Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Csaba Szabo | -- | 1862 | 2022-11-04 06:33:11 | | | |
| 2 | Sirius Huang | Meta information modification | 1862 | 2022-11-04 06:53:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lugata, J.K.; Ortega, A.D.S.V.; Szabó, C. The Antioxidant System of Poultry with a Focus on Methinonine. Encyclopedia. Available online: https://encyclopedia.pub/entry/32852 (accessed on 08 February 2026).
Lugata JK, Ortega ADSV, Szabó C. The Antioxidant System of Poultry with a Focus on Methinonine. Encyclopedia. Available at: https://encyclopedia.pub/entry/32852. Accessed February 08, 2026.
Lugata, James Kachungwa, Arth David Sol Valmoria Ortega, Csaba Szabó. "The Antioxidant System of Poultry with a Focus on Methinonine" Encyclopedia, https://encyclopedia.pub/entry/32852 (accessed February 08, 2026).
Lugata, J.K., Ortega, A.D.S.V., & Szabó, C. (2022, November 04). The Antioxidant System of Poultry with a Focus on Methinonine. In Encyclopedia. https://encyclopedia.pub/entry/32852
Lugata, James Kachungwa, et al. "The Antioxidant System of Poultry with a Focus on Methinonine." Encyclopedia. Web. 04 November, 2022.
Copy Citation
The physiological status of poultry can be disturbed by different stressors that may lead to oxidative stress conditions. Oxidative stress activates defense systems, which mitigates the adverse effects. Several lines of the poultry defense system exist, including enzyme systems such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and non-enzymatic antioxidants such as Glutathione (GSH). Methionine—a vital amino acid in poultry nutrition—plays a significant role in protein synthesis, transsulfuration, and transmethylation and is also involved in several biochemical pathway activations that can affect the antioxidant system.
poultry
antioxidant defense system
methionine sources
oxidative stress
1. Introduction
Poultry production is affected by many factors, mainly genetics, environment, and nutrition. About 85% of a broiler’s performance is due to genetics, while the environment and nutrition contribute by 15% [1]. However, the environment and nutrition should be optimal to allow the animal to express its maximum genetic potential [2][3]. Nutrition, especially amino acids, and energy supply greatly influence the performance of both genetically improved and autochthonous poultry by modulating different pathways responsible for maintaining the physiological status [4][5][6]. Maintaining normal physiological status, especially in intensive poultry production, is nearly impossible due to stress factors such as high temperature (in summer and tropical regions), high stocking density, and diseases [7][8][9]. Stress can be defined as a “nonspecific response of the body to any demand”, while a stressor can be defined as “an agent that produces stress at any time” [10]. Stress activates the defense system mechanism, resulting in increased demand for energy, amino acids, vitamins, and trace minerals and, consequently, poor production performance. The effects of stress are mainly managed or avoided by dietary supplementation with antioxidants or environmental management. Oxidative stress caused by an environmental stressor alters an animal’s physiological status [11]. Oxidative stress occurs when there is an overproduction of reactive oxygen species (ROS) or an insufficient antioxidant defense system [12]. Mitigation strategies involve elevated dietary supplementation of nutrients in antioxidant defense systems, such as vitamins, trace minerals, and amino acids.
Studies with amino acid supplementation in poultry have been conducted for about half a century [13][14]. Due to the importance of amino acids in poultry production, many studies have been conducted regarding the appropriate amount of different amino acids in the diet [15][16][17]. In most poultry diets, methionine (Met) is the first limiting amino acid which plays a vital role in the biosynthesis of other essential molecules such as cysteine, carnitine, and taurine, and is converted to S-adenosylmethionine (SAM), which serves as a methyl donor in the birds’ metabolism [17]. Birds cannot synthesize a sufficient amount of Met to sustain average growth; hence it must be supplemented in their diet [18][19][20]. As Met is involved in several routes in antioxidant defense systems, oxidative stress can raise requirements.
