1. Hydrothermal Method
The hydrothermal method is one of the most rapidly and commonly growing methods for the synthesis of metal oxide nanoparticles, especially tin oxide nanoparticles. This method of synthesis has received more attention and increased in significance in the last few years because of certain added advantages such as the ease of controlling reaction conditions (e.g., pressure, temperature, pH), and metal oxides of varied morphologies are produced by altering the reaction conditions. Sun et al.
[1] used a facile one-step hydrothermal route to synthesize SnO
2 nanostructures by calcining precipitates composed of 2D nanosheets with high porosity. The morphological evolution of SnO
2 samples with time and the entire possible growth mechanism were ascribed to the self-assembly and nucleation of building blocks. The final morphology, i.e., random porous structures in the nanosheets of the product, was attributed to the concentration of the precursor. The experimental results reveal a possible growth mechanism for the as-prepared SnO
2 nanostructures. Moreover, it was observed that the SnO
2 nanostructures which were annealed at 600 °C for 2 h showed higher gas sensing properties as compared to the sensor SnO
2 nanoparticles prepared conventionally. This change in sensing was ascribed to the unique flower-like structures, which usually facilitate mass transportation and gas diffusion in sensing materials.
Chiu et al.
[2] developed a hydrothermal method for the synthesis of nanocrystalline SnO
2 nanoparticles with an average grain size of 3.0 ± 0.5 nm. As-synthesized SnO
2 particles were annealed at 300 °C for 1 h under 10% H
2/Ar. An atomic ratio of 2.3/1 (O/Sn) was found for the SnO
2 nanoparticles prepared thermally, and an atomic ratio of 1.2/1 was found for the as-synthesized SnO
2 nanoparticles. For the thermally treated SnO
2, a surface area of about 92 m
2/g was observed as compared to the 130 m
2/g for the SnO
2 nanoparticles synthesized at 150 °C with rough undefined morphology. In the as-synthesized SnO
2, the oxygen ions are replaced by the chloride ions; the thermally treated SnO
2 acts as a better sensor for ethanol compared to the as-synthesized SnO
2. For the as-synthesized SnO
2 sensor, the sensitivity of SnO
2 nanoparticles can be enhanced by heating simply for 5 min at 350 °C to remove Cl
− partly. Consequently, efficient gas sensing in response to ethanol was revealed for the SnO
2 gas sensor with a detection limit of as low as 1.7 ppm.
Suematsu et al.
[3] used a hydrothermal route to synthesize SnO
2 clustered nanoparticles from SnO
2.nH
2O where preformed nanocrystals (ca. 5 nm) agglomerated at pH 9.3 to form large secondary nanocrystals (ca. 45 nm). Adding Pd-[(NH
3)
2(NO
2)
2] to the precursor solution resulted in the formation of Pd-loaded clusters of SnO
2 nanoparticles. Toluene was sensed by the application of highly fabricated films of clustered SnO
2 nanoparticles with spherical morphology. The spin coating method was used to develop highly porous gas sensing films by loading Pd with the clustered SnO
2 particles. An improved response of sensors at 300 °C to H
2 and CO was observed for the sensor devices using the porous films. The sensor response was increased by increasing the film porosity, which enhanced the diffusivity through the sensing films. Note that the sensor response was further increased by loading Pd onto the clustered nanospheres as a result of electrical and catalytic sensitization effects. More importantly, the Pd-loaded SnO
2 nanoparticles showed increased toluene sensitivity due to an increase in gas diffusivity through the sensing film which helped in its detection at very low ppb levels.
Shi et al.
[4] prepared SnO
2 nanotubes on a hard template of polycarbonate by employing a hydrothermal route at low temperatures. The obtained nanostructures with nanotube morphology were found to be a few nanometers in size with fine particles. It was observed that as the reaction temperature increased gradually, the size of the SnO
2 nanostructures also showed an increase. The SnO
2 nanocrystals showed that the band gap of these nanostructures increased from 3.75 eV with a particle size of 5.6 nm to 3.99 eV with a particle size of 3.3 nm. The results showed that the SnO
2 nanotubes can have potential applications for gas sensors with enhanced gas sensitivity.
Xue et al.
