to the potent glucose 6-phosphate inhibition product, considered an important regulator, is a characteristic shown in isoenzymes of type I, II, III and also in 50 kD hexokinases of lower organisms such as the starfish and the parasite Schistosoma mansoni [18].
The activity of hexokinase type I has been found predominantly in the brain; In addition, it has been shown to be associated with mitochondria, more specifically with the outer membrane26. The association of this glycolytic enzyme to the mitochondria, the primary site of oxidative metabolism, has been the subject of many speculations. Cellular increase in phosphorus concentration, due to the hydrolytic increase of high energy phosphorus compounds, is shown when there is an increase in phosphofructokinase activity, decreasing the cellular concentration of glucose 6-phosphate; this is typically observed in periods of increased energy demand and increased glycolytic metabolism. It has been considered that HK I has a catabolic role, introducing glucose into the glycolytic metabolism, with the consequence of generating ATP . In contrast, it has been suggested that the function of the isoenzyme type II, and probably, type III, have an anabolic repercussion, since they provide glucose 6P, for the synthesis of glycogen or the synthesis of lipids. Over-expression of HK I in epithelial cells conferred protection against cell death induced by oxidants. This protection is glucose dependent, suggesting that the increase in glucose 6-phosphate may be the mechanism of cellular protection by HK I 28. The expression of HK II in lung epithelial cells shows the same protection mechanism. The mechanism by which HK I and HK II protect from cell death is not yet known [19].
Isoenzyme type II also includes the amino-terminal sequence determinant for the binding of hexokinase to mitochondria. Both HK I and HK II are expressed at high levels in some cancer cells, intervening in an irregular growth of these cells. As for isozyme type III, it is distinguished by having a high affinity for glucose, but a low affinity for the other substrate: ATP. As it is also less sensitive to inhibition by glucose 6-phosphate, it is the only one to show inhibition at high glucose levels. In addition, it lacks the amino-terminal hydrophobic sequence, so it does not bind to mitochondria. Its location is peri-nuclear, and it does not have the classical amino acid sequence of nuclear labeling [20]. However, it has been located in the cytoplasm and in soluble fractions of homogenized tissues. Isoenzyme type IV, known as glucokinase, is 50 kDa 30-32. It has been found in nucleus, cytosol, liver and pancreas cells, and is not significantly affected by glucose 6-phosphate concentrations. In cells of the pancreas, a small change in glucokinase activity affects insulin secretion stimulated by glucose. In addition, it has been established that glucose phosphorylation is a key point for the control of glycolytic flow in B cells.
b) VDAC and hexokinase, interaction and its relation as enhancing cancer cell growth
Since HK is a HIF-1 target gene its expression is enhanced during hypoxia, as previously mentioned this protein binds to the VDAC porin protein in mitocondrial outer membrane. This interaction provides HK access to ATP. VDAC and HK strong interaction is associated with the role of VDAC porins in the regulation of glycolysis and mitochondrial respiration. During this association HK is low sensitive to glucose-6-phosphate, avoiding inhibition of its activity. It is well known that HK is highly expressed in tumor cells, putative mechanisms. HK is highly expressed in tumor cells, since VDAC availability to ANT, ADP and ATP transporter closer to the inner mitochondrial membrane. Providing a remarkable scenario for cancer cells growth. In order to supplying ATP, HK-VDAC interaction functions as a gate for the entry of metabolites required for proliferative cellular growth of cancer cells. Disruption of this interaction might be relevant for therapeutic purposes.
5. Perspectives of the Role of VDAC as a Target for Therapy in Cancer and Neurodegeneration Diseases
VDAC has been a striking target for both anti cancer and neurodegenerative diseases including Alzheimer, Parkinson, and myocardial diseases. Evidence suggests that VDAC is involved in the pathology process of multiple neurodegerative diseases. VDAC overexpression has been associated with beta amyloid toxicity, while, downregulation of this gene has been reported in the frontal cortex and thalamus of Alzheimer's disease patients. VDAC2 high expression has been found in the temporal cortex. It is remarkable to notice that conductance of VDAC could be modulate by interation between VDAC1 and the beta amyloid protein. This interaction promotes the dissociation of VDAC1-HK resulting in serial events of cell death mediated by apoptosis. Disadvantages of this alterations in compounds related to VDAC availability and activity might have an impact on the proliferation or death of cancer cells. As any drug, anti cancer medical strategies could lead to the expression of other diseases, as it is the case of cardiac diseases. Hearth muscle cells produce high amounts of ATP modifying the VDAC control of the ATP and Ca2 + flux and the association between VDAC and Bcl2 may be linked to cardio-protection.
Potential chemicals have been identified for their relevance in the direct interaction with VDAC activity as possible cancer and neurodegenerative treatments. Among them, Avicins function closing VDAC, Acrolein carbonylating VDAC, Erastin binding to VDAC2, Endostatin inducing PTP opening, Fluoxetine and Cisplatin inhibiting of PTP opening and apoptosis, Furanonaphthoquinones inducing VDAC dependent apoptosis, Oblimersen blocking channel activity, AKOS-022, VBIT-3/VIBT-4, preventing VDAC1 oligomerization and consequently apoptosis.
