
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mustapha Benkhalifa | -- | 1751 | 2022-11-02 09:53:27 | | | |
| 2 | Vivi Li | -1 word(s) | 1750 | 2022-11-03 02:28:47 | | |
Video Upload Options
After more than four decades of assisted reproductive technology (ART) practice worldwide, more than 60% of women undergoing in vitro fertilization (IVF) treatments fail to become pregnant after the first embryo transfer and nearly 20% of patients are suffering from unexplained recurrent implantation failures (RIFs) and repeated pregnancy loss (RPL). The literature reported different causes of RIF–RPL, mainly multifactorial, endometrial and idiopathic. RIF remains a black box because of the complicated categorization and causes of this physio-pathological dysregulation of implantation and pregnancy process after ovarian stimulation. Many options were suggested as solutions to treat RIF–RPL with controversial results on their usefulness.
1. Introduction
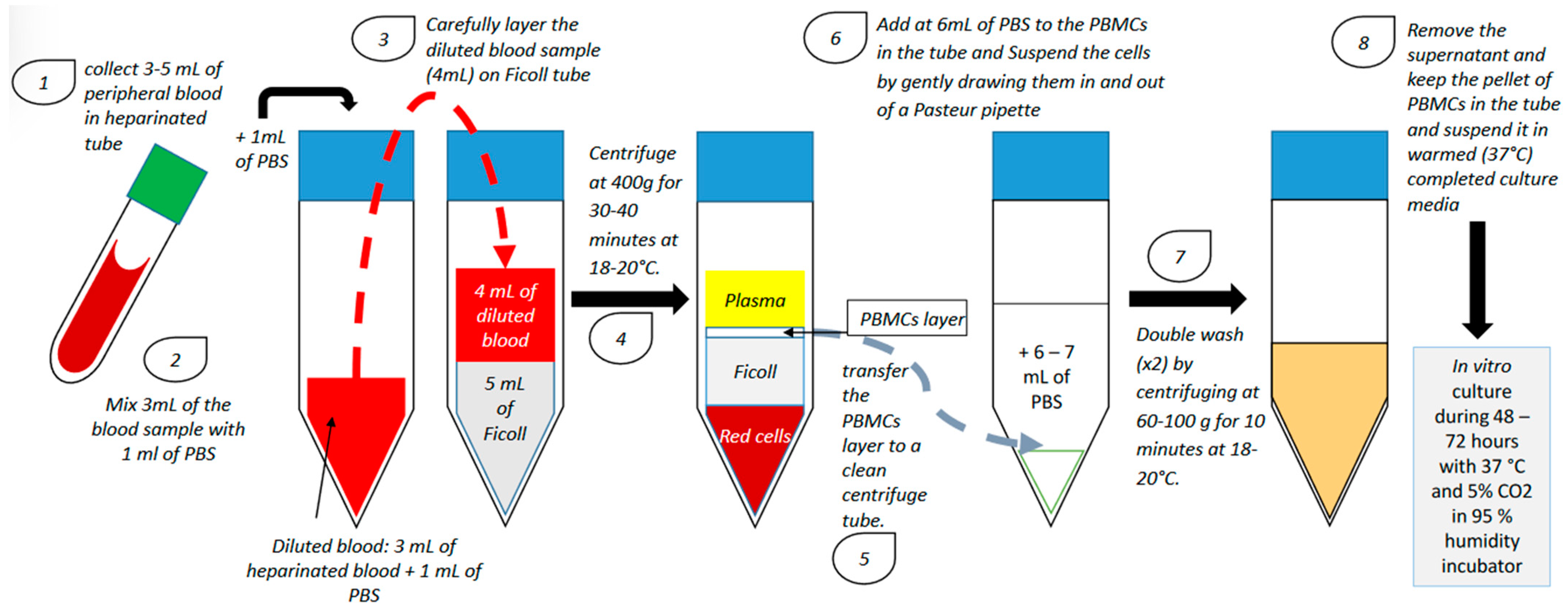
2. Endometrium Immunomodulation via Intrauterine Insemination of Activated Autologous Peripheral Blood Mononuclear Cells (PBMCs)
| Study | Number of Previous Failed IVF Cycles | Sample Size | Day of Blood Collection | PBMCs Co-cultured with | Duration of PBMC Culture | Number of PBMCs Administered In Utero | Transfer Type | Stage of Embryo | Implantation Rate (Control vs. Case) | Clinical Pregnancy Rate (Control vs. Case) | Miscarriage Rate (Control vs. Case) | Live Birth Rate (Control vs. Case) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Case | ||||||||||||
| Yoshioka et al., 2006 [7] | ≥4 | 18 | 17 | On the day of oocyte retrieval | hCG: 5 IU/mL | 48 h | 20 × 106 | Fresh | 1, 2 or 3 blastocysts | 4.1% vs. 23.4% (p = 0.0034) | 11.1% vs. 41.2% (p = 0.042) | Not specified | 7.6% vs. 55.6% (p = 0.013) |
| Okitsu et al., 2011 [16] | ≥1 | 170 | 83 | On the day following ovulation or the day after | Not activated | No culture | 30 × 106 | Frozen/ thawed |
early cleavage embryo or blastocyst | ≥1 RIF: 21.1% vs. 21.6% (ns); 3 RIF: 9.38% vs. 25.0% (p = 0.041) | ≥1 RIF: 32.9% vs. 34.9% (ns); ≥3 RIF: 16.7% vs. 42.1% (p = 0.039) | Not specified | ≥ 1 RIF: 21.8% vs. 21.7% (ns); ≥3 RIF: 11.1% vs. 21.2% (ns) |
| Makrigiannakis et al., 2015 [9] | ≥3 | 45 | 45 | On the day of oocyte retrieval | CRH: 107 M/1.106 cells/mL | 48 h | 20 × 106 + 107 CRH | Fresh | 2 or 3 blastocysts (grade 3BB and above) | Not specified | 0% vs. 44.44% (p < 0.001) | Not specified | Not specified |