[5] employed a simple one-step hydrothermal route and successfully synthesized a highly sensitive Pt@SnO
2 nanorod-based gas sensor. The morphology of synthesized nanoparticles obtained were nanorods with microstructures. At a high temperature of about 300 °C, upon exposure to 200 ppm of ethanol, the sensor sensitivity reached 39.5. This can be attributed to the influence of electrical and chemical contributions of Pt, because of which the sensor displays high gas sensitivity. Further, when the sensor is heated to 200 °C, opposite variations of resistances are observed, which may be attributed to the surface oxygen ions having different temperature-dependence. The results reveal that the synthesized nanostructures are potential sensors with high performance capacity.
Xu et al.
[6] for the first time synthesized Scandia-doped tin oxide powders in the presence of urea by a two-step hydrothermal method (produces weakly agglomerated nanocrystallites) followed by calcination between 500 and 1200 °C. When Sc
2O
3 is added as a dopant in appropriate amounts in nanosized SnO
2, as was revealed by the textural studies, it can retard grain growth and stabilize the surface area to withstand high calcination temperatures below 1000 °C with discrete undefined morphology. The sensing measurements of CO gas reveal that sensitivity of SnO
2 can improve significantly when Sc is incorporated at the surface of the nanocrystal, and at 800 °C, a pellet sample with 10 mol% of Scandia content displays enhanced sensing properties in response to CO in the operating temperature range of 300–400 °C.
Li et al.
[7] synthesized WO
3 and SnO
2 hollow spheres by a simple hydrothermal method followed by calcination. For synthesis, 0.5 mmol of Na
2SnO
3, 5 g of glucose and 1 mmol of Na
2WO
4 were mixed in 50 mL of distilled water. After this, the solution was then transferred into a stainless Teflon-lined autoclave and stirred for several minutes, sealed and heated at a temperature of 200 °C for 20 h. In this approach, extrinsic sensing behaviors with WO
3 and SnO
2 hollow-sphere-based gas sensors were observed. These responses obtained were treated as pseudo-sensing responses, and these results were considered as the interactions between adsorbed water and target gas. With the increase in temperature, the response of the pseudo-p-type sensor to ethanol can be converted into normal n-type as a result of transitions of sensing mechanisms. The WO
3-SnO
2 composites showed an enhanced sensing property which accounts for the extrinsic sensing behaviors. The results reveal that humidity has a counterproductive effect on gas sensing, and they open up a new promising way to produce gas sensors with reduced operation temperature or synthesize gas sensors at room temperature with little power consumption.
2. Polymeric Citrate Precursor Method
Another method used to synthesize metal oxides is a low-temperature polymeric citrate precursor (PCP) method. In this method, a multifunctional organic acid (e.g., malic acid, citric acid) chelates with the metal ion and results in the formation of a stable metal complex along with a diol such as ethylene glycol which is called gel. Random distribution of cations starts occurring in the starting solution as a result of gel formation. When this solution is heated to a high temperature, the organic moieties are removed from the gel component, which results in the formation of highly crystalline, very fine, homogeneous oxide powders at lesser temperatures as compared to other solid-state techniques. Jiang et al.
[8] employed a simple PCP route for the synthesis of SnO
2 microstructures followed by a suitable thermal treatment through a surfactant-assisted and solvent-induced assembly technique. In this method, 1.46 g of H
2C
2O
4 was dispersed into a mixed solution containing 40 mL of polyethylene glycol (PEG-600) and 120 mL of ethanol under constant stirring. Further, 1.78 g of SnCl
2·2H
2O was added to the solution after H
2C
2O
4 was completely dissolved, followed by dropwise addition of 16 mL of deionized water. After centrifugation and stirring, the obtained product was washed with distilled water and ethanol several times. The products obtained were annealed at 200 °C for 2 h to transform them into flower-like SnO
2 nano/microstructure morphology. Gas sensing applications of as-obtained SnO
2 microstructures towards CO and H
2 reveal excellent sensing properties with an extremely low detecting limit (5 ppm) and notable sensitivity with short response/recovery times and good reproducibility, which is ascribed to the unique flower-like structure with three-dimensional geometry of SnO
2 nanostructures and NPs which is considered as an important constituent for improved gas sensing performance.
Hidalgo et al.
