Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatima Al Hameli | -- | 3541 | 2022-11-03 10:18:24 | | | |
| 2 | Peter Tang | Meta information modification | 3541 | 2022-11-04 02:18:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hameli, F.A.; Belhaj, H.; Dhuhoori, M.A. CO2 Sequestration in Geological Formations. Encyclopedia. Available online: https://encyclopedia.pub/entry/32707 (accessed on 07 February 2026).
Hameli FA, Belhaj H, Dhuhoori MA. CO2 Sequestration in Geological Formations. Encyclopedia. Available at: https://encyclopedia.pub/entry/32707. Accessed February 07, 2026.
Hameli, Fatima Al, Hadi Belhaj, Mohammed Al Dhuhoori. "CO2 Sequestration in Geological Formations" Encyclopedia, https://encyclopedia.pub/entry/32707 (accessed February 07, 2026).
Hameli, F.A., Belhaj, H., & Dhuhoori, M.A. (2022, November 03). CO2 Sequestration in Geological Formations. In Encyclopedia. https://encyclopedia.pub/entry/32707
Hameli, Fatima Al, et al. "CO2 Sequestration in Geological Formations." Encyclopedia. Web. 03 November, 2022.
Copy Citation
The geological storage of carbon dioxide (CO2) is the most effective and, in many cases, the only viable short- to medium-term alternative for considerably moving towards CO2 sequestration in geological sinks and, thus, lowering net carbon emissions into the atmosphere.
CO2 geological storage
CO2 sequestration
trapping mechanisms
storage capacity
1. Introduction
Carbon capture and storage (CCS) is a critical method for reducing human CO2 emissions into the atmosphere [1]. Continuously growing CO2 emissions have been identified as a major potential cause of global concern, whereas CO2 geological sequestration (CGS) provides a viable strategy for addressing this massive environmental crisis that the world is now facing. Since the beginning of industrialization in the 19th century, the quantity of CO2 in the atmosphere has been growing significantly at an alarming rate [2]. As a result of this incremental rise, the world’s climate may be impacted, as shown by an increase in global temperatures and an increase in local weather extremes. Energy combustion and industrial processes contributed to a rise in global CO2 emissions in 2021, resulting in the highest year of CO2 emissions on record. The International Energy Agency’s (IEA) detailed region-by-region and fuel-by-fuel analysis, which draws on the most recent official national data as well as publicly available energy, economic, and weather data, estimates that emissions will reach 36.3 gigatons (Gt) by 2030, representing a 6% increase from 2020. According to the IEA (2022), emissions rose by around 2.1 Gt compared to the baseline year of 2020. In absolute terms, this places the year 2021 above 2010 as the year with the biggest year-on-year growth in energy-related CO2 emissions. The spike in emissions in 2021 more than negated the 1.9 Gt drop in emissions caused by the pandemic that occurred in 2020. CO2 emissions increased by about 180 megatons (Mt) in 2021, compared to the pre-pandemic level in 2019.
The purpose of this research is to shed light on the crucial role of CCS in lowering CO2 emissions by providing an in-depth examination of the relevant environmental and global metrics of CO2 and the integrated role of the CCS system. Several examples are also provided with an emphasis on the geological sequestration of CO2. Geological CO2 sinks include ocean storage, deep saline formations, depleted oil and gas reservoirs, formations that require CO2-enhanced oil recovery (CO2-EOR), unmineable coal seams, and organic-rich shales. They may contain hundreds of gigatons of carbon (GtC) or more. There are many trapping mechanisms that may be utilized to store CO2 in geologic formations, with the specific process depending on the kind of formation. These geological sinks have a wide range of potential capabilities, each of which is vastly different in terms of the storage capacity potential. As a result, a matrix evaluation of the CO2-trapping processes is created, demonstrating its significance in understanding how these trapping mechanisms interact. These CO2-trapping mechanisms play a crucial role in CO2 storage potential from a constitutive standpoint, and yet they also provide a baseline for modeling CO2 sequestration. Chemical trapping, physicochemical trapping, and physical trapping are the three main trapping mechanisms that set the fundamental baseline. Furthermore, there is a need for enhanced storage capacity estimations at the global, regional, and local levels, as well as a better knowledge of long-term storage, migration, and leakage processes, in order for CCS to be successful.