The optimal Met requirements have been established for carcass quality, productive performance, and growth performance [21][22][23][24][25]. In poultry, there are different Met requirements for different species and breeds due to the breed’s different growth rates and genetic potential [26]. For example, according to NRC [27], the Met requirement for commercial broilers at the starter, grower, and finisher phases are 0.50%, 0.38%, and 0.32%, respectively. Growing turkeys require a recommended Met of 0.55%, 0.45%, 0.40%, and 0.35% for 1–4 weeks, 5–8 weeks, 9–12 weeks, and 13–16 weeks, respectively [27]. Several studies have been conducted to evaluate the effects of varying the optimal Met recommended for growth rate, feed intake, feed conversion ratio, and meat quality on the antioxidant status of poultry [8][20][23][25]. From the above-cited studies and NRC 1994, commercial broilers do not need more than 0.5%, and 0.38% Met for optimum performance in the starter and grower period. However, higher Met levels have been used to evaluate the antioxidant status of broilers (Figure 1). Met levels in starter diets ranging from 0.2 to 1.32% and in grower diets from 0.24 to 1.3%, along with the Met to Lys ratio of 20 to 110%, have been used in experiments on antioxidant responses of commercial broilers under stress (Figure 1). The increased dietary Met levels improved the antioxidant status with no adverse effect on the growth performance, indicating that a high level of Met is needed to facilitate antioxidant function.

Figure 1. Dietary total Met levels and Met to Lysine ratios used in starter and grower diets from various studies conducted in broilers. (A) Relationship of total dietary Met content to Met/Lys ratio in the starter phase of broilers. (B) Relationship of total dietary Met content and Met/Lys ratio in the grower phase of broilers. (1 NRC 1994 Methionine to Lysine ratio recommendations, 2 NRC 1994 Met recommendation in broilers diet in respective feeding phase).
2. Methionine Supplementation of Poultry
Met as a feed-grade amino acid in poultry diets was first introduced in 1951. Since then, a variety of Met sources have been used to supplement bird diets, such as L-methionine (LM) [28][29][30], DL-methionine (DLM), a combination of the L and D enantiomers [30][31][32][33][34], the Met analog DL-2-hydroxy-4-(methylthio) butanoic acid (DL-HMTBA), also known as MHA, which is sold as a calcium salt (MHA-Ca) or as a free acid (MHA-FA) [35][36][37][38][39][40]. In contrast to DLM and DL-HMTBA, mostly employed as Met sources in chicken feeds, LM is a naturally occurring form of Met that may be utilized directly by birds. These sources differ in bioavailability depending on their metabolism in poultry [41][42], therefore differing in their effect on performance. While L-Met and D-Met are actively absorbed (transported against a concentration gradient), MHAs are passively absorbed through diffusion from a more concentrated medium to a less concentrated medium [35]. Physiologically, D-isomer amino acids cannot be utilized in the animal cell; instead, they must be converted to the corresponding L-isomer before being utilized in protein synthesis [43]. DLM and HMTBA must be converted to LM to be utilized in the body, mainly by the liver and kidney as the main organ [17][42]. Some experiments showed that D-Met is 90 to 100% as efficacious as L-Met, whereas HMTBA is 65 to 100% as efficacious as DL-Met [44].
3. The Antioxidant System of Poultry
Stress control, which may be due to management, nutrition, technology, and environmental elements, as well as internal stress, is one of the unavoidable issues in poultry production. One typical source of stress is an excessive generation of free radicals, which causes oxidative stress at the molecular level [45]. As a result, animals’ antioxidant defense mechanisms developed over time to provide sufficient protection in an environment with high oxygen levels. There are multiple lines of antioxidant defense in the antioxidant defense network. The first line comprises antioxidant enzymes that are in charge of detoxifying the superoxide radical (the prominent biological radical) and its metabolic products. Superoxide dismutase, glutathione peroxidases (six different types in avian species), and catalase are all part of the first line of antioxidant defense [46][47]. Some metals, such as free iron and copper, which play a significant role in catalyzing free radical production and metal-binding protein, are included in the first line of antioxidant defense [46]. Chain-breaking antioxidants (such as vitamin E, carotenoids, ascorbic acid, glutathione, and uric acid) are responsible for chain oxidation reaction limitation and termination in the second line of antioxidant defense [46].
Specific enzymes that deal with the damage caused by free radicals and hazardous products of their metabolism make up the third level of antioxidant defense (e.g., methionine sulfoxide reductase, and DNA-repair enzymes) or removal (phospholipases, proteasomes) [44][45]. Protective protein modifications that prevent inactivation, such as glutathionylation and other modifications, comprise the fourth stage of antioxidant defense. Apoptosis, interestingly, can be included in the antioxidant defense network to cope with terminally damaged cells that cannot be restored. In general, the integrated antioxidant defense network’s principal purpose is to maintain optimum redox status—the balance between the process of production and inactivation/detoxification of ROS/Reactive Nitrogen species (RNS) [46][47].