[9] demonstrated a simple polymeric precursor method for the synthesis of SnO
2-NiO nanopowders with different compositions. In this method, cationic precursors were added to a citric acid and ethylene glycol solution. Sn
2-(C
6O
7H
4).H
2O (tin citrate was prepared by SnCl
2.H
2O) and Fe(NO
3)
3.9H
2O were used as the precursors, and HNO
3 was added to this system to obtain the desired solubilization of citrate ions in the whole system. To obtain the desired molar concentrations, the appropriate amounts of precursors were calculated. After this, the solution was heated at 180–200 °C to promote the polyesterification between ethylene glycol and citric acid resulting in the formation of a polymer chain with sites available to react with the present ions. Then the liquid precursor was heated at 450 °C for 4 h, and a powder rich in carbon was obtained, further ground and then again heat treated at 500 °C for 5 h to guarantee total carbon elimination from the compound with nearly spherical morphology. In the SnO
2-NiO system, the separation of Ni is used to obtain a rapid sensor response to SO
2. Compared to pure SnO
2, response to SO
2 is enhanced in sensitivity and speed with reliable operation at room temperature for SnO
2 films containing 1 mol% of Ni. The sensor measurements and drift results showed a completely reversible reaction at this composition with an enhanced electrical response at this temperature.
Leite et al.
[10] demonstrated a simple polymeric route for the synthesis of undoped and Nb
2O
5-doped tin oxide. This method is based on the chelation of cations by a hydro carboxylic acid such as citric acid. The citrate solution is then mixed with ethylene glycol through a polyesterification reaction to enhance polymerization. The reaction occurs after the water has been eliminated at a temperature ranging from 90 °C to 120 °C.
Preliminary gas sensing measurements with doped SnO2 and undoped SnO2 thin films were tested for ethanol. The prepared suspensions were deposited on an alumina substrate by spin coating and were then sintered at 500 °C. Gas sensing tests showed that both powders offer a good response with roughly spherical morphology. However, the sensor response time of doped SnO2 was shorter. The preliminary results depict that doped SnO2 has good sensing properties. In other words, Nb2O5 can be used to control particle size during the synthesis process, which will produce a material with good potential applications in sensor technology.