1.1. Global Measures
The consumption of conventional energy sources results in CO2 emissions, with power generation emitting the highest CO2, followed by industry and transportation vehicles, as shown in Figure 1.
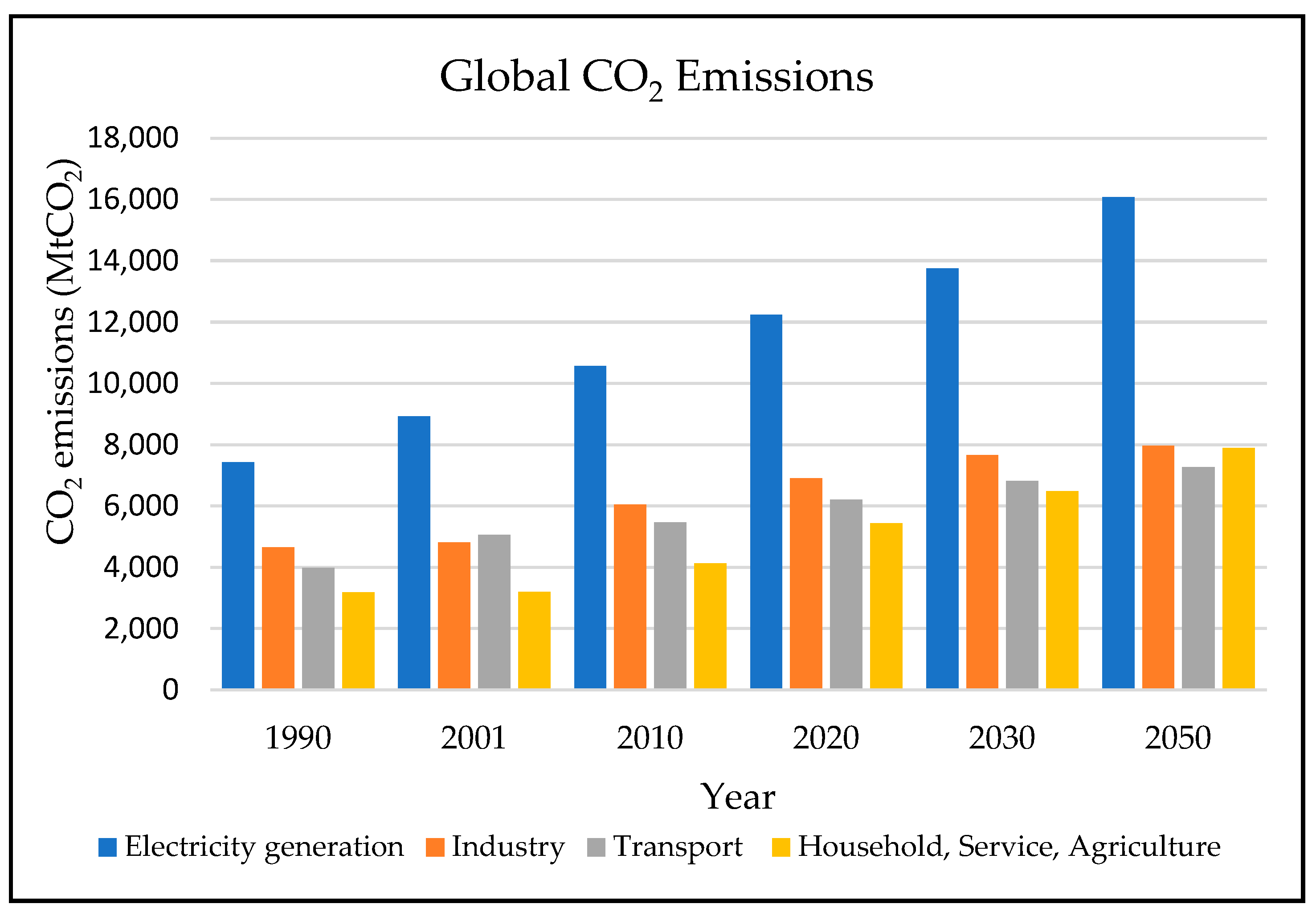
Figure 1. Global CO2 emissions (data source: World Energy Technology Outlook 2050 [3]).
Lapillonne et al. [3] provided the data statistics on CO2 worldwide emissions and sequestration. However, CO2 emissions into the atmosphere could be reduced by reducing the need for fossil fuel combustion through more efficient energy use, substituting biofuel or hydrogen for fossil fuels in transportation and electric power generation, substituting natural gas for coal in electric power generation, and capturing and sequestering CO2 in geological formations.
