
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jesus Lozano-Fernandez | -- | 2390 | 2022-11-03 12:03:38 | | | |
| 2 | Beatrix Zheng | + 3 word(s) | 2393 | 2022-11-04 04:28:49 | | |
Video Upload Options
Arthropods, the most diverse form of macroscopic life in the history of the Earth, originated in the sea. Since the early Cambrian, at least ~518 million years ago, these animals have dominated the oceans of the world. By the Silurian–Devonian, the fossil record attests to arthropods becoming the first animals to colonize land, However, a growing body of molecular dating and palaeontological evidence suggests that the three major terrestrial arthropod groups (myriapods, hexapods, and arachnids), as well as vascular plants, may have invaded land as early as the Cambrian–Ordovician. These dates precede the oldest fossil evidence of those groups and suggest an unrecorded continental “Cambrian explosion” a hundred million years prior to the formation of early complex terrestrial ecosystems in the Silurian–Devonian.
1. Arthropod Origins
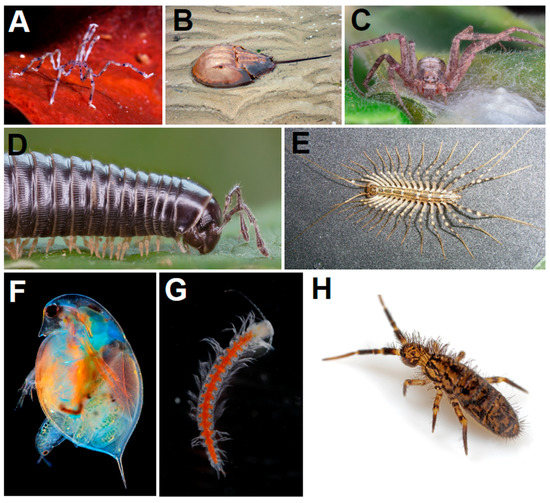
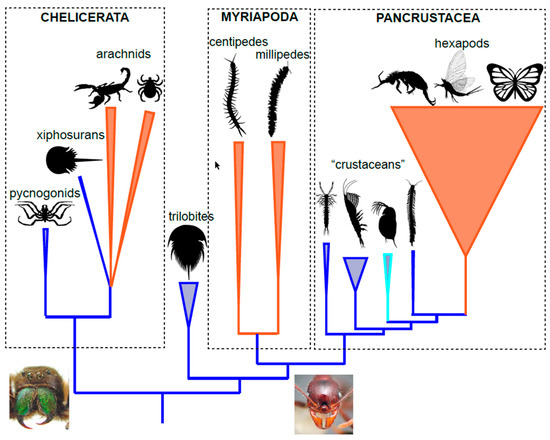
2. Arthropod Phylogeny
3. Myriapods
4. Pancrustacea (Hexapoda)
5. Pancrustacea (Isopods)
6. Arachnids
References
- Edgecombe, G.D. Arthropod Phylogeny: An Overview from the Perspectives of Morphology, Molecular Data and the Fossil Record. Arthropod Struct. Dev. 2010, 39, 74–87.
- Grimaldi, D.; Engel, M.S. Evolution of the Insects, 1st ed.; Cambridge University Press: Cambridge, UK, 2005.
- Govorushko, S. Economic and Ecological Importance of Termites: A Global Review. Entomol. Sci. 2019, 22, 21–35.
- Brusca, R.C.; Brusca, G.J. Invertebrates, 2nd ed.; Sinauer Associates, Incorporated: Sunderland, MA, USA, 2003; ISBN 978-0-87893-099-9.
- Little, C. The Colonisation of Land: Origins and Adaptations of Terrestrial Animals; Cambridge University Press: Cambridge, UK, 1983; ISBN 978-0-521-25218-8.
- Dunlop, J. A Trigonotarbid Arachnid from the Upper Silurian of Shropshire. Palaeontology 1996, 39, 605–614.
- Jeram, A.J.; Selden, P.A.; Edwards, D. Land Animals in the Silurian: Arachnids and Myriapods from Shropshire, England. Science 1990, 250, 658–661.
