Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronan Lordan | -- | 4812 | 2022-11-02 03:46:49 | | | |
| 2 | Conner Chen | + 7 word(s) | 4819 | 2022-11-02 04:59:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lordan, R.; Zabetakis, I. Cadmium and Human Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/32383 (accessed on 07 February 2026).
Lordan R, Zabetakis I. Cadmium and Human Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/32383. Accessed February 07, 2026.
Lordan, Ronan, Ioannis Zabetakis. "Cadmium and Human Health" Encyclopedia, https://encyclopedia.pub/entry/32383 (accessed February 07, 2026).
Lordan, R., & Zabetakis, I. (2022, November 02). Cadmium and Human Health. In Encyclopedia. https://encyclopedia.pub/entry/32383
Lordan, Ronan and Ioannis Zabetakis. "Cadmium and Human Health." Encyclopedia. Web. 02 November, 2022.
Copy Citation
Cadmium is ubiquitous in the diet, with the highest levels present in grains, leafy greens, and shellfish. Cadmium is a major health risk globally and is associated with pollution and anthropogenic activity. It is important to understand the dietary sources of cadmium, how they are monitored, and the effect of cadmium exposure to human health.
cadmium
crab
crustaceans
heavy metal toxicity
nutrition
1. Cadmium Exposure in Humans
Understanding all possible routes of human cadmium exposure is important to consider when assessing one’s risk of excessive exposure. Cadmium exposure occurs through three possible routes: dermal, gastrointestinal, or pulmonary. Inhalation of cadmium by industrial workers or smokers is a significant exposure risk, but for the general population of non-smokers, exposure most commonly occurs via ingestion of contaminated foods or water [1]. Previously, it was suggested that atmospheric changes in cadmium levels due to increased pollution may affect blood cadmium levels. However, a recent study found that ingestion of dietary cadmium has a stronger impact on blood cadmium levels [2], likely due to the biomagnification of cadmium in dietary sources versus occasional acute exposure from atmospheric pollution. Therefore, it is important to consider dietary sources of cadmium, which may contain excessive cadmium, so that people and public health authorities can decide whether to mitigate excessive cadmium exposure risk by eliminating or reducing these food sources in the diet. Notably, cadmium is found ubiquitously in nature, and not all anthropogenic sources are the result of industrial emissions. For example, it has been documented that metal pollution can occur due to mining, aquaculture, wastewater treatment, crop farming, and animal breeding [3][4].
Diet is the most prevalent source of cadmium exposure in the general population, and it is also a source that can be mitigated to reduce cadmium exposure. It is estimated that daily dietary cadmium intake in unpolluted European areas can vary from 0.1 to 0.45 µg/kg bodyweight. However, in polluted areas, the total intake may be significantly more than the tolerable daily cadmium intake and reach several hundred µg/day [5][6]. This has major implications when considering the overall intake of cadmium from various foods, which tends to govern what limits are applied to the cadmium content of foods intended for human consumption. Lifestyle choices are certainly one of the biggest determinants of cadmium exposure. Smoking is a significant modifiable risk factor for cadmium exposure, as the tobacco plant accumulates cadmium from the soil into its leaves with great efficiency [7]. The United States national geometric mean blood cadmium level for non-smoking adults is 0.47 µg/L, whereas the mean of smokers is approximately thrice as high at 1.58 µg/L [8]. Smoking is estimated to at least double the body burden of cadmium exposure in one’s lifetime. Cadmium oxide (CdO) is a highly bioavailable form of cadmium that is responsible for the high concentrations of cadmium in the blood, urine, and tissues of smokers compared with non-smokers [9][10].
Whether there are specific foods that one should avoid, or dietary alterations required to reduce a person’s exposure to dietary cadmium is a topic of interest. The European Food Safety Authority (EFSA) noted that it is not the foods with the highest cadmium levels but, rather, the foods that are consumed in larger quantities most often that have the largest impact on dietary exposure to cadmium [11]. EFSA, using the food description and classification system FoodEx, determined that dietary cadmium exposure in European populations mainly originated from grains and grain-derived products (26.9%), vegetables and vegetable products (16.0%), and starchy roots and tubers (13.2%). In more detail, the following food categories contributed the most to dietary cadmium exposure across all age groups: potatoes (13.2%), bread and rolls (11.7%), fine bakery goods (5.1%), chocolate products (4.3%), leafy vegetables (3.9%), and molluscs (3.2%). However, it was noted that crustaceans were among a group of foods that exceeded 100 μg/kg, along with algal formulations, cocoa powder, offal, some seafood, mushrooms, and water molluscs [12]. Lifetime cadmium dietary exposure for Europeans is estimated to be approximately 2 μg/kg bodyweight/week (averaged for all age groups)—within the EFSA’s tolerable weekly intake (TWI) of 2.5 µg/kg bodyweight/week.