1.2. Environmental Measures
CO2 emissions are directly linked to rising environmental problems [4]. CO2 levels have risen to their highest level in recent decades and are a significant contributor to global warming greenhouse gas emissions, making up over 55% of all emissions [5]. As a result, CO2 emissions are a major contributor to global warming and climate change [6]. Climate change has become the greatest threat to human civilization in conjunction with the rise in CO2 emissions. Excess CO2 and other greenhouse gases in the atmosphere have already warmed the Earth’s temperature by around 1.8 °F (1 °C) on average, and even if emissions were to halt immediately, more warming would still occur owing to existing greenhouse gases in the atmosphere [7]. Therefore, concerns about environmental protection prompted the introduction of CO2 sequestration, which is expanding in conjunction with energy output, as seen in Figure 2. By 2050, it is expected that the CO2 emissions will rise drastically due to increased utilization of transportation, electricity, manufacturing, constructions, and other sources of fugitive emissions [8]. As a result, the produced CO2 must be captured and stored such that the whole process is almost free of CO2 emissions into the environment. This may be accomplished by using advanced technologies and ensuring that leakage into the atmosphere is minimized. Another form that contributes to CO2 reduction measures suggests combining electric vehicles (EVs) with renewable energy such as photovoltaics (PV), or wind power is yet another method that may be used to lessen the detrimental effects that CO2 emissions have on the environment [9]. Furthermore, CO2 emissions must be severely reduced in order to avoid the economic and human consequences of catastrophic climate change. Reduced mitigation costs and more adaptability in decreasing greenhouse gas emissions are also feasible results of CCS utilization [10].
Figure 2. Global CO2 emissions in conjunction with CO2 sequestration from 1990 to 2050 (data source: World Energy Technology Outlook 2050 [3]).
1.3. Role of CCS
CO2 capture, sequestration, and storage in geological formations may aid significantly in reducing the anthropogenic CO2 emissions. CO2 is extracted from a power plant’s flue gas (“capture”) and then compressed and transferred through pipelines. At a nearby location, the CO2 is injected into a geological formation through a deep borehole (“sequestration” or “storage”). Carbon capture is technically accomplished through cryogenic separation, adsorption/abstraction, and membrane separation [11], but one of the most cutting-edge methods for CO2 storage is injection into deep saline aquifers, as well as deep coal-bed methane and ocean storage (all of which can be used to store CO2). However, carbon capture is the most costly component of CCS, since the CO2 extraction from flue gas and subsequent compression requires either modifying an existing power plant or altering the design of a new power plant. Whether converted or integrated into a new construction, this new equipment involves capital inputs and operating expenses that dramatically raise the price of the energy produced [12]. However, the widespread use of CCS would rely on the maturity of the technology, prices, overall potential, diffusion, and transfer of the technology to emerging nations and their ability to implement the technology, regulatory elements, environmental concerns, and public perceptions. CCS can only reduce emissions to the environment by a certain percentage depending on the amount of CO2 it is able to capture, transport, and store, as well as any leakage that occurs during transportation and the amount of CO2 that is able to be stored for an extended period of time [10]. Current CCS research aims to enhance the separation process and to produce innovative materials that can be employed as effectively as is feasible in the capture process. When it comes to carbon sequestration, a wide range of geological formations have the ability to trap significant amounts of CO2 and are widely distributed and categorized according to the CO2 sequestration varieties in Table 1. CO2 geological sequestration options provide significant storage capacity potential for storing CO2, and these geological sinks vary in terms of their location, type of the formation, and the types of trapping mechanisms that contribute to the CO2 storage. Ocean storage, deep saline formations, depleted oil and gas reservoirs, formations that require CO2-EOR, unminable coal seams, and organic-rich shales are all examples of geological CO2 sinks. Collectively, they can hold hundreds of GtC. There is a variety of different trapping methods that may be used to store CO2 in geologic formations, with the precise mechanism being dependent on the type of formation. These geological sinks provide a variety of potential capacities, each of which differs greatly from the others. At an estimated 40,000 GtC, the world’s oceans have the greatest storage capacity of any natural or manmade system, and have been demonstrated to have the greatest potential as a sink for anthropogenic CO2. Marchetti [13] was the first to propose the method of direct injection of liquefied CO2 into deep ocean waters to improve the degree of CO2 isolation [14]. In addition, deep saline aquifers provide up to 10,000 Gt of potential CO2 sequestration storage area on a global scale [15]. In the 1990s, Canada became the first country to inject CO2 into a deep saline formation due to the need to dispose of “acid gas”, or