- Shear, W.; Selden, P. Eoarthropleura (Arthropoda, Arthropleurida) from the Silurian of Britain and the Devonian of North America. Neues Jahrb. Geol. Palaontol. Abh. 1995, 196, 347–375.
- Shear, W.A.; Jeram, A.J.; Selden, P. Centiped Legs (Arthropoda, Chilopoda, Scutigeromorpha) from the Silurian and Devonian of Britain and the Devonian of North America. Am. Mus. Novit. 1998, 3231, 1–16.
- Dunlop, J.A. A Replacement Name for the Trigonotarbid Arachnid Eotarbus Dunlop. Palaeontology 1999, 42, 191.
- Brookfield, M.E.; Catlos, E.J.; Suarez, S.E. Myriapod Divergence Times Differ between Molecular Clock and Fossil Evidence: U/Pb Zircon Ages of the Earliest Fossil Millipede-Bearing Sediments and Their Significance. Hist. Biol. 2020, 10, 2014–2018.
- Wilson, H.M.; Anderson, L.I. Morphology and Taxonomy of Paleozoic Millipedes (Diplopoda: Chilognatha: Archipolypoda) from Scotland. J. Paleontol. 2004, 78, 169–184.
- Marshall, J.E.A. Palynology of the Stonehaven Group, Scotland: Evidence for a Mid Silurian Age and Its Geological Implications. Geol. Mag. 1991, 128, 283–286.
- Wellman, C.H. A Land Plant Microfossil Assemblage of Mid Silurian Age from the Stonehaven Group, Scotland. J. Micropalaeontol. 1993, 12, 47–66.
- Howard, R.J.; Puttick, M.N.; Edgecombe, G.D.; Lozano-Fernandez, J. Arachnid Monophyly: Morphological, Palaeontological and Molecular Support for a Single Terrestrialization within Chelicerata. Arthropod Struct. Devel. 2020, 59, 100997.
- Suarez, S.E.; Brookfield, M.E.; Catlos, E.J.; Stöckli, D.F. A U-Pb Zircon Age Constraint on the Oldest-Recorded Air-Breathing Land Animal. PLoS ONE 2017, 12, e0179262.
- Kutscher, F. Friedrich Beiträge Zur Sedimentation Und Fossilführung Des Hunsrückschiefers 32. Palaeoscorpius devonicus, Ein Devonischer Skorpion. Jahrb. Nassau. Ver. Naturkd. 1971, 101, 191.
- Lehmann, W.M. Palaeoscorpius devonicus Ng, n. Sp., Ein Skorpion Aus Dem Rheinischen Unterdevon. N. Jahrb. Geol. Palaontol. Monat. 1944, 7, 177–185.
- Kühl, G.; Bergmann, A.; Dunlop, J.; Garwood, R.J.; Rust, J. Redescription and Palaeobiology of Palaeoscorpius devonicus Lehmann, 1944 from the Lower Devonian Hunsrück Slate of Germany. Palaeontology 2012, 55, 775–787.
- Zrzavý, J.; Hypša, V.; Vlášková, M. Arthropod Phylogeny: Taxonomic Congruence, Total Evidence and Conditional Combination Approaches to Morphological and Molecular Data Sets. In Arthropod Relationships; Fortey, R.A., Thomas, R.H., Eds.; The Systematics Association Special Volume Series; Springer: Dordrecht, The Netherlands, 1998; pp. 97–107. ISBN 978-94-011-4904-4.
- Giribet, G.; Edgecombe, G.D. The Phylogeny and Evolutionary History of Arthropods. Curr. Biol. 2019, 29, R592–R602.
- van Straalen, N.M. Evolutionary Terrestrialization Scenarios for Soil Invertebrates. Pedobiologia 2021, 87–88, 150753.
- Legg, D.A.; Sutton, M.D.; Edgecombe, G.D. Arthropod Fossil Data Increase Congruence of Morphological and Molecular Phylogenies. Nat. Commun. 2013, 4, 2485.
