
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karolina Szewczyk-Golec | -- | 1446 | 2022-11-02 11:50:17 | | | |
| 2 | Peter Tang | + 5 word(s) | 1451 | 2022-11-03 02:48:34 | | |
Video Upload Options
More and more data indicate the participation of inflammation in carcinogenesis, as evidenced by the formation of neoplasms at the site of infection, irritation, or chronic inflammation. In the tumor microenvironment, apart from neoplastic cells, the presence of immune cells, fibroblasts, endothelial cells, growth factors, pro-angiogenic factors, as well as cytokines and chemokines has been demonstrated. Components within the tumor may predispose to the neutralization of malignant tumor cells, but as a result of oncogenic evolution, transformed cells may escape from the control of the immune system and then antitumor activity is suppressed. If the elimination of tumor cells by the immune system is no longer possible, cells and factors forming the tumor microenvironment can help tumor cells to survive and avoid apoptosis, induce angiogenesis, as well as stimulate metastasis. Intensive production of reactive oxygen and nitrogen species, which is enhanced by immune cells infiltrating the inflammatory focus, may damage normal tissues and, consequently, initiate compensatory cell proliferation. These processes can lead to the duplication and accumulation of DNA damage, gene mutations and the stimulation of internal and external carcinogens. DNA damage can also be caused by cytokines, for example interleukins 22 (IL-22), which activates the response to abnormal DNA by regulating the expression of numerous genes.
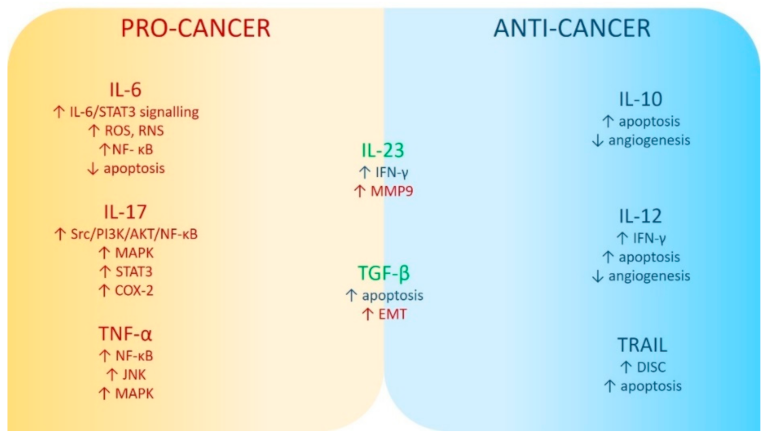
1. Introduction
2. Interleukin 6
3. Tumor Necrosis Factor Alpha
4. Interleukin 17
5. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand
6. Interleukin 10
7. Interleukin 12
8. Transforming Growth Factor β
References
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41.
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899.
- Huang, S. Regulation of Metastases by Signal Transducer and Activator of Transcription 3 Signaling Pathway: Clinical Implications. Clin. Cancer Res. 2007, 13, 1362–1366.
- Naugler, W.E.; Karin, M. NF-ΚB and Cancer—Identifying Targets and Mechanisms. Curr. Opin. Genet. Dev. 2008, 18, 19–26.
- Lin, W.-W.; Karin, M. A Cytokine-Mediated Link between Innate Immunity, Inflammation, and Cancer. J. Clin. Investig. 2007, 117, 1175–1183.
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398.
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, And Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295.
- Ting, E.Y.-C.; Yang, A.C.; Tsai, S.-J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194.
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of Interleukin-6 in Cancer Progression and Therapeutic Resistance. Tumor Biol. 2016, 37, 11553–11572.
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148.
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100.
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 1–19.
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.; Vallabhapurapu, S. Article IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113.
- Sethi, G. TNF: A Master Switch for Inflammation to Cancer. Front. Biosci. 2008, 13, 5094–5107.
- Wang, X.; Lin, Y. Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288.
- Lubecka-Macura, A.; Kohut, M. TNF Superfamily—Mechanisms of Action, Biologic Funtions and Therapeutic Possibilities. Gastroenterol. Rev. 2010, 6, 303–309.
- Kim, J.J.; Lee, S.B.; Park, J.K.; Yoo, Y.D. TNF-α-Induced ROS Production Triggering Apoptosis Is Directly Linked to Romo1 and Bcl-XL. Cell Death Differ. 2010, 17, 1420–1434.
- Song, Y.; Yang, M.; Zhang, H.; Sun, Y.; Tao, Y.; Li, H.; Zhang, J.; Li, Y.; Yang, J. IL-17 Affects the Progression, Metastasis, and Recurrence of Laryngeal Cancer via the Inhibition of Apoptosis through Activation of the PI3K/AKT/FAS/FASL Pathways. J. Immunol. Res. 2020, 2020, 2953191.
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The Role of Interleukin-17 in Tumor Development and Progression. J. Exp. Med. 2020, 217, e20190297.
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s Path in the Immune System. Immunology 2009, 127, 145–154.
- Thapa, B.; Remant, K.C.; Uludağ, H. TRAIL Therapy and Prospective Developments for Cancer Treatment. J. Control. Release 2020, 326, 335–349.
- Wong, S.H.M.; Kong, W.Y.; Fang, C.-M.; Loh, H.-S.; Chuah, L.-H.; Abdullah, S.; Ngai, S.C. The TRAIL to Cancer Therapy: Hindrances and Potential Solutions. Crit. Rev. Oncol. Hematol. 2019, 143, 81–94.
- LeBlanc, H.N.; Ashkenazi, A. Apo2L/TRAIL and Its Death and Decoy Receptors. Cell Death Differ. 2003, 10, 66–75.
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs Less Travelled: TRAIL in Cancer Biology and Therapy. Nat. Rev. Cancer 2017, 17, 352–366.
- Gonzalez-Garza, M.T.; Cruz-Vega, D.E.; Maldonado-Bernal, C. IL10 as Cancer Biomarker. In Translational Research in Cancer; IntechOpen: London, UK, 2021.
- Qiao, J.; Liu, Z.; Dong, C.; Luan, Y.; Zhang, A.; Moore, C.; Fu, K.; Peng, J.; Wang, Y.; Ren, Z.; et al. Targeting Tumors with IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis. Cancer Cell 2019, 35, 901–915.
- Kundu, N.; Fulton, A.M. Interleukin-10 Inhibits Tumor Metastasis, Downregulates MHC Class I, and Enhances NK Lysis. Cell. Immunol. 1997, 180, 55–61.
- Oft, M. IL-10: Master Switch from Tumor-Promoting Inflammation to Antitumor Immunity. Cancer Immunol. Res. 2014, 2, 194–199.
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597.
- Trinchieri, G. Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nat. Rev. Immunol. 2003, 3, 133–146.
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167.
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-Beta Signaling in Cancer Treatment. Curr. Pharm. Des. 2014, 20, 2934–2947.
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172.
- Peinado, H.; Quintanilla, M.; Cano, A. Transforming Growth Factor β-1 Induces Snail Transcription Factor in Epithelial Cell Lines. Mechanisms for Epithelial Mesenchymal Transitions. J. Biol. Chem. 2003, 278, 21113–21123.
- Hao, Y.; Baker, D.; ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767.
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and Molecular Basis for the Regulation of Inflammation by TGF-β. J. Biochem. 2010, 147, 781–792.