The antioxidant defense system in commercial poultry production requires external assistance, such as dietary supplementation of traditional antioxidants such as vitamin E or other nutrients with regulatory functions in the antioxidant defenses, such as taurine, carnitine, and branched-chain amino acids.
4. How Antioxidants Network Work under Oxidative Stress
When ROS/RNS overpowers the antioxidant defense system’s ability, oxidative stress damages several biological compounds such as polyunsaturated fatty acids, proteins, and DNA [48]. In response to oxidative stress, the antioxidant defense system employs some strategies to restore the situation to normal [46] (Figure 2). Foremost, they reduce the formation of free radicals, decreasing the activities of enzymes involved in ROS/RNS formation, such as NADPH oxidase and xanthine oxidase, and by the action of iron and copper bound to protein which prevents the formation of new free radicals. Second, the critical maintenance of mitochondrial integrity is the biological system’s principal source of free radicals. Third, crucial elements of an antioxidant defense strategy include detoxification/decomposition of free radicals and non-radical harmful chemicals (SOD, GPx, catalase, etc.) and scavenging free radicals (e.g., vitamin E, vitamin C, GSH, coenzyme Q). Fourth, vitamin E’s biological antioxidant effectiveness may be increased by the recycling mechanism that maintains it active (ascorbic acid, thioredoxin reductase (TrxR), vitamins B1 and B2). Fifth, redox signaling, transcription factor (Nrf2), and vitagene activation are the main components of the anti-stress strategy. Additional protective molecules with antioxidant and detoxifying properties are also produced. Sixth, enzymatic mechanisms (heat shock proteins, HSP; methionine sulfoxide reductase, Msr; DNA repair enzymes, etc.) are involved in repairing damaged molecules, after which the damaged molecules are removed to prevent accumulation. Last but not least, the antioxidant defense network consists of apoptosis, autophagy, and other procedures that eliminate fatally injured cells and stop the damage from spreading to neighboring cells and tissues [46].
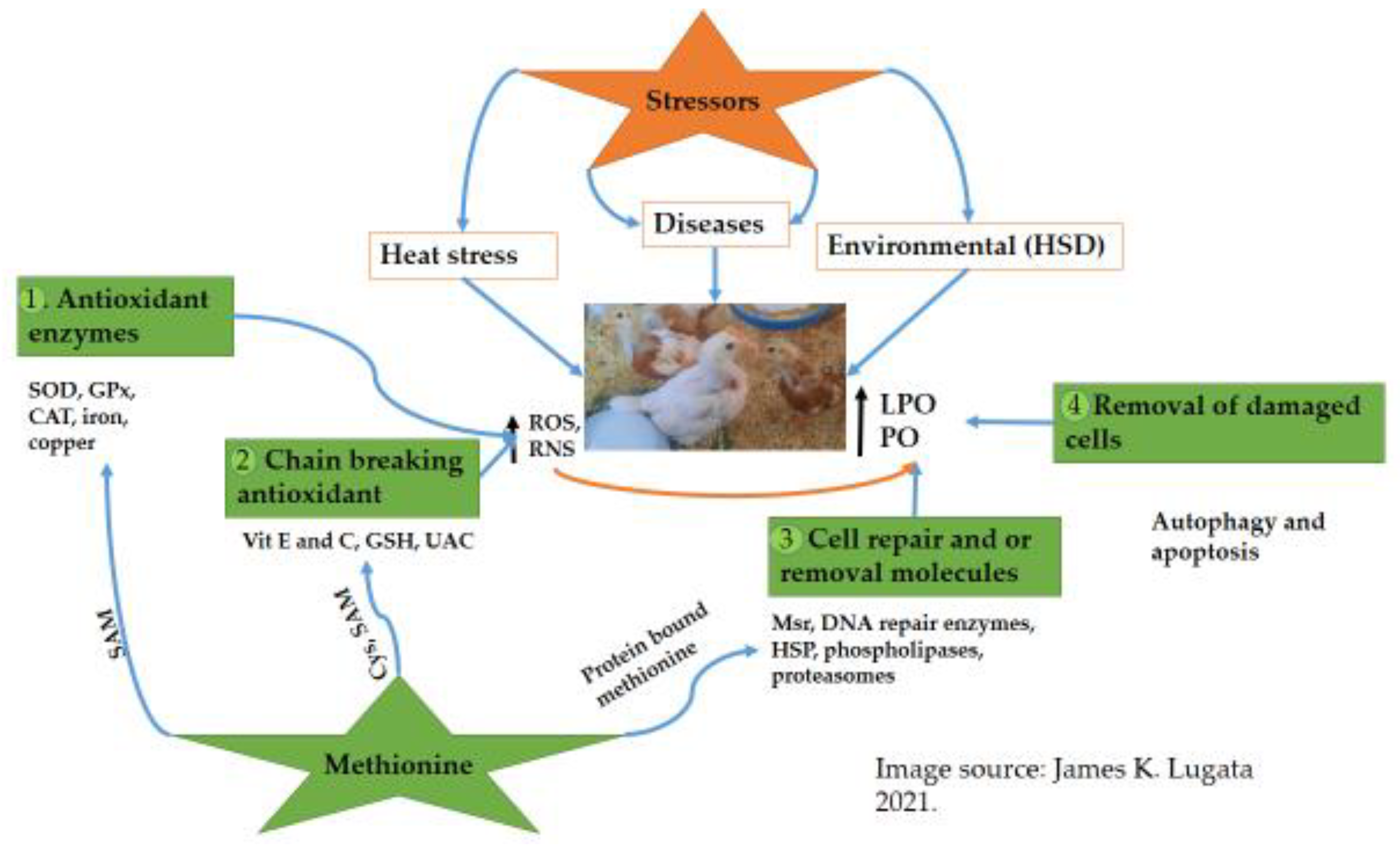