| Madkour et al., 2016 [10] | ≥2 | 27 | 27 | On the day of ovulation induction | Complete culture medium + 75 IU of hMG | 72 h | 1 × 106 | Fresh | 1, 2 or 3 early cleavage embryos | ≥2 RIF: 9% vs. 22% (p = 0.02); 2 RIF vs. ≥ 3 RIF: 15% vs. 35% (p = 0.09) | ≥2 RIF: 15% vs. 44% (p = 0.045); 2 RIF vs. ≥ 3 RIF: 29% vs. 70% (p = 0.04) | ≥2 RIF: 17% vs. 75% (p = 0.08) 2 RIF vs. ≥ 3 RIF: 20% vs. 14% (p = 0.8) | Not specified |
| Yu et al., 2016 [11] | ≥3 | 105 | 93 | On the day following ovulation | hCG: 10 IU/mL | 24 h | 10–20 × 106 | Frozen/ thawed |
early cleavage embryo | 11.43% vs. 23.66% (p < 0.05) | 20.95% vs. 46.24% (p < 0.05) | 31.8% vs. 20.9% (ns) | 14.28% vs. 34.41% (p < 0.05) |
| Li et al., 2017 [12] | ≥1 | 339 | 294 | Two days before embryo transfer | hCG: 10 IU/mL | 24 h | 10–20 × 106 | Fresh and frozen/ thawed |
2 or 3 early cleavage embryos or 2 or 3 grade 2 blastocysts at day 5 and 3BB and above at day 6 | 1 RIF: 32.33% vs. 29.35% (ns); 2 RIF: 27.74% vs. 35.98% (p = 0.048); 3 RIF: 26.23% vs. 23.20% (ns); ≥4 RIF: 4.88% vs. 22.00% (p = 0.014) | 1 RIF: 41.23% vs. 43.75% (ns); 2 RIF: 42.18% vs. 48.15% (p = 0.016); 3 RIF: 36.84% vs. 42.22% (ns); ≥4 RIF: 14.29% vs. 39.58% (p = 0.038) | Not specified | 1 RIF: 36.84% vs. 37.5% (ns); 2 RIF: 33.33% vs. 34.26% (ns); 3 RIF: 24.56% vs. 28.89% (ns); ≥4 RIF: 9.58% vs. 33.33% (p = 0.038) |
| Makrigiannakis et al., 2019 [13] | ≥3 | 26 | 26 | On the day of oocyte retrieval | CRH: 107 M/1.106 cells/mL | 48 h | 20 × 106 + 107 M CRH | Fresh | 2 or 3 grade 1 or 2 early cleavage embryos | Not specified | 0% vs. 57,69% (p < 0.01) | Not specified | Not specified |
| Nobijari et al., 2019 [15] | ≥1 | 128 | 122 | 5 days before the frozen/thawed embryo transfer | CRH (concentration not specified) | 48–72 h | 20 × 106 + 107 M CRH | Frozen/ thawed |
early cleavage embryo or blastocyst | Not specified | <3 RIF: 30.4% vs. 30.8% (p = 0.91); ≥3 RIF: 19,7% vs. 38,6% (p = 0.01) | Not specified | Not specified |
| Pourmoghadam et al., 2020 [14] | ≥3 | 50 | 50 | On the day of ovulation induction | hCG: 10 IU/mL daily | 48 h | 15–20 × 106 | Frozen/ thawed |
early cleavage embryo or blastocyst | Not specified | 22% vs. 42% (p = 0.032) | 24% vs. 8% (p = 0.029) | 20% vs. 38% (p = 0.047) |
3. Immunoregulation of the Endometrium during Embryo Implantation: Biological Function and Molecular Pathway
References
- Benkhalifa, M.; Zayani, Y.; Bach, V.; Copin, H.; Feki, M.; Benkhalifa, M.; Allal-Elasmi, M. Does the dysregulation of matrix metalloproteinases contribute to recurrent implantation failure? Expert Rev. Proteom. 2018, 15, 311–323.
