Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Inês Alexandra Marques | -- | 1174 | 2022-11-02 11:12:13 | | | |
| 2 | Peter Tang | -58 word(s) | 1116 | 2022-11-03 02:37:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marques, I.A.; Fernandes, C.; Tavares, N.T.; Pires, A.S.; Abrantes, A.M.; Botelho, M.F. Magnetic-Based Three-Dimensional Cell Culture Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/32547 (accessed on 13 January 2026).
Marques IA, Fernandes C, Tavares NT, Pires AS, Abrantes AM, Botelho MF. Magnetic-Based Three-Dimensional Cell Culture Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/32547. Accessed January 13, 2026.
Marques, Inês Alexandra, Carolina Fernandes, Nuno Tiago Tavares, Ana Salomé Pires, Ana Margarida Abrantes, Maria Filomena Botelho. "Magnetic-Based Three-Dimensional Cell Culture Technology" Encyclopedia, https://encyclopedia.pub/entry/32547 (accessed January 13, 2026).
Marques, I.A., Fernandes, C., Tavares, N.T., Pires, A.S., Abrantes, A.M., & Botelho, M.F. (2022, November 02). Magnetic-Based Three-Dimensional Cell Culture Technology. In Encyclopedia. https://encyclopedia.pub/entry/32547
Marques, Inês Alexandra, et al. "Magnetic-Based Three-Dimensional Cell Culture Technology." Encyclopedia. Web. 02 November, 2022.
Copy Citation
Cell-based assays, conducted on monolayer (2D) cultured cells, are an unquestionably valuable tool for biomedical research. However, three-dimensional (3D) cell culture models have gained relevance due to the advantages of better mimicking the microenvironment and tissue microarchitecture in vivo. Magnetic-based 3D (m3D) cell culture systems can be used for this purpose. These systems are based on exposing magnetized cells to magnetic fields by levitation, bioprinting, or ring formation to promote cell aggregation into 3D structures.
3D cell culture
magnetic nanoparticles
spheroid
magnetic levitation
1. Introduction
Pre-clinical cell-based assays have been a fundamental tool for biomedical, pharmaceutical, and biotechnology research, namely for the development of new diagnostic methods, drug discovery and screening, disease study, tissue engineering, and regenerative medicine, among others [1][2]. The use of cell-based assays allows us to minimize the extensive, expensive, and ethical-related issues associated with the use of animal models for research purposes [2]. So far, most of these assays are still widely based on bidimensional (2D) cell culture models, considering the low cost, simplicity, and reproducibility [3][4]. The standard process for drug development starts by testing the drug in a 2D cell culture model followed by animal testing and lastly, the clinical trials phase. However, about 90% of new drugs fail in clinical trials, mostly due to the ineffectiveness of existing preclinical tests in representing the complex and natural human microenvironment and, consequently, in predicting the human biological response to molecules [5].
2. Three-Dimensional (3D) Cell Culture Systems
Three-dimensional (3D) cell culture models are an emerging area with several benefits and advantages to overcome the aforementioned 2D cell culture limitations. Generally, 3D cultures allow for conducting cancer research and drug screening in a microarchitecture that is more similar to human tissue. The genetic analysis of samples allowed us to establish a greater correlation between the profiles of human tissues and 3D cell cultures, compared to animal models [4]. Thus, 3D cell cultures present several advantages: (i) a microenvironment and microarchitecture similar to in vivo, presenting more biological and physiological relevancy; (ii) a complex structure with several cell–cell and cell–extracellular matrix (ECM) interactions, closely mimicking the intracellular communications; and (iii) the possibility to have different access to nutrients and oxygen as occurring in human tissues. All these attributes make the 3D systems a good simulator both to move forward in translational preclinical research, as well as to allow reduction in the use of animal models [6][7].
The term 3D culture has been widely used in the bibliography to describe several types of 3D structures. Diverse sources can be used for 3D cultures such as cell lines, stem cells, primary tissues, and embryonic whole organs, among others. Based on cellular origin, aggregate morphology, and culture methods, Weiswald and colleagues proposed a classification of 3D structures into four distinct classes: multicellular tumor spheroids, tumorspheres, tissue-derived tumorspheres, and organotypic multicellular spheroids. However, the same terminology is still widely used for different groups, such as the use of the term spheroid to refer either to multicellular spheroids or to tumorspheres [8]. Spheroids are derived from primary sources or cell lines and do not have the ability to differentiate or self-organize, allowing for an easy distinction between them and the organoids. Therefore, spheroids have the advantage of allowing the removal or addition of cell types and elements relevant to the type of investigation to be carried out. Organotypic multicellular spheroids, often called organoids, are derived from biopsies or tissues and have the capability to self-organize into differentiated and complex structures, capable of recapitulating some physiological functions similar to the source. Considering the diversity of cell types that constitute the spheroids, they can be classified as homotypic or heterotypic, also known as co-cultures, if they are composed of only one or more than one cell type, respectively [6][9].