- Ballesteros, J.A.; Santibáñez-López, C.E.; Baker, C.M.; Benavides, L.R.; Cunha, T.J.; Gainett, G.; Ontano, A.Z.; Setton, E.V.W.; Arango, C.P.; Gavish-Regev, E.; et al. Comprehensive Species Sampling and Sophisticated Algorithmic Approaches Refute the Monophyly of Arachnida. Mol. Biol. Evol. 2022, 39, msac021.
- Tihelka, E.; Cai, C.; Giacomelli, M.; Lozano-Fernandez, J.; Rota-Stabelli, O.; Huang, D.; Engel, M.S.; Donoghue, P.C.J.; Pisani, D. The Evolution of Insect Biodiversity. Curr. Biol. 2021, 31, R1299–R1311.
- Bäcker, H.; Fanenbruck, M.; Wägele, J.W. A Forgotten Homology Supporting the Monophyly of Tracheata: The Subcoxa of Insects and Myriapods Re-Visited. Zool. Anz. J. Comp. Zool. 2008, 247, 185–207.
- Giribet, G.; Edgecombe, G.D. Reevaluating the Arthropod Tree of Life. Annu. Rev. Entomol. 2012, 57, 167–186.
- Fernández, R.; Edgecombe, G.D.; Giribet, G.; Edgecombe, G.D.; Giribet, G. Phylogenomics Illuminates the Backbone of the Myriapoda Tree of Life and Reconciles Morphological and Molecular Phylogenies. Sci. Rep. 2018, 8, 83.
- Rota-Stabelli, O.; Campbell, L.; Brinkmann, H.; Edgecombe, G.D.; Longhorn, S.J.; Peterson, K.J.; Pisani, D.; Philippe, H.; Telford, M.J. A Congruent Solution to Arthropod Phylogeny: Phylogenomics, MicroRNAs and Morphology Support Monophyletic Mandibulata. Proc. R. Soc. B 2011, 278, 298–306.
- Fernández, R.; Edgecombe, G.D.; Giribet, G. Exploring Phylogenetic Relationships within Myriapoda and the Effects of Matrix Composition and Occupancy on Phylogenomic Reconstruction. Syst. Biol. 2016, 65, 871–889.
- Szucsich, N.U.; Bartel, D.; Blanke, A.; Böhm, A.; Donath, A.; Fukui, M.; Grove, S.; Liu, S.; Macek, O.; Machida, R.; et al. Four Myriapod Relatives—But Who Are Sisters? No End to Debates on Relationships among the Four Major Myriapod Subgroups. BMC Evol. Biol. 2020, 20, 144.
- Wang, J.; Bai, Y.; Zhao, H.; Mu, R.; Dong, Y. Reinvestigating the Phylogeny of Myriapoda with More Extensive Taxon Sampling and Novel Genetic Perspective. PeerJ 2021, 9, e12691.
- Benavides, L.R.; Edgecombe, G.D.; Giribet, G. Re-Evaluating and Dating Myriapod Diversification with Phylotranscriptomics under a Regime of Dense Taxon Sampling. Mol. Phylogenetics Evol. 2022, 178, 107621.
- Schwentner, M.; Combosch, D.J.; Nelson, J.P.; Giribet, G. A Phylogenomic Solution to the Origin of Insects by Resolving Crustacean-Hexapod Relationships. Curr. Biol. 2017, 27, 1818–1824.
- Regier, J.C.; Shultz, J.W.; Zwick, A.; Hussey, A.; Ball, B.; Wetzer, R.; Martin, J.W.; Cunningham, C.W.; Shultz, J.W.; Zwick, A.; et al. Arthropod Relationships Revealed by Phylogenomic Analysis of Nuclear Protein-Coding Sequences. Nature 2010, 463, 1079–1083.
- Lozano-Fernandez, J.; Giacomelli, M.; Fleming, J.F.; Chen, A.; Vinther, J.; Thomsen, P.F.; Glenner, H.; Palero, F.; Legg, D.A.; Iliffe, T.M.; et al. Pancrustacean Evolution Illuminated by Taxon-Rich Genomic-Scale Data Sets with an Expanded Remipede Sampling. Genome Biol. Evol. 2019, 11, 2055–2070.
