More and more data indicate the participation of inflammation in carcinogenesis, as evidenced by the formation of neoplasms at the site of infection, irritation, or chronic inflammation. In the tumor microenvironment, apart from neoplastic cells, the presence of immune cells, fibroblasts, endothelial cells, growth factors, pro-angiogenic factors, as well as cytokines and chemokines has been demonstrated. Components within the tumor may predispose to the neutralization of malignant tumor cells, but as a result of oncogenic evolution, transformed cells may escape from the control of the immune system and then antitumor activity is suppressed. If the elimination of tumor cells by the immune system is no longer possible, cells and factors forming the tumor microenvironment can help tumor cells to survive and avoid apoptosis, induce angiogenesis, as well as stimulate metastasis. Intensive production of reactive oxygen and nitrogen species, which is enhanced by immune cells infiltrating the inflammatory focus, may damage normal tissues and, consequently, initiate compensatory cell proliferation. These processes can lead to the duplication and accumulation of DNA damage, gene mutations and the stimulation of internal and external carcinogens. DNA damage can also be caused by cytokines, for example IL-22, which activates the response to abnormal DNA by regulating the expression of numerous genes.
1. Introduction
Every inflammatory factor has specific functions in the stages of neoplastic tumor development, namely at the stage of initiation, promotion, progression and metastasis [
80]. The most important association between cancer and inflammation is related to the activity of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB), the signal transducer 3 (STAT3) pathway and an activator of transcription [
81]. These signaling pathways control genes necessary for angiogenesis and affect the ability of cancer cells to metastasize [
82,
83]. Cytokines released by inflammatory, immune, and neoplastic cells may have pro-cancer, but also anti-tumor effects, depending on their profile [
4]. TNF-α, IL-6, and IL-17 primarily accelerate tumor progression, while cytokines normally leading to the tumor suppression include, among others, pro-apoptotic ligand inducing apoptosis associated with TNF (TRAIL, TNF-related apoptosis-inducing ligand), anti-inflammatory IL-10 and IL-12, activating cytotoxic T lymphocytes and NK cells, and regulating the expression of cytotoxic mediators [
4,
81]. Transforming growth factor beta (TGF-β) and IL-23 may play a role in both promoting tumor progression and suppressing tumor [
4].
2. Interleukin 6
IL-6 is secreted mainly by monocytes, and also by macrophages, Kupffer cells, keratinocytes, endothelial cells, B and T cells, and cancer cells [
84]. Increased production of IL-6 is the result of infection or tissue damage, and it performs its functions by binding to the IL-6 receptor [
84,
85]. This cytokine has pleiotropic effects including acute phase protein production, hematopoiesis, osteoclast activation, proliferation, and differentiation of B lymphocytes to produce antibodies [
86]. Pro-tumor activity has been shown in mouse plasmacytoma and human myeloma, in cancer of the lung, breast, colon, prostate, ovary, pancreas, lung, cervix, and renal cell carcinoma [
87]. IL-6 enhances its action promoting the survival of neoplastic cells by activating IL-6/STAT3 signaling [
88]. It is of major importance in promoting tumor growth, it induces the release of reactive oxygen and nitrogen species, and is a suppressor of apoptosis [
79,
84,
89]. IL-6 enhances the proliferation of colorectal cancer cell lines in vitro, resulting in an increase in NF-kB and STAT3, which induce colorectal cancer cell growth. Inhibition of STAT3 in intestinal epithelial cells effectively stopped tumor induction and growth [
84,
90].
3. Tumor Necrosis Factor Alpha
TNF-α, released mainly by monocytes and lymphocytes is a multi-directional cytokine [
91]. As a pro-inflammatory cytokine, it may be involved in inflammatory-related cancer induction, survival, proliferation, differentiation, and even in cell death [
92]. By inducing genes encoding nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) dependent anti-apoptotic molecules, it promotes the survival of cancer cells. By permanently activating the N-terminal c-JUN (JNK)-dependent signaling pathway through stimulation of the JNK-phosphorylating kinase complex, mitogen-activated protein kinases (MAPKs) contribute to cell death [
93]. In addition, the pro-apoptotic function of TNF-α is enhanced by the production of reactive oxygen and nitrogen species [
94]. It has been suggested that the properties of TNF contributing to the death of neoplastic cells may constitute a potential cancer therapy [
92].
4. Interleukin 17
IL-17 is a potent pro-inflammatory cytokine released by T lymphocytes, type 3 innate lymphoid cells, δγT lymphocytes, and NK cells [
95]. IL-17 may participate in both the early and late stages of cancer development and in its growth, as well as it may contribute to the initiation of metastatic processes [
96]. It is an important pro-inflammatory cytokine enhancing the release of numerous pro-inflammatory cytokines and chemokines (TNF-α, IL-6, IL-1β) and thus intensifying inflammation [
4]. IL-17 activates the Src/PI3K/AKT/NF-κB, MAPK, STAT3, and Cox-2 pathways that play a significant role in oncogenesis, angiogenesis, and metastasis. Additionally, it induces tumor growth and angiogenesis [
95,
96].
5. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand
TRAIL can be expressed by activated T lymphocytes, NK cells, dendritic cells, and macrophages [
97]. It is a molecule that belongs to the TNF superfamily and binds to cell surface receptors, namely DR4 (TRAILR-1) and DR5 (TRAIL-2) [
98]. Forming a death-inducing signaling complex (DISC), it triggers the apoptotic cascade independently from p53 [
99]. It is a powerful anti-cancer agent, inducing apoptosis in various types of cancer cells, while having low impact on healthy cells [
100]. However, it is not a perfect anticancer drug because malignant neoplasms develop mechanisms of resistance to apoptosis, which significantly hinders effective therapy [
99,
101].
6. Interleukin 10
IL-10 is an immunosuppressive cytokine with a strong anti-inflammatory effect, produced mainly by regulatory T cells, helper T cells, as well as monocytes, mast cells, activated T, and B lymphocytes [
102]. Lack of IL-10 is associated with the occurrence of autoimmune and inflammatory diseases, as confirmed by experimental studies in the model of IL-10 deficient mice, in which spontaneously developed enteritis and administration of recombinant IL-10 showed therapeutic efficacy [
103]. IL-10 inhibits tumor growth and progression, modulates apoptosis, and inhibits angiogenesis during tumor regression [
104]. However, it is possible that this cytokine plays a dual role in oncogenesis. At the time of tumor formation, it stimulates the death of cancer cells, but for cancer cells that have escaped from the control of the host’s immune system, IL-10 may be a strong cancer promoter [
105]. Activation of the STAT3 pathway and induction of anti-apoptotic genes play a role in the proliferation and survival of neoplastic cells [
4].
7. Interleukin 12
IL-12 is a potent, pro-inflammatory cytokine released by antigen presenting cells, usually in response to microbial pathogens [
106]. IL-12 receptors are mainly expressed on CD8 + T cells, NK cells, and NKT cells and they significantly affect the activity of this cytokine [
107]. L-12 is responsible for a wide variety of functions, such as induction and enhancement of cellular immunity, induction, and differentiation of TH1 cells, enhancement of T and NK cell activation and cytotoxic capacity, inhibition of tumor-associated immunosuppressive cells, and myeloid suppressor cells [
108]. IL-12 shows its anti-tumor activity also by increasing the production of interferon γ (IFN-γ), which is an anti-angiogenic, cytostatic, and cytotoxic molecule; it can also induce apoptosis of cancer cells and control tumor growth [
106,
108].
8. Transforming Growth Factor β
Transforming growth factor β (TGF-β) is produced mainly by macrophages and neutrophils. Like IL-6 and TNF-α, it is involved in cancer stromal activation, immune escape, angiogenesis, migration, and differentiation of many cell types and inhibition of apoptotic pathways [
109,
110]. TGF-β acts as a potent tumor suppressor in normal and precancerous cells by inhibiting the G1 phase of the cell cycle, inducing apoptosis in early cancer and autophagy in some cancer cells. In the later stages of cancer, when tumor cells have acquired oncogenic mutations and/or have lost gene suppressor function and have become resistant to TGF-β-induced growth arrest, this cytokine leads to tumor promotion by inducing epithelial–mesenchymal transition (EMT) [
111,
112]. The primary signaling pathway of TGF-β is the binding of the cytokine to the TGF-βR receptor (TGF-βRI or TGF-βRII) and subsequent activation of the transcription factors Smad. There are other mechanisms of action of TGF-β, e.g., it induces rapid activation of extracellular kinase regulated by Ras signals (Erk), acts through TGF-β-activated kinases (MAPK), namely 4-c-Jun kinase N-terminal (TAK1-MKK4-JNK), TAK1-MKK3/6-p38, Rho-Rac-cdc42, and the signaling pathway of phosphatidylinositol 3-kinase (PI3K) and Akt protein kinase [
113].
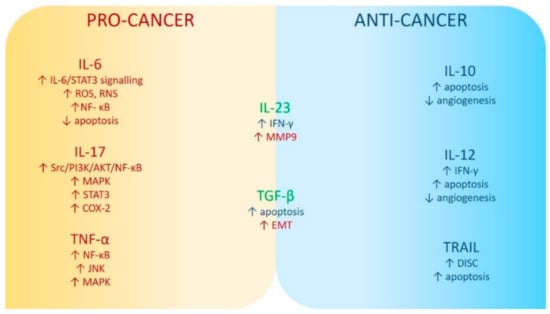
Figure 1 summarizes possible pro- and anti-carcinogenic mechanisms of selected inflammatory factors.
Figure 1. Mechanisms of pro- and anti-carcinogenic activity of selected inflammatory factors. Abbreviations used: Akt (PKB): protein kinase B; COX2: cyclooxygenase 2; DISC: death-inducing signaling complex; EMT: epithelial–mesenchymal transition; IL-6: interleukin 6; IL-17: interleukin 17; IL-23: interleukin 23; IFN-γ: interferon-gamma; JNK: c-Jun N-terminal kinases; MAPK: mitogen-activated protein kinases; MMP9: matrix metallopeptidase 9; NF-кB: nuclear factor kappa B; PI3K: phosphatidilinositol 3-kinases; RNS: reactive nitrogen species; ROS: reactive oxygen species; Src (short from sarcoma): proto-oncogene tyrosine–protein kinase; STAT3: signal transducer and activator of transcription 3; TNF-α: tumor necrosis factor α. The upwards arrow indicates an increase in the parameter’s level; the downwards arrow indicates a decrease in the parameter’s level.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102660