Several 3D cell models have been proposed, such as the hanging drop technique, the use of scaffolds, hydrogels or cell sheets, microfluid systems, rotatory and bioreactor systems, and, more recently, the magnetic-based 3D (m3D) cell culture [2][6][10][11][12]. Cell cultures in 3D structures mimic better the in vivo cellular microenvironment, namely the cell–cell and cell–ECM interactions, and their morphological, physiological, and transcriptional responses. Thus, the 3D cultures emerge as a bridge from conventional 2D cell cultures to in vivo experiments and human clinical trials, allowing for the reduction in animal experiments [1][13]. The 3D cultures also provide a more proper model for cell growth, mimicking in vivo signaling pathways, gene expression, molecular mechanisms, and 3D structure [14].
3. Magnetic-Based 3D (m3D) Cell Culture Technology
This magnetic-based technology consists of incubating cells in 2D apparatus with magnetic nanoparticles (MNP), named NanoShuttleTM-PL, composed of a mixture of iron oxide, gold, and poly-L-lysine nanoparticles. These MNP will bind electrostatically and non-specifically to the cell membrane to magnetize them. Then, magnetized cells are enzymatically dissociated and transferred to a 3D apparatus, according to the methodology described for each m3D method. Then, cells will be exposed to magnetic fields generated by neodymium magnets to induce them to aggregate into 3D structures [1].
Currently, there are three types of magnetic-based models available, presented in Figure 1, all starting from the magnetization of the cells (Figure 1A) to create different final structures by levitation, bioprinting, or ring formation. The m3D levitation method (Figure 1B) consists of seeding the cells and then placing the magnets atop the plates to promote the levitation of magnetized cells in a liquid–air interface where they will aggregate into 3D structures by cell–cell and cell–extracellular matrix interactions [1][15]. The m3D bioprinting method (Figure 1C) consists of placing the magnets under the plates, where magnetized cells were seeded, to promote cell aggregation and matrix formation by printing 3D structures at the bottom of each well. The most recent m3D method, ring formation (Figure 1D), consists of firstly levitating the magnetized cells to allow for the formation of aggregates with ECM, followed by a second step to disintegrate these 3D cultures into dispersed cells, and, lastly, placing these plates atop a magnetic unit with ring-shaped neodymium magnets to induce cell aggregation in a toroidal shape [1]. These magnetic-based techniques are an easy procedure to implement and standardize using diverse cell types, allowing for a fast and consistent spheroid formation as well as a controlled cellular movement and aggregation [1][13][15] Moreover, since spheroids are able to produce their own endogenous ECM during their formation and aggregation process, there is no need to use an artificial matrix [1][15].