- Lozano-Fernandez, J.; Carton, R.; Tanner, A.R.; Puttick, M.N.; Blaxter, M.; Vinther, J.; Olesen, J.; Giribet, G.; Edgecombe, G.D.; Pisani, D. A Molecular Palaeobiological Exploration of Arthropod Terrestrialization. Phil. Trans. Roy. Soc. B. 2016, 371, 20150133.
- Yager, J. Remipedia, a New Class of Crustacea from a Marine Cave in the Bahamas. J. Crustacean Biol. 1981, 1, 328–333.
- Schmalfuss, H. World Catalog of Terrestrial Isopods (Isopoda: Oniscidea). Stuttg. Beitr. Naturkd. A 2003, 654, 341.
- Richardson, A.; Araujo, P.B. Lifestyles of Terrestrial Crustacean. In The Natural History of the Crustacea. Lifestyles and Feeding Biology; Oxford University Press: Oxford, UK, 2015; pp. 299–336.
- Broly, P.; Deville, P.; Maillet, S. The Origin of Terrestrial Isopods (Crustacea: Isopoda: Oniscidea). Evol. Ecol. 2013, 27, 461–476.
- Elisabeth, H. Evolutionary Adaptation of Oniscidean Isopods to Terrestrial Life: Structure, Physiology and Behavior. Terr. Arthropod Rev. 2011, 4, 95–130.
- Schmidt, C. Phylogeny of the Terrestrial Isopoda (Oniscidea): A Review. Arthr. Syst. Phyl. 2008, 66, 191–226.
- Dimitriou, A.C.; Taiti, S.; Sfenthourakis, S. Genetic Evidence against Monophyly of Oniscidea Implies a Need to Revise Scenarios for the Origin of Terrestrial Isopods. Sci. Rep. 2019, 9, 18508.
- Tabacaru, I.; Giurginca, A. The Monophyly and the Classification of the Terrestrial Isopods (Crustacea, Isopoda, Oniscidea). Trav. Inst. Speol. Emile Racovitza 2021, 59, 3–23.
- Selden, P.A.; Jeram, A.J. Palaeophysiology of Terrestrialisation in the Chelicerata. Earth Environ. Sci. Trans. R. Soc. Edinb. 1989, 80, 303–310.
- Zhang, Z.-Q. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa 2013, 3703, 1–82.
- Ballesteros, J.A.; Sharma, P.P. A Critical Appraisal of the Placement of Xiphosura (Chelicerata) with Account of Known Sources of Phylogenetic Error. Syst. Biol. 2019, 68, 896–917.
- Shultz, J.W. A Phylogenetic Analysis of the Arachnid Orders Based on Morphological Characters. Zool. J. Linn. Soc. 2007, 150, 221–265.
- Shultz, J.W. Evolutionary Morphology and Phylogeny of Arachnida. Cladistics 1990, 6, 1–38.
- Bicknell, R.D.C.; Pates, S. Pictorial Atlas of Fossil and Extant Horseshoe Crabs, with Focus on Xiphosurida. Front. Earth Sci. 2020, 8, 98.
- Gould, S.J. Dollo on Dollo’s Law: Irreversibility and the Status of Evolutionary Laws. J. Hist. Biol. 1970, 3, 189–212.
- Lamsdell, J.C. Evolutionary History of the Dynamic Horseshoe Crab. Int. Wader Stud. 2019, 21, 1–15.
- Bicknell, R.D.C.; Kimmig, J.; Budd, G.E.; Legg, D.A.; Bader, K.S.; Haug, C.; Kaiser, D.; Laibl, L.; Tashman, J.N.; Campione, N.E. Habitat and Developmental Constraints Drove 330 Million Years of Horseshoe Crab Evolution. Biol. J. Linn. Soc. 2022, 136, 155–172.
- Lozano-Fernandez, J.; Tanner, A.R.; Giacomelli, M.; Carton, R.; Vinther, J.; Edgecombe, G.D.; Pisani, D. Increasing Species Sampling in Chelicerate Genomic-Scale Datasets Provides Support for Monophyly of Acari and Arachnida. Nat. Commun. 2019, 10, 2295.