H2S and CO2 mixes, from sour gas wells [16]. Furthermore, CO2 sequestration as a concept for depleted oil and gas reservoirs emerged in the past as a result of the reservoirs’ proven reliability as long-term storage sites for hydrocarbons and acid gases. Up to 90% of the entire volume injected is composed of CO2, making disposal of acid gas a primary goal. Acid gas is a byproduct of oil and gas extraction and refining that contains CO2, H2S, and other substances. In addition, CO2-EOR was first used in 1983, and oil production stabilized shortly afterwards. To retrieve the oil, most EOR operations require “blowing down” the reservoir pressure, which releases CO2, with some of the injected CO2 remaining dissolved in the immobile oil [17]. This temporarily stores a large quantity of CO2 that was injected into the reservoir. Another kind of geological sink is the sequestration of CO2 in unmineable coal seams; the coal surface has a preferential chemical attraction for CO2 adsorption over methane with a ratio of 2:1. As a result, coalbed methane (CBM) recovery may be improved using CO2 sequestration. Furthermore, despite having relatively low porosities and permeabilities, several organic-rich shale formations are used as geological sinks for CO2 and methane generation [18]. However, recent scientific developments have boosted the prospect of using shales and other tight formations to minimize fluid leak-off into the reservoir using the application of dual-porosity models, which can be applicable for potential CO2 storage [19]. Another kind of sequestration is terrestrial, in which CO2 is absorbed by the trees and plants through photosynthesis and stored as carbon in soils and biomass [20]. Terrestrial sequestration is an example of biological sequestration. In addition to these options, a new technique known as direct air capture emerged in 2019, and the newest facility was already operational by September 2021 [21]. Direct air capture captures CO2 from the environment using chemical processes. As air passes over the chemicals, they selectively react with and remove CO2 while allowing the other components of air to flow through [22]. This form of CO2 sequestration comes under using innovate technologies to reduce the anthropogenic CO2 emissions. In addition to the research made by Herzog and Golomb [23], the following table has been modified to account for biological and technological options for CO2 sequestration (see Table 1).
Table 1. Worldwide potential reservoir capacity for CO2 sequestration.
|
CO2 Sequestration Options |
Storage Capacity * |
References |
|---|---|---|
|
Geological CO2 Sequestration: |
||
|
Ocean |
1000–10,000 + GtC |
|
|
Deep saline formations |
100–10,000 GtC |
|
|
Depleted oil and gas reservoirs |
100–1000 GtC |
|
|
CO2-EOR |
61–123 GtC |
[17] |
|
Coal seams |
10–1000 GtC |
|
|
Organic-rich shales |
2.5–25 GtC |
[18] |
|
Biological CO2 Sequestration: |
||
|
Terrestrial |
10–100 GtC |
[20] |
|
Technological CO2 Sequestration: |
||
|
Direct air capture (DAC) |
<0.1 GtC |
|
* 1 GtC = 1 billion tons of carbon.
2. Components of the CCS System
CCS technology is expected to lower atmospheric concentrations of greenhouse gases. Compressing, transporting, and using the collected CO2 for procedures such as injection into deep underground geological formations for long-term storage, and injection into existing oil fields for further hydrocarbon recovery, are all included in CCS [24]. The CCS system comprises many fundamental components, each of which must be understood in order to fully understand the technologies used in the CCS system. These components include the following: (i) capture, (ii) transport, (iii) injection, and/or (iv) storage, which is also known as “sequestration”, and (v) monitoring [25]. In 2009, Herzog [25] provided a concise description of the component of the CCS system as follows: capturing CO2 from an effluent stream and compressing it to a liquid or supercritical state is known as capture. Currently, the resultant CO2 concentration is more than 99% in the majority of situations; however, lesser amounts may be tolerable. Capture is often necessary in order to be able to transport and store CO2 in an economically viable manner. The transportation of CO2, which is defined as the transportation of CO2 from its source to a storage reservoir, is considered the second component of the CCS system. CO2 can be transported by trucks, rails, and ships; however, the most cost-effective method of moving big volumes is through pipelines. These transportation techniques are regarded as practical, but the sheer amount of CO2 to be carried from the capture site will certainly require the building of local and regional infrastructure for appropriate transportation. This approach will lower the shipping cost substantially while also removing the concerns associated with hydrate crystallization [17]. Furthermore, carbon injection, the fourth CCS component, is defined as the act of injecting CO2 into a storage reservoir. Geological formations are the primary storage reservoirs now under consideration. The final component of the CCS system is associated with monitoring the CO2 once it has been injected into the ground. Despite the fact that CO2 is neither harmful nor combustible, it nonetheless presents a risk to the environment, as well as to health and safety. One of the primary goals of monitoring is to ensure that the CO2 sequestration process is successful, which means that a substantial amount of the CO2 is kept out of the atmosphere for hundreds of years or centuries.