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1747.
- Simon, A.; Laufer, N. Repeated implantation failure: Clinical approach. Fertil. Steril. 2012, 97, 1039–1043.
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121.
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38.
- Cimadomo, D.; Craciunas, L.; Vermeulen, N.; Vomstein, K.; Toth, B. Definition, diagnostic and therapeutic options in recurrent implantation failure: An international survey of clinicians and embryologists. Hum. Reprod. 2021, 36, 305–317.
- Yoshioka, S.; Fujiwara, H.; Nakayama, T.; Kosaka, K.; Mori, T.; Fujii, S. Intrauterine administration of autologous peripheral blood mononuclear cells promotes implantation rates in patients with repeated failure of IVF-embryo transfer. Hum. Reprod. 2006, 21, 3290–3294.
- Bielfeld, A.P.; Pour, S.J.; Poschmann, G.; Stühler, K.; Krüssel, J.S.; Baston-Büst, D.M. A Proteome Approach Reveals Differences between Fertile Women and Patients with Repeated Implantation Failure on Endometrial Level⁻Does hCG Render the Endometrium of RIF Patients? Int J. Mol. Sci. 2019, 2, 425.
- Makrigiannakis, A.; BenKhalifa, M.; Vrekoussis, T.; Mahjub, S.; Kalantaridou, S.N.; Gurgan, T. Repeated implantation failure: A new potential treatment option. Eur. J. Clin. Investig. 2015, 45, 380–384.
- Madkour, A.; Bouamoud, N.; Louanjli, N.; Kaarouch, I.; Copin, H.; Benkhalifa, M.; Sefrioui, O.; Madkour, L. Intrauterine insemination of cultured peripheral blood mononuclear cells prior to embryo transfer improves clinical outcome for patients with repeated implantation failures. Zygote 2016, 24, 58–69.
- Yu, N.; Zhang, B.; Xu, M.; Wang, S.; Liu, R.; Wu, J.; Yang, J.; Feng, L. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: A prospective randomized study. Am. J. Reprod. Immunol. 2016, 76, 212–216.
- Li, S.; Wang, J.; Cheng, Y.; Zhou, D.; Yin, T.; Xu, W.; Yu, N.; Yang, J. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 2017, 119, 15–22.
- Makrigiannakis, A.; Vrekoussis, T.; Makrygiannakis, F.; Ruso, H.; Kalantaridou, S.N.; Gurgan, T. Intrauterine CRH-treated PBMC in repeated implantation failure. Eur. J. Clin. Investig. 2019, 49, e13084.
- Pourmoghadam, Z.; Soltani-Zangbar, M.S.; Sheikhansari, G.; Azizi, R.; Eghbal-Fard, S.; Mohammadi, H.; Siahmansouri, H.; Aghebati-Maleki, L.; Danaii, S.; Mehdizadeh, A.; et al. Intrauterine administration of autologous hCG- activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; A double-blind, randomized control trial study. J. Reprod. Immunol. 2020, 142, 103182.
- Nobijari, F.F.; Arefi, S.S.; Moini, A.; Taheripanah, R.; Fazeli, E.; Kharazi, H.; Hosseini, S.Z.; Hosseini, A.; Valojerdi, M.R.; Copin, H. Endometrium immunomodulation by intrauterine insemination administration of treated peripheral blood mononuclear cell prior frozen/thawed embryos in patients with repeated implantation failure. Zygote 2019, 27, 214–218.
- Okitsu, O.; Kiyokawa, M.; Oda, T.; Miyake, K. Intrauterine administration of autologous blood mononuclear cells increases clinical pregnancy rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 2011, 92, 82–87.
- Qin, Q.; Chang, H.; Zhou, S.; Zhang, S.; Yuan, D.; Yu, L.L.; Qu, T. Intrauterine administration of peripheral blood mononuclear cells activated by human chorionic gonadotropin in patients with repeated implantation failure: A meta-analysis. J. Reprod. Immunol. 2021, 145, 103323.
- Zenclussen, A.C.; Hämmerling, G.J. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front. Immunol. 2015, 6, 321.
- King, A.; Balendran, N.; Wooding, P.; Carter, N.P.; Loke, Y.W. CD3- leukocytes present in the human uterus during early placentation: Phenotypic and morphologic characterization of the CD56++ population. Dev. Immunol. 1991, 1, 169–190.