In Ireland, weekly adult intake of cadmium has been estimated to be between 1.1 and 2.5 µg/kg bodyweight/week, which is between 44 and 62% of the EFSA’s TWI [13]. These findings indicate that the majority of Irish people are not exposed to excess dietary cadmium levels. These findings are supported by the National Adult Nutrition Survey, which examined urinary cadmium excretion in the general population They and that 95% of participants had urinary cadmium levels below the 1 µg cadmium/g creatinine that the EFSA has deemed safe [14]. The main cadmium-contributing foods in the Irish diet were cereals (39%), vegetables (36%), and dairy (12%), where fish and shellfish only accounted for approximately 1% [13], likely due to the low consumption of fish and shellfish in Ireland.
In the United States, a recent study was conducted to determine the intake and sources of cadmium [15]. The average intake of dietary cadmium in the general population was 4.6 µg/day, or 0.54 µg/kg body weight/week—that is, approximately 22% of the tolerable weekly intake (TWI), which is considered to be 2.5 µg/kg body weight/week. However, certain demographics—such as elderly men, those who were well-educated and had a high income, and those with high adiposity—had higher levels of cadmium intake [15]. The food groups that contributed the most to the majority of the cadmium intake in the United States were cereals and bread (34%), leafy vegetables (20%), potatoes (11%), legumes and nuts (7%), and root vegetables (6%). Notably, the individual foods that contributed the most to the overall cadmium intake included lettuce (14%), spaghetti (8%), bread (7%), and potatoes (6%).
Interestingly, but unsurprisingly, due to the many cultures that coexist in the United States, there were ethnic and cultural differences in cadmium intake due to differences in dietary preferences. Lettuce was a major cadmium source for Caucasian and Black populations, whereas tortillas were the main source for Hispanics, and rice was the top contributor to the Asian population. Notably, the trends of cadmium intake in the United States seem to be very similar overall to those in the European Union. This is also unsurprising because despite there being many culinary differences in the foods and cultures of the US and Europe, the prevailing dietary pattern in both regions is the so-called “Western Diet” characterised by highly processed foods [16]. Amongst the Asian populations of the United States, smoking was the main exposure route for cadmium, followed by dietary exposure [17], which increases one’s risk for many non-communicable diseases—including cardiovascular, renal, and pulmonary diseases [16][18]. It is notable that fish and shellfish comprise a low contribution of cadmium to the American diet, but fish consumption is traditionally low in the United States [19].
Looking further afield, there are similarities between the so-called Western countries and Asian countries such as the People’s Republic of China (PRC) and South Korea. The average total daily cadmium intake in healthy Koreans is estimated to be 20.8 µg/day [20]. Notably, the food groups recorded are starkly different to the food groups associated with higher cadmium exposure in Europe or the United States. In particular, they seem to be culturally relevant. For example, there are much higher levels of rice (40.3%), as was noted in Asian groups from the United States cohort [15], but also higher intake of seafood and specific foods associated with Korean cuisine, such as kimchi and seaweed. Indeed, crab in this case was shown to contribute 8.6% of the cumulative cadmium intake in this South Korean population.
