After more than four decades of assisted reproductive technology (ART) practice worldwide, today more than 60% of women undergoing in vitro fertilization (IVF) treatments fail to become pregnant after the first embryo transfer and nearly 20% of patients are suffering from unexplained recurrent implantation failures (RIFs) and repeated pregnancy loss (RPL). The literature reported different causes of RIF–RPL, mainly multifactorial, endometrial and idiopathic. RIF remains a black box because of the complicated categorization and causes of this physio-pathological dysregulation of implantation and pregnancy process after ovarian stimulation. Many options were suggested as solutions to treat RIF–RPL with controversial results on their usefulness.
- infertility
- assisted reproductive technology
- implantation failure
- endometrium immunomodulation
1. Introduction
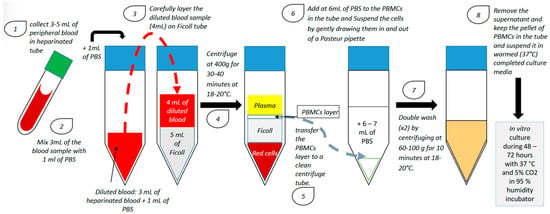
2. Endometrium Immunomodulation via Intrauterine Insemination of Activated Autologous Peripheral Blood Mononuclear Cells (PBMCs)
| Study | Number of Previous Failed IVF Cycles | Sample Size | Day of Blood Collection | PBMCs Co-cultured with | Duration of PBMC Culture | Number of PBMCs Administered In Utero | Transfer Type | Stage of Embryo | Implantation Rate (Control vs. Case) | Clinical Pregnancy Rate (Control vs. Case) | Miscarriage Rate (Control vs. Case) | Live Birth Rate (Control vs. Case) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Case | ||||||||||||
| Yoshioka et al., 2006 [7] | ≥4 | 18 | 17 | On the day of oocyte retrieval | hCG: 5 IU/mL | 48 h | 20 × 106 | Fresh | 1, 2 or 3 blastocysts | 4.1% vs. 23.4% (p = 0.0034) | 11.1% vs. 41.2% (p = 0.042) | Not specified | 7.6% vs. 55.6% (p = 0.013) |
| Okitsu et al., 2011 [16] | ≥1 | 170 | 83 | On the day following ovulation or the day after | Not activated | No culture | 30 × 106 | Frozen/ thawed |
early cleavage embryo or blastocyst | ≥1 RIF: 21.1% vs. 21.6% (ns); 3 RIF: 9.38% vs. 25.0% (p = 0.041) | ≥1 RIF: 32.9% vs. 34.9% (ns); ≥3 RIF: 16.7% vs. 42.1% (p = 0.039) | Not specified | ≥ 1 RIF: 21.8% vs. 21.7% (ns); ≥3 RIF: 11.1% vs. 21.2% (ns) |
| Makrigiannakis et al., 2015 [9] | ≥3 | 45 | 45 | On the day of oocyte retrieval | CRH: 107 M/1.106 cells/mL | 48 h | 20 × 106 + 107 CRH | Fresh | 2 or 3 blastocysts (grade 3BB and above) | Not specified | 0% vs. 44.44% (p < 0.001) | Not specified | Not specified |
| Madkour et al., 2016 [10] | ≥2 | 27 | 27 | On the day of ovulation induction | Complete culture medium + 75 IU of hMG | 72 h | 1 × 106 | Fresh | 1, 2 or 3 early cleavage embryos | ≥2 RIF: 9% vs. 22% (p = 0.02); 2 RIF vs. ≥ 3 RIF: 15% vs. 35% (p = 0.09) | ≥2 RIF: 15% vs. 44% (p = 0.045); 2 RIF vs. ≥ 3 RIF: 29% vs. 70% (p = 0.04) | ≥2 RIF: 17% vs. 75% (p = 0.08) 2 RIF vs. ≥ 3 RIF: 20% vs. 14% (p = 0.8) | Not specified |
| Yu et al., 2016 [11] | ≥3 | 105 | 93 | On the day following ovulation | hCG: 10 IU/mL | 24 h | 10–20 × 106 | Frozen/ thawed |
early cleavage embryo | 11.43% vs. 23.66% (p < 0.05) | 20.95% vs. 46.24% (p < 0.05) | 31.8% vs. 20.9% (ns) | 14.28% vs. 34.41% (p < 0.05) |
| Li et al., 2017 [12] | ≥1 | 339 | 294 | Two days before embryo transfer | hCG: 10 IU/mL | 24 h | 10–20 × 106 | Fresh and frozen/ thawed |
2 or 3 early cleavage embryos or 2 or 3 grade 2 blastocysts at day 5 and 3BB and above at day 6 | 1 RIF: 32.33% vs. 29.35% (ns); 2 RIF: 27.74% vs. 35.98% (p = 0.048); 3 RIF: 26.23% vs. 23.20% (ns); ≥4 RIF: 4.88% vs. 22.00% (p = 0.014) | 1 RIF: 41.23% vs. 43.75% (ns); 2 RIF: 42.18% vs. 48.15% (p = 0.016); 3 RIF: 36.84% vs. 42.22% (ns); ≥4 RIF: 14.29% vs. 39.58% (p = 0.038) | Not specified | 1 RIF: 36.84% vs. 37.5% (ns); 2 RIF: 33.33% vs. 34.26% (ns); 3 RIF: 24.56% vs. 28.89% (ns); ≥4 RIF: 9.58% vs. 33.33% (p = 0.038) |
| Makrigiannakis et al., 2019 [13] | ≥3 | 26 | 26 | On the day of oocyte retrieval | CRH: 107 M/1.106 cells/mL | 48 h | 20 × 106 + 107 M CRH | Fresh | 2 or 3 grade 1 or 2 early cleavage embryos | Not specified | 0% vs. 57,69% (p < 0.01) | Not specified | Not specified |
| Nobijari et al., 2019 [15] | ≥1 | 128 | 122 | 5 days before the frozen/thawed embryo transfer | CRH (concentration not specified) | 48–72 h | 20 × 106 + 107 M CRH | Frozen/ thawed |
early cleavage embryo or blastocyst | Not specified | <3 RIF: 30.4% vs. 30.8% (p = 0.91); ≥3 RIF: 19,7% vs. 38,6% (p = 0.01) | Not specified | Not specified |
| Pourmoghadam et al., 2020 [14] | ≥3 | 50 | 50 | On the day of ovulation induction | hCG: 10 IU/mL daily | 48 h | 15–20 × 106 | Frozen/ thawed |
early cleavage embryo or blastocyst | Not specified | 22% vs. 42% (p = 0.032) | 24% vs. 8% (p = 0.029) | 20% vs. 38% (p = 0.047) |
3. Immunoregulation of the Endometrium during Embryo Implantation: Biological Function and Molecular Pathway
This entry is adapted from the peer-reviewed paper 10.3390/ijms232112787