Since several studies have indicated that the hexokinase-VDAC interaction can inhibit apoptosis in mammalian cells, including tumor cells, an evident treatment approach could be disruption the proteins complex . Several separation strategies have been validated to promote apoptosis in tested cells; for example methyl jasmonate (a stress hormone in plants). VDAC appear to be a potential therapeutic target for both cancer and neurodegenerative diseases. Since the knowledges about the functional properties of VDAC protein are still insufficient, VDAC as a pharmacological target is still an open and promising area for future studies.
References
- Matthew J Young; Denice C Bay; Deborah A Court; Georg Hausner; The evolutionary history of mitochondrial porins. BMC Evolutionary Biology 2006, 7, 31, 10.1186/1471-2148-7-31.
- Roland Benz; Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes 1994, 1197, 167-196, 10.1016/0304-4157(94)90004-3.
- V Shoshan-Barmatz; A Israelson; D Brdiczka; S S Sheu; The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death.. IJMS 2005, 12, NA, NA.
- Shay Geula; H. Naveed; J. Liang; V. Shoshan-Barmatz; Structure-based Analysis of VDAC1 Protein: DEFINING OLIGOMER CONTACT SITES. Journal of Biological Chemistry 2011, 287, 2179-2190, 10.1074/jbc.M111.268920.
- M.K. Desai; R.N. Mishra; D. Verma; S. Nair; S.K. Sopory; M.K. Reddy; Structural and functional analysis of a salt stress inducible gene encoding voltage dependent anion channel (VDAC) from pearl millet (Pennisetum glaucum). Plant Physiology and Biochemistry 2006, 44, 483-493, 10.1016/j.plaphy.2006.08.008.
- Fawaz Al Bitar; Nancy Roosens; Mathias Smeyers; Marc Vauterin; Jos Van Boxtel; Michel Jacobs; Fabrice Homblé; Sequence analysis, transcriptional and posttranscriptional regulation of the rice vdac family. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 2002, 1625, 43-51, 10.1016/s0167-4781(02)00590-0.
- Sebastian Hiller; R. G. Garces; T. J. Malia; V. Y. Orekhov; M. Colombini; G. Wagner; Solution Structure of the Integral Human Membrane Protein VDAC-1 in Detergent Micelles. Science 2008, 321, 1206-1210, 10.1126/science.1161302.
- Jeffrey S. Armstrong; Mitochondrial membrane permeabilization: the sine qua non for cell death. BioEssays 2006, 28, 253-260, 10.1002/bies.20370.
- James N. Weiss; Paavo Korge; Henry M. Honda; Peipei Ping; Role of the Mitochondrial Permeability Transition in Myocardial Disease. Circulation Research 2003, 93, 292-301, 10.1161/01.res.0000087542.26971.d4.
- Yoshihide Tsujimoto; Shigeomi Shimizu; The voltage-dependent anion channel: an essential player in apoptosis. Biochimie 2002, 84, 187-193, 10.1016/s0300-9084(02)01370-6.
- J. G. Pastorino; Nataly Shulga; Jan B. Hoek; Mitochondrial Binding of Hexokinase II Inhibits Bax-induced Cytochrome c Release and Apoptosis. Journal of Biological Chemistry 2001, 277, 7610-7618, 10.1074/jbc.m109950200.
- Nathan Majewski; Veronique Nogueira; Prashanth Bhaskar; Platina E. Coy; Jennifer E. Skeen; Kathrin Gottlob; Navdeep S. Chandel; Craig B. Thompson; R. Brooks Robey; Nissim Hay; et al. Hexokinase-Mitochondria Interaction Mediated by Akt Is Required to Inhibit Apoptosis in the Presence or Absence of Bax and Bak. Molecular Cell 2004, 16, 819-830, 10.1016/j.molcel.2004.11.014.
- A. Godbole; J. Varghese; A. Sarin; M.K. Mathew; VDAC is a conserved element of death pathways in plant and animal systems. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2003, 1642, 87-96, 10.1016/s0167-4889(03)00102-2.
- Bart W. Hoogenboom; Kitaru Suda; Andreas Engel; Dimitrios Fotiadis; The Supramolecular Assemblies of Voltage-dependent Anion Channels in the Native Membrane. Journal of Molecular Biology 2007, 370, 246-255, 10.1016/j.jmb.2007.04.073.
- Camillo Rosano; Molecular model of hexokinase binding to the outer mitochondrial membrane porin (VDAC1): Implication for the design of new cancer therapies. Mitochondrion 2011, 11, 513-519, 10.1016/j.mito.2011.01.012.
- John E. Wilson; Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. The Journal of Experimental Biology 2003, 206, 2049-2057, 10.1242/jeb.00241.
- Koichiro Nabe; Yutaka Seino; [Hexokinase].. Nihon rinsho. Japanese journal of clinical medicine 2002, 60, na, na.
- A G Tielens; J M Van Den Heuvel; H J Van Mazijk; J E Wilson; C B Shoemaker; The 50-kDa glucose 6-phosphate-sensitive hexokinase of Schistosoma mansoni.. Journal of Biological Chemistry 1994, 269, na, na.
- J. E. Wilson; An introduction to the isoenzymes of mammalian hexokinase types I-III. Biochemical Society Transactions 1997, 25, 103-107, 10.1042/bst0250103.
- J E Wilson; An introduction to the isoenzymes of mammalian hexokinase types I-III.. Biochemical Society Transactions 1997, 25, na, na.