Figure 2. Overview of the effect of stressors, the antioxidant system function, and the influences of Met on poultry’s antioxidant system/status. Adapted from [17][46][49]. The stressors greatly affect poultry production, including heat stress, diseases, environmental, technological, and nutrition, by altering the physiological status due to an increase in the production of Reactive Oxygen Species (ROS) and Reactive Nitrogen species (RNS). The poultry antioxidant defense system plays a great role in detoxifying and preventing ROS’s effect. Four levels of antioxidant defense have been suggested. (1). The first level deals with detoxifying free radicals at the beginning of forming (superoxidase dismutase -SOD, glutathione peroxidase -GPX, catalase -CAT, and chelating metals such as iron and copper). (2). The second level of antioxidant defense deals with scavenging the free radicals (vitamin E and C, glutathione -GSH, and uric acid -UAC). (3). The third level involves cell repairs and removing the damaged molecules; this includes methionine sulfoxide reductase (Msr), heat shock proteins (HSPs), and DNA-repairing enzymes. (4). The 4-fourth level deals with removing damaged cells and preventing the spread of the damage (autophagy and apoptosis). Met, directly and indirectly, influences all the major antioxidant defense levels through the ROS scavenging role of protein Met residues and cysteine (Cys) and SAM, which then influence the activity of the antioxidant enzymes such as SOD and CAT.
A growing body of research suggests that all antioxidants in the body operate collectively as a “team” to maintain adaptive homeostasis. There are cooperative interactions when one team member helps another to work more effectively. All cell compartments, including the mitochondria, nucleus, and cytoplasm, have antioxidant defense mechanisms that are expressed tissue-specifically. These mechanisms include internal antioxidants (AO enzymes, GSH, CoQ, uric acid, carnitine, taurine, etc.) and antioxidants consumed through diet (vitamin E, carotenoids, synthetic antioxidants, carnitine, silymarin, etc.). The degree of stress affects the expression and activity of numerous antioxidant enzymes, including SOD, GPx, and other selenoproteins [46].
References
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Growth, Livability, and Feed Conversion of 1957 versus 2001 Broilers When Fed Representative 1957 and 2001 Broiler Diets. Poult. Sci. 2003, 82, 1500–1508.
- Djumadil, N.; Syafie, Y. Analyses of Factors Affecting the Production of Broiler Chickens in Ternate. In Proceedings of the 5th International Conference on Food, Agriculture and Natural Resources (FANRes 2019), Ternate, Indonesia, 17–18 September 2019; Atlantis Press: Dordrecht, The Netherlands, 2020.