- Koopman, L.A.; Kopcow, H.D.; Rybalov, B.; Boyson, J.E.; Orange, J.S.; Schatz, F.; Masch, R.; Lockwood, C.J.; Schachter, A.D.; Park, P.J.; et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 2003, 198, 1201–1212.
- Mori, M.; Bogdan, A.; Balassa, T.; Csabai, T.; Szekeres-Bartho, J. The deciduas-the maternal bed embracing the embryo-maintains the pregnancy. Semin. Immunopathol. 2016, 38, 635–649.
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465.
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55.
- Timeva, T.; Shterev, A.; Kyurkchiev, S. Recurrent Implantation Failure: The Role of the Endometrium. J. Reprod. Infertil. 2014, 15, 173–183.
- Helige, C.; Ahammer, H.; Hammer, A.; Huppertz, B.; Frank, H.G.; Dohr, G. Trophoblastic invasion in vitro and in vivo: Similarities and differences. Hum. Reprod. 2008, 23, 2282–2291.
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. Update 2012, 18, 458–471.
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125.
- Huang, X.; Venet, F.; Chung, C.S.; Lomas-Neira, J.; Ayala, A. Changes in dendritic cell function in the immune response to sepsis: Cell- & tissue-based therapy. Expert Opin. Biol. Ther. 2007, 7, 929–938.
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905.
- Péguet-Navarro, J.; Dalbiez-Gauthier, C.; Rattis, F.M.; Van Kooten, C.; Banchereau, J.; Schmitt, D. Functional expression of CD40 antigen on human epidermal Langerhans cells. J. Immunol. 1995, 155, 4241–4247.
- Rattis, F.M.; Péguet-Navarro, J.; Staquet, M.J.; Dezutter-Dambuyant, C.; Courtellemont, P.; Redziniak, G.; Schmitt, D. Expression and function of B7-1 (CD80) and B7-2 (CD86) on human epidermal Langerhans cells. Eur. J. Immunol. 1996, 26, 449–453.
- Tagliani, E.; Erlebacher, A. Dendritic cell function at the maternal–fetal interface. Expert Rev. Clin. Immunol. 2011, 7, 593–602.
- Tafuri, A.; Alferink, J.; Möller, P.; Hämmerling, G.J.; Arnold, B. T cell awareness of paternal alloantigensduring pregnancy. Science 1995, 270, 630–633.
- Hudic, I.; Fatušic, Z. Progesterone-induced blocking factor (PIBF) and Th1/Th2 cytokine in women with threatened spontaneous abortion. J. Perinat. Med. 2009, 37, 338–342.
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338.
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87.
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309.
- Deshmukh, H.; Way, S.S. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu. Rev. Pathol. 2019, 14, 185–210.
- Liang, P.Y.; Diao, L.H.; Huang, C.Y.; Lian, R.C.; Chen, X.; Li, G.G.; Zhao, J.; Li, Y.Y.; He, X.B.; Zeng, Y. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod. Biomed. Online 2015, 31, 823–826.
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235.
- Von Wolff, M.; Thaler, C.J.; Strowitzki, T.; Broome, J.; Stolz, W.; Tabibzadeh, S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: Dysregulation in habitual abortion. Mol. Hum. Reprod. 2000, 6, 627–634.
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Dolati, S.; Farzadi, L.; Ghasemzadeh, A.; Hamdi, K.; Younesi, V.; Nouri, M.; et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst. Biol. Reprod. Med. 2017, 63, 350–359.
- Teles, A.; Schumacher, A.; Kühnle, M.C.; Linzke, N.; Thuere, C.; Reichardt, P.; Tadokoro, C.E.; Hämmerling, G.J.; Zenclussen, A.C. Control of uterine microenvironment by foxp3 (+) cells facilitates embryo implantation. Front. Immunol. 2013, 4, 158.
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1⁄Th ⁄Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610.
- Tedesco, S.; Adorni, M.P.; Ronda, N.; Cappellari, R.; Mioni, R.; Barbot, M.; Pinelli, S.; Plebani, M.; Bolego, C.; Scaroni, C.; et al. Activation profiles of monocyte-macrophages and HDL function in healthy women in relation to menstrual cycle and in polycystic ovary syndrome patients. Endocrine 2019, 66, 360–369.
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298.
- Cervar, M.; Blaschitz, A.; Dohr, G.; Desoye, G. Paracrine regulation of distinct trophoblast functions in vitro by placental macrophages. Cell Tissue Res. 1999, 295, 297–305.
- Tonello, A.; Poli, G. Tubal ectopic pregnancy: Macrophages under the microscope. Hum. Reprod. 2007, 22, 2577–2584.