3. Carbon Sequestration in Unconventional Reservoirs
Unconventional reservoirs are most likely abundant, but their nature and distribution are not fully known. They are known to exist in vast quantities, but do not readily flow toward current wells for commercial recovery. Naik [26] added that unconventional reservoirs are less prevalent and less well known than traditional petroleum reservoirs such as sandstone and carbonate, fractured, or tight reservoirs. However, unconventional petroleum reservoirs are becoming an increasingly significant source of petroleum supply. Tight reservoirs do not have natural fissures, yet they are unable to be economically produced without the use of hydraulic fracturing. Unconventional reservoirs include tar, bitumen, and heavy oil reservoirs, as well as coalbed methane, shale, and basin-center gas reservoirs. In order to be economically viable, unconventional reservoirs must depend on evolving exploration tactics and novel production technology. All of these reservoirs are becoming more major contributors to the world’s oil and gas reserves and production as a collective. It is a common perception that unconventional reservoirs, such as fractured and tight reservoirs, are more expensive and riskier than conventional reservoirs. In addition, geologists have discovered that techniques such as regional facies mapping and sequence stratigraphy, which are useful for locating and delineating conventional reservoirs, are often ineffective for locating and delineating fractured, tight, and unconventional reservoirs, according to their findings. Engineers are wary of them because they are difficult to analyze and because recovery strategies must be carefully selected and deployed in order to minimize production difficulties. As a result of recent technological developments, an increasing number of these accumulations are becoming economically viable. Coupling the application of CO2 and storage in unconventional formations could combine the potential to store the CO2 underground and obtain the remaining bypassed oil or gas that has been left behind.
There are a number of CO2-assisted recovery technologies, such as supercritical extraction, CO2 injection via huff and puff, or CO2 flooding, that take advantage of the favorable physical and chemical properties of CO2 under reservoir conditions, where it typically exists above its critical point (31.1 °C, 7.38 mpa). Swelling of the oil phase, as well as the reduction of viscosity and interfacial tension (IFT), contribute to the improved displacement of residual oil that would otherwise remain unrecovered, especially with the high diffusivity, low viscosity, and higher miscibility of supercritical CO2 (sc-CO2) [27]. CO2 preferentially adsorbs on organic matter in coalbeds, resulting in the desorption of CH4 and so boosting methane recovery via increased gas recovery in unconventional organic-rich formations such as coalbeds [28]. In gas shale formations, this method has also been successful [29][30]. As a consequence, the geological storage of CO2 in conjunction with EOR recovery might have the dual advantage of increasing hydrocarbon recovery factors while also reducing greenhouse gas emissions [31].
4. Trapping Mechanisms
The ultimate distribution of CO2 in a reservoir is the result of many factors. Structural or stratigraphic trapping, residual trapping, mobility trapping, and mineral trapping are some of the methods. These mechanisms kick in at various points during the CO2 mitigation process’s overall lifetime. For example, structural trapping is in charge of initial CO2 containment and safe storage. Residual and solubility trapping are critical in the dispersion and migration of the CO2 plume, and they help to accelerate geochemical trapping when the CO2 comes into contact with more rock minerals as it expands outwards in the reservoir layer [32]. When geochemical trapping or mining starts, CO2 will no longer be able to exit the reservoir in any way, and the CO2 geological storage may be considered secure since leakage concerns are reduced [17]. The geological and petrophysical properties of the target formation influence CO2 storage capacity, confinement, and injectivity. The supercritical CO2 injected underground is safely trapped by three key trapping methods: (1) chemical trapping, (2) physicochemical trapping, and (3) physical trapping. The effectiveness of the storage process is decided by a combination of trapping processes to ensure long-term storage [32]. Figure 3 displays a matrix of the different CO2-trapping systems, which is important for understanding how these trapping mechanisms interact. In terms of their mechanisms, the chemical and physical trapping methods have a common element, which includes physicochemical trapping. Hydrodynamic trapping is a type of physicochemical trapping that occurs on both the chemical and physical scales. In the following sections, an in-depth explanation is provided for each kind of trapping mechanism.