- Baracho, M.S.; Nääs, I.A.; Lima, N.D.S.; Cordeiro, A.F.S.; Moura, D.J. Factors Affecting Broiler Production: A Meta-Analysis. Braz. J. Poult. Sci. 2019, 21, 001–010.
- Zhao, P.Y.; Kim, I.H. Effect of Diets with Different Energy and Lysophospholipids Levels on Performance, Nutrient Metabolism, and Body Composition in Broilers. Poult. Sci. 2017, 96, 1341–1347.
- Das, T.K.; Mondal, M.K.; Biswas, P.; Bairagi, B.; Samanta, C.C. Influence of Level of Dietary Inorganic and Organic Copper and Energy Level on the Performance and Nutrient Utilization of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2009, 23, 82–89.
- Maharjan, P.; Mullenix, G.; Hilton, K.; Caldas, J.; Beitia, A.; Weil, J.; Suesuttajit, N.; Kalinowski, A.; Yacoubi, N.; Naranjo, V.; et al. Effect of Digestible Amino Acids to Energy Ratios on Performance and Yield of Two Broiler Lines Housed in Different Grow-out Environmental Temperatures. Poult. Sci. 2020, 99, 6884–6898.
- Liu, G.; Magnuson, A.D.; Sun, T.; Tolba, S.A.; Starkey, C.; Whelan, R.; Lei, X.G. Supplemental Methionine Exerted Chemical Form-Dependent Effects on Antioxidant Status, Inflammation-Related Gene Expression, and Fatty Acid Profiles of Broiler Chicks Raised at High Ambient Temperature. J. Anim. Sci. 2019, 97, 4883–4894.
- Magnuson, A.D.; Liu, G.; Sun, T.; Tolba, S.A.; Xi, L.; Whelan, R.; Lei, X.G. Supplemental Methionine and Stocking Density Affect Antioxidant Status, Fatty Acid Profiles, and Growth Performance of Broiler Chickens. J. Anim. Sci. 2020, 98, skaa092.
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369.
- Selye, H. Forty Years of Stress Research: Principal Remaining Problems and Misconceptions. Can. Med. Assoc. J. 1976, 115, 53–56.
- Yunianto, V.D.; Hayashi, K.; Kaneda, S.; Ohtsuka, A.; Tomita, Y. Effect of Environmental Temperature on Muscle Protein Turnover and Heat Production in Tube-Fed Broiler Chickens. Br. J. Nutr. 1997, 77, 897–909.
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015.
- Fry, J.L.; Stadelman, W.J. The Effect of Dietary Methionine on the Methionine and Cystine Content of Poultry Meat. Poult. Sci. 1960, 39, 614–617.
- Graber, G.; Scott, H.M.; Baker, D.H. Sulfur Amino Acid Nutrition of the Growing Chick: Effect of Age on the Dietary Methionine Requirement. Poult. Sci. 1971, 50, 854–858.
- Bao, Y. Amino Acid Nutrition and Chicken Gut Health. Worlds Poult. Sci. J. 2020, 76, 563–576.
- Stipanuk, M.H. Metabolism of Sulfur-Containing Amino Acids: How the Body Copes with Excess Methionine, Cysteine, and Sulfide. J. Nutr. 2020, 150, 2494S–2505S.
- Zhang, S.; Saremi, B.; Gilbert, E.R.; Wong, E.A. Physiological and Biochemical Aspects of Methionine Isomers and a Methionine Analogue in Broilers. Poult. Sci. 2017, 96, 425–439.
- Ren, Z.; Bütz, D.E.; Whelan, R.; Naranjo, V.; Arendt, M.K.; Ramuta, M.D.; Yang, X.; Crenshaw, T.D.; Cook, M.E. Effects of Dietary Methionine plus Cysteine Levels on Growth Performance and Intestinal Antibody Production in Broilers during Eimeria Challenge. Poult. Sci. 2020, 99, 374–384.
- Sigolo, S.; Deldar, E.; Seidavi, A.; Bouyeh, M.; Gallo, A.; Prandini, A. Effects of Dietary Surpluses of Methionine and Lysine on Growth Performance, Blood Serum Parameters, Immune Responses, and Carcass Traits of Broilers. J. Appl. Anim. Res. 2019, 47, 146–153.
- Zhang, S.; Gilbert, E.R.; Saremi, B.; Wong, E.A. Supplemental Methionine Sources Have a Neutral Impact on Oxidative Status in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1274–1283.