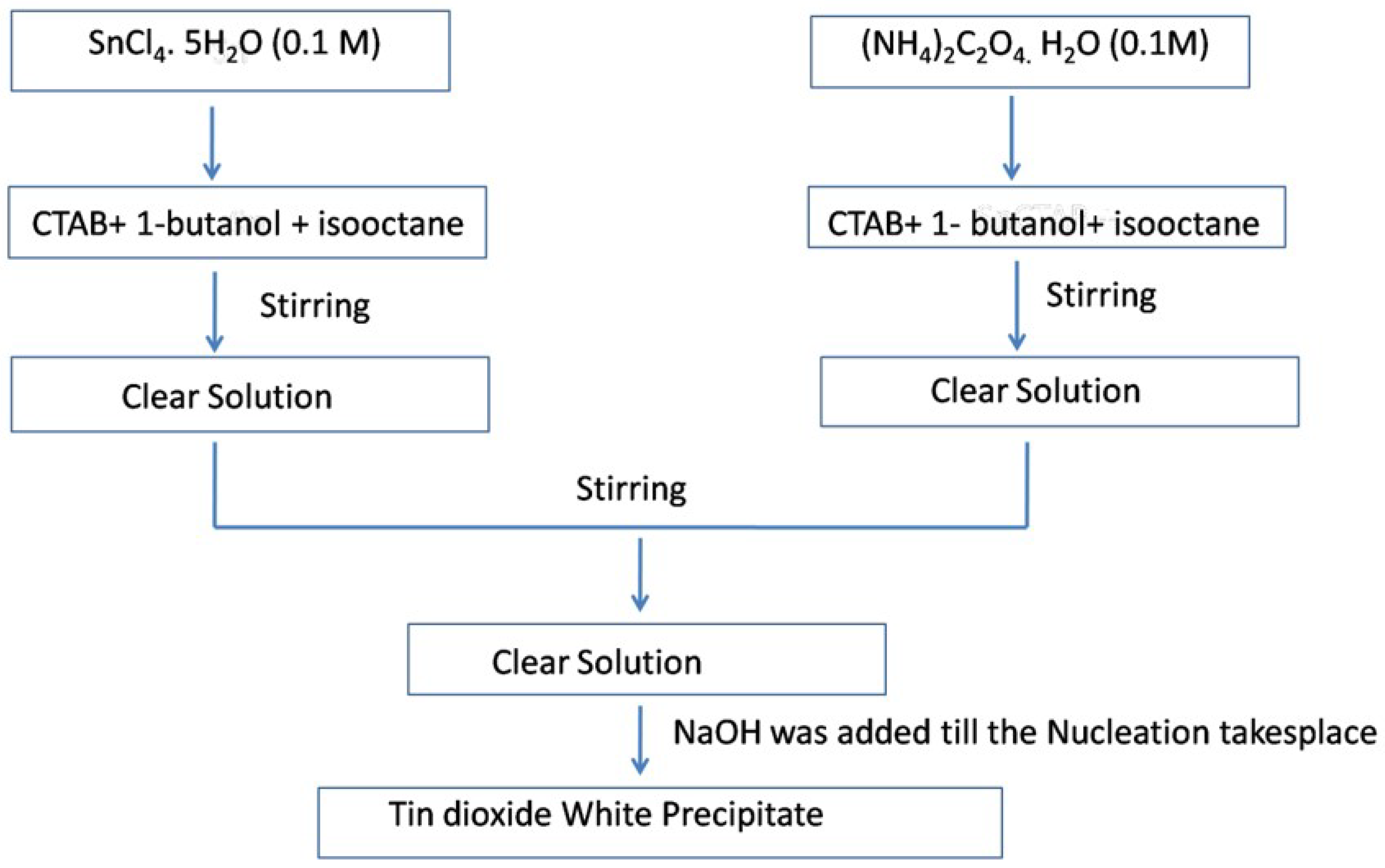
3. Microemulsion or Reverse Micellar Method
The microemulsion method is another significant procedure for the synthesis of metal oxides; in this method, polar and nonpolar solvents of two immiscible liquids are mixed together by the addition of surfactant in a vessel, which results in the formation of an oil-in-water (O/W) microemulsion
[11].
Figure 1a depicts the structure of a microemulsion consisting of a phase encapsulated by a hydrophilic polar head group of a surfactant directed inwards and a chain of long hydrophobic hydrocarbons (which are nonpolar) directed outwards towards the oil phase
[12]. In the reverse micelle or microemulsion method, the polar head which is water soluble forms a water pool content that is characterized by a W
0 ratio, i.e., the concentration of water to the concentration of surfactant. When W
0 ˂ 15, reverse micelles are formed, and when W
0 > 15, a microemulsion is formed. The water pool has a size range of 10–15 nm. These reverse micelles have great control over the shape and size of nanoparticles and thus can act as uniformly sized nanoreactors. The important feature of this method is that different shapes of micro emulsions in the phase diagram can be obtained, and the morphologies of the final material, as shown in
Figure 1b, can be chosen at different positions in the phase diagram below.
Figure 1. (a) Schematic of W/O microemulsion or reverse micelle; (b) mechanistic steps involved in the reverse micellar method.
Ahmed et al.
[13] used the reverse micellar route to synthesize SnO
2 nanoparticles, using CTAB as the surfactant. Monophasic tin oxide nanoparticles with rough undefined morphology were found to be crystalline after heating at 500 °C and using NH
3 as a precipitating agent. Gas sensing measurements of the as-prepared SnO
2 showed enhanced sensitivity towards n-butanol as compared to the other solution phase techniques such as the co-precipitation method used for the preparation of SnO
2 polycrystalline samples. The presence of a high oxygen vacancy level in the nanoparticles resulted in enhanced gas sensitivity, as reported in the literature
[13]. The schematic procedure for the synthesis of SnO
2 nanoparticles using reverse micelles is shown in
Figure 2.
Figure 2. Flow chart for the synthesis of SnO2 nanoparticles at 500 °C.
Gyger et al.