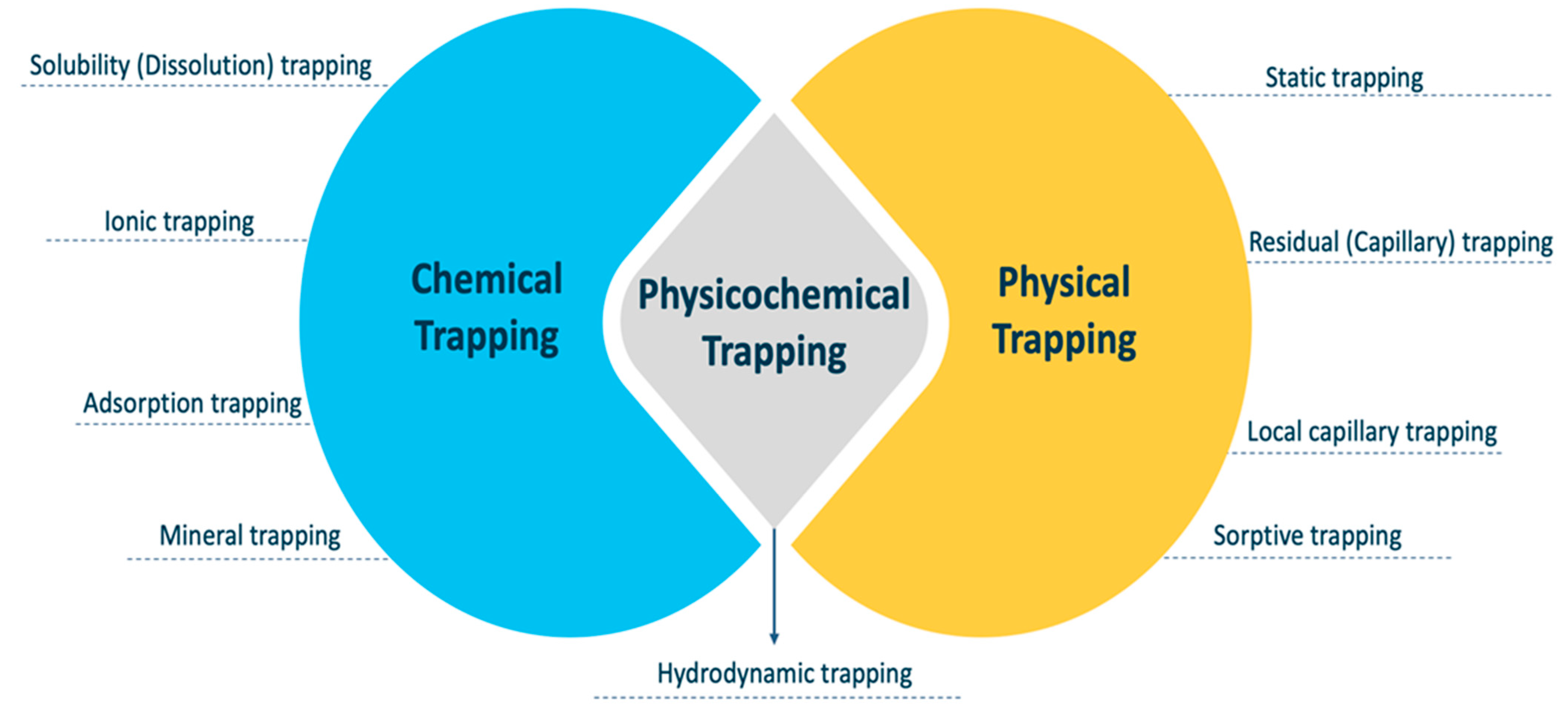
Figure 3. CO2-trapping mechanisms in geological formations.