- Akter, N.; Islam, M.; Zaman, S.; Jahan, I.; Hossain, M. The Impact of Different Levels of L-Methionine (L-Met) on Carcass Yield Traits, Serum Metabolites, Tibial Characters, and Profitability of Broilers Fed Conventional Diet. J. Adv. Vet. Anim. Res. 2020, 7, 253.
- Esteve-Garcia, E.; Khan, D.R. Relative Bioavailability of DL and L-Methionine in Broilers. Open J. Anim. Sci. 2018, 8, 151–162.
- Jankowski, J.; Ognik, K.; Kubińska, M.; Czech, A.; Juśkiewicz, J.; Zduńczyk, Z. The Effect of DL-, L-Isomers and DL-Hydroxy Analog Administered at 2 Levels as Dietary Sources of Methionine on the Metabolic and Antioxidant Parameters and Growth Performance of Turkeys. Poult. Sci. 2017, 96, 3229–3238.
- Murawska, D.; Kubińska, M.; Gesek, M.; Zduńczyk, Z.; Brzostowska, U.; Jankowski, J. The Effect of Different Dietary Levels and Sources of Methionine on the Growth Performance of Turkeys, Carcass and Meat Quality. Ann. Anim. Sci. 2018, 18, 525–540.
- Park, I.; Pasquetti, T.; Malheiros, R.D.; Ferket, P.R.; Kim, S.W. Effects of Supplemental L-Methionine on Growth Performance and Redox Status of Turkey Poults Compared with the Use of DL-Methionine. Poult. Sci. 2018, 97, 102–109.
- Li, L.; Abouelezz, K.F.M.; Cheng, Z.; Gad-Elkareem, A.E.G.; Fan, Q.; Ding, F.; Gao, J.; Jiang, S.; Jiang, Z. Modelling Methionine Requirements of Fast- and Slow-Growing Chinese Yellow-Feathered Chickens during the Starter Phase. Animals 2020, 10, 443.
- National Research Council (U.S.). Nutrient Requirements of Poultry. In Nutrient Requirements of Domestic Animals, 9th ed.; National Academy Press: Washington, DC, USA, 1994; ISBN 978-0-309-04892-7.
- Rehman, A.U.; Arif, M.; Husnain, M.M.; Alagawany, M.; Abd El-Hack, M.E.; Taha, A.E.; Elnesr, S.S.; Abdel-Latif, M.A.; Othman, S.I.; Allam, A.A. Growth Performance of Broilers as Influenced by Different Levels and Sources of Methionine Plus Cysteine. Animals 2019, 9, 1056.
- Tian, Q.Y.; Zeng, Z.K.; Zhang, Y.X.; Long, S.F.; Piao, X.S. Effect of L- or DL-Methionine Supplementation on Nitrogen Retention, Serum Amino Acid Concentrations and Blood Metabolites Profile in Starter Pigs. Asian-Australas. J. Anim. Sci. 2016, 29, 689–694.
- Ullrich, C.; Langeheine, M.; Brehm, R.; Taube, V.; Rosillo Galera, M.; Rohn, K.; Popp, J.; Visscher, C. Influence of Different Methionine Sources on Performance and Slaughter Characteristics of Broilers. Animals 2019, 9, 984.
- Agostini, P.S.; Dalibard, P.; Mercier, Y.; Van der Aar, P.; Van der Klis, J.D. Comparison of Methionine Sources around Requirement Levels Using a Methionine Efficacy Method in 0 to 28 Day Old Broilers. Poult. Sci. 2016, 95, 560–569.
- Fouad, A.M.; Ruan, D.; Lin, Y.C.; Zheng, C.T.; Zhang, H.X.; Chen, W.; Wang, S.; Xia, W.G.; Li, Y. Effects of Dietary Methionine on Performance, Egg Quality and Glutathione Redox System in Egg-Laying Ducks. Br. Poult. Sci. 2016, 57, 818–823.
- Zduńczyk, Z.; Jankowski, J.; Kubińska, M.; Ognik, K.; Czech, A.; Juśkiewicz, J. The Effect of Different Dietary Levels of Dl-Methionine and Dl-Methionine Hydroxy Analogue on the Antioxidant and Immune Status of Young Turkeys. Arch. Anim. Nutr. 2017, 71, 347–361.