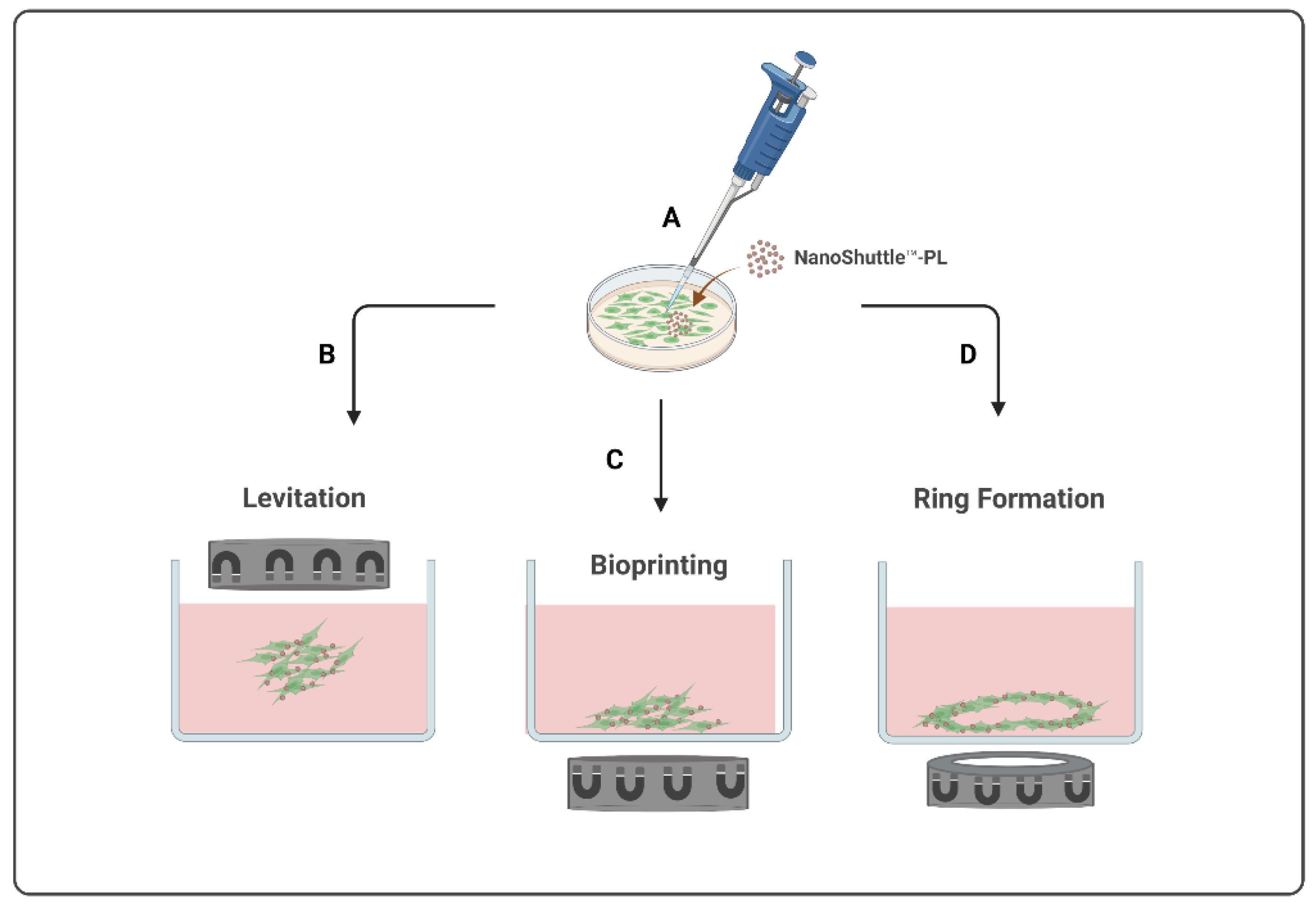
Figure 1. Schematic illustration of magnetic-based 3D (m3D) systems to develop 3D structures. This method starts with the magnetization of the cells (A) to generate 3D structures by levitation (B), bioprinting (C), or by ring formation (D).
These advantages have driven the development of studies using m3D-based cultures over the years for a variety of research purposes [16].
References
- Caleffi, J.T.; Aal, M.C.E.; Gallindo, H.D.O.M.; Caxali, G.H.; Crulhas, B.P.; Ribeiro, A.O.; Souza, G.R.; Delella, F.K. Magnetic 3D Cell Culture: State of the Art and Current Advances. Life Sci. 2021, 286, 120028.
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 1–15.
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226.
- Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Could 3D Models of Cancer Enhance Drug Screening? Biomaterials 2020, 232, 119744.
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218.
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190.
- Lee, K.-H.; Kim, T.-H. Recent Advances in Multicellular Tumor Spheroid Generation for Drug Screening. Biosensors 2021, 11, 445.
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15.
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 1–14.
- Chen, M.Y.; Skewes, J.; Desselle, M.; Wong, C.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Current Applications of Three-Dimensional Printing in Urology. BJU Int. 2020, 125, 17–27.
- Shokoohmand, A.; Ren, J.; Baldwin, J.; Atack, A.; Shafiee, A.; Theodoropoulos, C.; Wille, M.-L.; Tran, P.A.; Bray, L.J.; Smith, D.; et al. Translational Models of Prostate Cancer Bone Metastasis. Biomaterials 2019, 7, 17535.
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as in Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186.
- Gaitán-Salvatella, I.; López-Villegas, E.O.; González-Alva, P.; Susate-Olmos, F.; Álvarez-Pérez, M.A. Case Report: Formation of 3D Osteoblast Spheroid Under Magnetic Levitation for Bone Tissue Engineering. Front. Mol. Biosci. 2021, 8, 672518.
- Sebők, C.; Tráj, P.; Vörösházi, J.; Mackei, M.; Papp, M.; Gálfi, P.; Neogrády, Z.; Mátis, G. Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures. Cells 2021, 10, 1910.
- Haisler, W.L.; Timm, D.M.; Gage, J.A.; Tseng, H.; Killian, T.C.; Souza, G.R. Three-Dimensional Cell Culturing by Magnetic Levitation. Nat. Protoc. 2013, 8, 1940–1949.
- Natânia de Souza-Araújo, C.; Rodrigues Tonetti, C.; Cardoso, M.R.; Lucci de Angelo Andrade, L.A.; Fernandes da Silva, R.; Romani Fernandes, L.G.; Guimarães, F. Three-Dimensional Cell Culture Based on Magnetic Fields to Assemble Low-Grade Ovarian Carcinoma Cell Aggregates Containing Lymphocytes. Cells 2020, 9, 635.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
841
Revisions:
2 times
(View History)
Update Date:
03 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