4.1. Chemical Trapping
Chemical entrapment occurs when CO2 undergoes a sequence of geo-chemical interactions with the formation brine and the rock, causing it to alter its physical and chemical characteristics and to cease to exist in either the mobile or immobile phase. This interaction guarantees that CO2 is no longer present as a distinct phase and boosts storage capacity significantly, making it an appropriate characteristic for long-term storage [24]. There are several trapping mechanisms that fall under geo-chemical trapping, including:
-
Dissolution (solubility) trapping;
-
Ionic trapping;
-
Adsorption trapping;
-
Mineral trapping.
4.2. Physicochemical Trapping
The bridge between the chemical and physical pathways is physicochemical entrapment. It is important to note that the hydrodynamic trapping aspect is present in all of these methods. The process of storing the CO2 via the interaction of all of the many processes that might occur along a migration route is referred to as hydrodynamic trapping [33].
Hydrodynamic Trapping
This trapping mechanism refers to the interaction of various processes when CO2 is injected into the reservoir or saline formations. It may move extremely slowly for a long time before being trapped by residual, solubility, or mineral trapping. It is the link between chemical and physical trapping mechanisms. In saline formations, hydrodynamic trapping may happen even if there is not a definitive closed trap; it only has to be in an area where fluids move extremely slowly over a very long distance. Because CO2 has a lower density than water, it is able to displace the salty formation water when it is injected into a formation. It then migrates upwards buoyantly because it is lighter than the water. When it reaches the top of the formation, it continues moving as a distinct phase until it is either trapped as residual CO2 saturation or in local structural or stratigraphic traps inside the formation that is sealing it. Over the course of a longer period of time, considerable amounts of CO2 will dissolve in the formation water and subsequently move with the groundwater [32]. When there is a large distance between the deep injection site and the end of the overlaying impermeable formation, such as when there are hundreds of kilometers between them, the amount of time it takes for fluid to travel from the deep basin to the surface may be measured in millions of years [34]. Many researchers and academics are of the opinion that hydrodynamic trapping may be placed in either the category of chemical or physical trapping. Nevertheless, it refers to the common bridging point between the systems that were previously discussed. When the CO2 is injected into the reservoirs, it has the potential to linger there for a significant amount of time while only moving extremely slowly. Eventually, it may get trapped due to residual (physical trapping), solubility, or mineral entrapment (chemical trapping).
4.3. Physical Trapping
Physical entrapment is a method in which CO2 retains its physical properties after being injected into an aquifer or reservoir, and the flow of CO2 is impeded by a physical low-permeability barrier. The physical trapping mechanisms may be subdivided into the following [24][35]:
-
Static trapping;
-
Residual (capillary) trapping;
-
Local capillary trapping;
-
Sorption trapping.
References
- Hepple, R.P.; Benson, S.M. Geologic storage of carbon dioxide as a climate change mitigation strategy: Performance requirements and the implications of surface seepage. Environ. Geol. 2005, 47, 576–585.
- Chaves, G. Simulation of CO2 Sequestration in Deep Saline Aquifers; New Mexico Institute of Mining and Technology: Socorro, New Mexico, 2011.
- Lapillonne, B.; Chateau, B.; Criqui, P.; Kitous, A.; Menanteau, P.; Mima, S.; Gusbin, D.; Gilis, S.; Soria, A.; Russ, P.; et al. World Energy Technology Outlook-2050-WETO-H2; No. halshs-00121063; Bruxelles: Luxembourg, 2007; Available online: https://halshs.archives-ouvertes.fr/halshs-00121063 (accessed on 12 April 2021).
- Zhang, B.; Wang, Z.; Wang, B. Energy production, economic growth and CO2 emission: Evidence from Pakistan. Nat. Hazards 2018, 90, 27–50.
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; 151p, ISBN 978-92-9169-143-2.
- Murshed, M.; Alam, R.; Ansarin, A. The environmental Kuznets curve hypothesis for Bangladesh: The importance of natural gas, liquefied petroleum gas, and hydropower consumption. Environ. Sci. Pollut. Res. 2021, 28, 17208–17227.
- Peridas, G.; Schmidt, B.M. The role of carbon capture and storage in the race to carbon neutrality. Electr. J. 2021, 34, 106996.