[14] synthesized SnO
2 nanoparticles by a simple microemulsion method. The preparation of samples was performed via water-in-oil microemulsion (w/o) by mixing 5 mL of hexanol as a co-surfactant and 1.82 gm CTAB as a surfactant in 50 mL of n-dodecane as the nonpolar phase, and further, demineralized water and 2 mL of a 5:1 mixture of methanol were added. In addition, 10 mL of a 0.01 M solution of Sn(Ot-Bu)
4 (ABCR, 99.99%) in dodecane was added dropwise to this micellar system, and the reaction mixture was left for 12 h to react. This resulted in the hydrolysis at the liquid–liquid phase boundary of the tin alcoholate, due to which polar-phase hollow spheres were established. The reaction was concluded by adding 20 mL of ethylene glycol. The resulting colorless precipitate was washed several times with ethanol followed by centrifugation, and the colorless SnO
2 nanoparticles were obtained. The nanoparticles obtained with hollow spherical morphology revealed a good sensor response to CO in a concentration range of 50 to 300 ppm that is relevant to the sensing applications. Zhang et al.
[15] employed a reverse-micelle-mediated solution route for the synthesis of SnO
2 one-dimensional nanocrystals. A typical assembly-mediated process was employed for the formation of SnO
2 hollow spheres. The crystal habit of the SnO
2 rutile phase and the reverse micelle nature are believed to be responsible for the growth behavior of 1-D SnO
2 nanocrystals. The temperature range of 220–240 °C resulted in the growth and formation of the morphology of SnO
2 nanowires, while the morphology of nanorods was obtained when the temperature of the reaction was below 220 °C. The aggregates of SnO
2 nanocrystals between dendrites and hollow spheres were regarded as the intermediates for the nanowires. The CO gas sensing measurements revealed that the hollow-structured products had a good sensitivity and stability. Compared with SnO
2 nanorod dendrites and nanowires, the specific surface area is believed to be the dominating factor for enhanced sensing activity. Liangyuan et al.
[16] synthesized nanocrystalline ZnO-SnO
2 nanocomposites as gas sensor materials by successfully employing a microemulsion synthesis route for controlled morphology and grain size. In this method, a water-in-oil microemulsion that contained a maximal amount of water and a minimal amount of surfactant was created. Then CTAB, n-octane, water and n-pentanol were taken in appropriate amounts to form a solution, and the ratio of water or CTAB to alcohol, the precursor salt concentration and the effects of the alcohol chain length on the stability and formation of microemulsions were studied. The morphology of the synthesized nanoparticles was spherical. The performance of the obtained nanocomposites was characterized for gas sensing measurements.
4. Sol–Gel Method
A sol is a colloidal suspension of a particle in a liquid. In a typical sol–gel process, hydrolysis reactions of a precursor form a colloidal suspension which includes metal–organic compounds such as metal alkoxides or generally inorganic metal salts. The sol–gel process involves the homogeneous solution of one or more selected alkoxides as the starting material because metal alkoxides on hydrolysis give oxide as the colloidal product which remains in the suspension rather than precipitation. In a sol–gel method, the formation of a concentrated suspension, called sol, of a metallic hydroxide is evaporated by dehydration and results in the formation of a semi-solid mass called gel. A varied number of mixed and pure oxides can be obtained by controlled heating of a gelatin material. This method has good control over the particle size and gel. The sol is heated to form gel, and gel on calcination gives the final product.
Zhang et al.
[17] used granulated tin to synthesize nanocrystalline SnO
2 particles by a simple sol–gel method. During the process, the granulated tin in HNO
3 solution obtained by dissolution is mixed with the citric acid which acts as a stabilizer and slows down the process of condensation and hydrolysis. SnO
2 nanocrystals obtained ranged from 2.8 to 5.1 nm in size and had a specific surface area ranging from 289 to 143 m
2g
−1 when different heating temperatures were employed. The obtained nanocrystallites displayed a reduction in particle size as well as lattice expansion. Further, the precursor condenses and hydrolyzes in an uncontrolled manner in the absence of citric acid, which results in the formation of larger and broader nanocrystals with roughly spherical morphology. This route can be employed to synthesize tin oxide doped nanocrystallites and also serves beneficial purposes in fields such as electronics and engineering and, above all, gas sensing. Rella et al.
[18] used a sol–gel technique to synthesize SnO
2-based thin films. During the process, thin films based on Pd-doped SnO
2 and undoped SnO
2 were prepared. Sensing measurements revealed that the sensitivity of the sensor towards CO is increased by the palladium doping, along with a decrease in the sensitivity maximum temperature. The surface area of SnO
2 is enhanced by Pd, which also helps in catalyzing the oxidation of CO.