- Murillo, M.G.; Jensen, L.S.; Ruff, M.D.; Rahn, A.P. Effect of Dietary Methionine Status on Response of Chicks to Coccidial Infection. Poult. Sci. 1976, 55, 642–649.
- Meirelles, H.T.; Albuquerque, R.; Borgatti, L.M.O.; Souza, L.W.O.; Meister, N.C.; Lima, F.R. Performance of Broilers Fed with Different Levels of Methionine Hydroxy Analogue and DL-Methionine. Braz. J. Poult. Sci. 2003, 5, 69–74.
- Swennen, Q.; Geraert, P.-A.; Mercier, Y.; Everaert, N.; Stinckens, A.; Willemsen, H.; Li, Y.; Decuypere, E.; Buyse, J. Effects of Dietary Protein Content and 2-Hydroxy-4-Methylthiobutanoic Acid or DL-Methionine Supplementation on Performance and Oxidative Status of Broiler Chickens. Br. J. Nutr. 2011, 106, 1845–1854.
- Yodseranee, R.; Bunchasak, C. Effects of Dietary Methionine Source on Productive Performance, Blood Chemical, and Hematological Profiles in Broiler Chickens under Tropical Conditions. Trop. Anim. Health Prod. 2012, 44, 1957–1963.
- Xiao, X.; Wang, Y.; Liu, W.; Ju, T.; Zhan, X. Effects of Different Methionine Sources on Production and Reproduction Performance, Egg Quality and Serum Biochemical Indices of Broiler Breeders. Asian-Australas. J. Anim. Sci. 2017, 30, 828–833.
- Albrecht, A.; Herbert, U.; Miskel, D.; Heinemann, C.; Braun, C.; Dohlen, S.; Zeitz, J.O.; Eder, K.; Saremi, B.; Kreyenschmidt, J. Effect of Methionine Supplementation in Chicken Feed on the Quality and Shelf Life of Fresh Poultry Meat. Poult. Sci. 2017, 96, 2853–2861.
- Jankowski, J.; Tykałowski, B.; Ognik, K.; Koncicki, A.; Kubińska, M.; Zduńczyk, Z. The Effect of Different Dietary Levels of DL-Methionine and DL-Hydroxy Analogue on the Antioxidant Status of Young Turkeys Infected with the Haemorrhagic Enteritis Virus. BMC Vet. Res. 2018, 14, 404.
- Wan, J.; Ding, X.; Wang, J.; Bai, S.; Peng, H.; Luo, Y.; Su, Z.; Xuan, Y.; Zhang, K. Dietary Methionine Source and Level Affect Hepatic Sulfur Amino Acid Metabolism of Broiler Breeder Hens. Anim. Sci. J. 2017, 88, 2016–2024.
- Sangali, C.P.; Bruno, L.D.G.; Nunes, R.V.; de Oliveira Neto, A.R.; Pozza, P.C.; de Oliveira, T.M.M.; Frank, R.; Schöne, R.A. Bioavailability of Different Methionine Sources for Growing Broilers. R. Bras. Zootec. 2014, 43, 140–145.
- Millecam, J.; Khan, D.R.; Dedeurwaerder, A.; Saremi, B. Optimal Methionine plus Cystine Requirements in Diets Supplemented with L-Methionine in Starter, Grower, and Finisher Broilers. Poult. Sci. 2021, 100, 910–917.
- Shen, Y.B.; Ferket, P.; Park, I.; Malheiros, R.D.; Kim, S.W. Effects of Feed Grade L-Methionine on Intestinal Redox Status, Intestinal Development, and Growth Performance of Young Chickens Compared with Conventional DL-Methionine. J. Anim. Sci. 2015, 93, 2977–2986.
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The Role of Methionine on Metabolism, Oxidative Stress, and Diseases. Amino Acids 2017, 49, 2091–2098.
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235.
- Horváth, M.; Babinszky, L. Impact of Selected Antioxidant Vitamins (Vitamin A, E and C) and Micro Minerals (Zn, Se) on the Antioxidant Status and Performance under High Environmental Temperature in Poultry. A Review. Acta Agric. Scand. Sect. A—Anim. Sci. 2018, 68, 152–160.
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 60.
- Luo, S.; Levine, R.L. Methionine in Proteins Defends against Oxidative Stress. FASEB J. 2009, 23, 464–472.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
987
Revisions:
2 times
(View History)
Update Date:
04 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