- Salvi, B.L.; Jindal, S. Recent developments and challenges ahead in carbon capture and sequestration technologies. SN Appl. Sci. 2019, 1, 1–20.
- Querini, F.; Dagostino, S.; Morel, S.; Rousseaux, P. Greenhouse gas emissions of electric vehicles associated with wind and photovoltaic electricity. Energy Procedia 2012, 20, 391–401.
- Rubin, E.; De Coninck, H. IPCC Special Report on Carbon Dioxide Capture and Storage; TNO (2004): Cost Curves for CO2 Storage, Part 2; Cambridge University Press: Cambridge, UK, 2005; p. 14.
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20.
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; Imperial College Press: London, UK, 2014; Volume 1.
- Marchetti, C. On geoengineering and the CO2 problem. Clim. Chang. 1977, 1, 59–68.
- Brewer, P.G. A changing ocean seen with clarity. Proc. Natl. Acad. Sci. USA 2009, 106, 12213–12214.
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175.
- Bachu, S.; Gunter, W.D. Overview of acid-gas injection operations in western Canada. In Greenhouse Gas Control Technologies; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; Volume 7, pp. 443–448.
- Vishal, V.; Singh, T. Geologic carbon sequestration. Environ. Geosci. 2016, 16.
- Carter, K.M.; Harper, J.A.; Schmid, K.W.; Kostelnik, J. Unconventional natural gas resources in Pennsylvania: The backstory of the modern Marcellus Shale play. Environ. Geosci. 2011, 18, 217–257.
- Al Hameli, F.; Suboyin, A.; Al Kobaisi, M.; Rahman, M.M.; Haroun, M. Modeling Fracture Propagation in a Dual-Porosity System: Pseudo-3D-Carter-Dual-Porosity Model. Energies 2022, 15, 6779.
- Sha, Z.; Bai, Y.; Li, R.; Lan, H.; Zhang, X.; Li, J.; Liu, X.; Chang, S.; Xie, Y. The global carbon sink potential of terrestrial vegetation can be increased substantially by optimal land management. Commun. Earth Environ. 2022, 3, 8.
- IEA. CO2 Emissions from Energy Combustion and Industrial Processes, 1900–2021; IEA: Paris, France; Available online: https://www.iea.org/data-and-statistics/charts/co2-emissions-from-energy-combustion-and-industrial-processes-1900-2021 (accessed on 12 April 2021).
- Azarabadi, H.; Lackner, K.S. Postcombustion capture or direct air capture in decarbonizing US natural gas power? Environ. Sci. Technol. 2020, 54, 5102–5111.
- Herzog, H.; Golomb, D. Carbon capture and storage from fossil fuel use. Encycl. Energy 2004, 1, 277–287.
- Ajayi, T.; Gomes, J.S.; Bera, A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Pet. Sci. 2019, 16, 1028–1063.
- Herzog, H. Carbon Dioxide Capture and Storage; Typset by SPi: Chennai, India, 2009; pp. 246–283.
- Naik, G.C. Tight gas reservoirs–an unconventional natural energy source for the future. Accessado Em 2003, 1, 2008.
- Lan, Y.; Yang, Z.; Wang, P.; Yan, Y.; Zhang, L.; Ran, J. A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding. Fuel 2019, 238, 412–424.
- Prusty, B.K. Sorption of methane and CO2 for enhanced coalbed methane recovery and carbon dioxide sequestration. J. Nat. Gas Chem. 2008, 17, 29–38.
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Enhanced gas recovery and CO2 storage in gas shales: A summary review of its status and potential. Energy Procedia 2014, 63, 5849–5857.
- Tao, Z.; Clarens, A. Estimating the carbon sequestration capacity of shale formations using methane production rates. Environ. Sci. Technol. 2013, 47, 11318–11325.
- Liu, J.; Xie, L.; Yao, Y.; Gan, Q.; Zhao, P.; Du, L. Preliminary study of influence factors and estimation model of the enhanced gas recovery stimulated by carbon dioxide utilization in shale. ACS Sustain. Chem. Eng. 2019, 7, 20114–20125.
- Metz, B.; Davidson, O.; De Coninck, H.C.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005.
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443.
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279.
- Jiang, X. A review of physical modelling and numerical simulation of long-term geological storage of CO2. Appl. Energy 2011, 88, 3557–3566.
More
Information
Subjects:
Engineering, Petroleum
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
04 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