Many parts of the PRC share similar food consumption patterns to South Korea and, thus, similar cadmium exposure [21]. In one study [21], freshwater crab and sea-caught crab samples obtained contained 0.101 ± 0.323 and 0.544 ± 1.203 mg/kg (mean ± standard deviation) cadmium, respectively, which were estimated to be consumed at 0.7 ± 7.3 and 0.8 ± 8.9 g/day by the general population. This contrasts with rice and wheat, which contain 0.062 ± 0.128 and 0.021 ± 0.026 mg/kg of cadmium and are consumed at 218 ± 174.5 and 145.4 ± 168 g/day (mean ± standard deviation), respectively. These data show that while it is true that crab contains higher concentrations of cadmium in mg/kg weight, its consumption levels are markedly different. A further examination of the specific food groups contributing dietary cadmium to the Chinese population is presented in Table 1. The interesting point of this research is that for the high-exposure subpopulation with cadmium exposure higher than the 95th percentile, rice was the largest contributor (58.6%), followed by shellfish (13.2%), and leafy vegetables (9.2%). This is a very small sub-fraction of the population that are exposed to such high levels of cadmium because of their dietary choices; it would be interesting to break down the subcategory of shellfish further to determine the impact that crab may have on the consumption of cadmium in this cohort. The study determined that the mean dietary cadmium exposure of the general Chinese population was 15.3 μg/kg body weight/month (30.6 μg/day for a 60 kg average body weight of adults). A similar study in Shanghai found that the average exposure to dietary and environmental cadmium was 167 µg/day (34% of the PTDI). Similarly, vegetables and rice were the main sources of dietary cadmium, and tobacco accounted for 25% of the total cadmium exposure from non-occupational sources [22]. Considering that almost 20% of agricultural soil in the PRC is contaminated with cadmium [23], it is likely that their dietary exposure to cadmium will only increase with their growing economy. Furthermore, the daily exposure to dietary cadmium in the Chinese population is significantly higher than that in either Europe or the United States.
Table 1. Data depicting the main contributors to dietary cadmium intake in (A) the general Chinese population and (B) the highly exposed Chinese population. Data adapted with permission from [21].
| General Population (A) | High-Exposure Population (B) * | ||
|---|---|---|---|
| Food Group | Percentage (%) Contribution of Dietary Cadmium Intake | Food Group | Percentage (%) Contribution of Dietary Cadmium Intake |
| Rice | 55.8 | Rice | 58.6 |
| Leafy vegetables | 10.5 | Leafy vegetables | 9.2 |
| Wheat flour | 11.8 | Wheat flour | 2 |
| Shellfish | 4.8 | Shellfish | 13.2 |
| Meat | 2.6 | Meat | 2 |
| Seaweed | 2.4 | Seaweed | 6.4 |
| Other vegetables | 2.4 | Other vegetables | 1.4 |
| Other cereals | 2.1 | Other cereals | 0.9 |
| Root and stalk vegetables | 2.0 | Root and stalk vegetables | 1.7 |
| Mushrooms | 1.1 | Mushrooms | 1.5 |
| Fish | 1.1 | Fish | 1 |
| Legumes | 0.9 | Legumes | 0.6 |
| Fruits | 0.6 | Fruits | 0.4 |
| Eggs | 0.6 | Eggs | 0.2 |
| Nuts | 0.4 | Nuts | 0.4 |
| Offal | 0.4 | Offal | 0.2 |
| Other | 0.5 | Other | 0.3 |
* The highly exposed population was determined to be those within the 95th percentile of the mean dietary cadmium exposure of the general Chinese population.
The total diet study (TDS) is a food safety monitoring program that is conducted by various food agencies, including the United States Food and Drug Administration (FDA), the Food Standards of Australia and New Zealand (FSANZ), and the European Food Safety Agency (EFSA) [24]. These are “market basket surveys” that collection of various food samples from groceries and retailers for the quantitation of food additives, pesticide residues, contaminants, nutrients and, of course, heavy metals [25][26]. The TDS provides a realistic approach to gauge the relative contribution of each food group and specific item to estimate the total intake of cadmium in the diet. Foods that were consumed in large quantities at high frequency contributed the most to cadmium intake [24]. Currently, TDS data are available for a limited number of countries, including Australia, the United States, France, Spain, Sweden, Chile, Denmark, and Serbia [10]. Overall, data from TDS show that these countries’ cadmium intake varies between 8 and 25 µg/day for the average consumer with staple foods (e.g., rice, wheat, and potatoes), which accounted for 40–60% of total dietary cadmium ingestion. Shellfish, crustaceans, molluscs, offal, and spinach were considered to be additional cadmium sources [10][24]. These types of studies are often thought to underestimate dietary cadmium, as they fail to demonstrate an association between estimated cadmium intake and the incidence of cancer and bone diseases [27][28][29][30].
Overall, these epidemiological and dietary studies demonstrate that dietary cadmium exposure is affected by many factors and that the main contributor of dietary cadmium in all instances around the world mostly originates from staple foods such as rice, wheat, and other grains. In Asian diets, seafood and shellfish were contributors to dietary cadmium intake, but this is not necessarily the main source in countries that consume Western diets. Overall, it seems that it is important to strike a balance and be cautious of foods that are potentially significant contributors of cadmium to the diet. Indeed, moderate consumption of shellfish should not significantly affect one’s risk of illness from cadmium ingestion, but further research specific to crab consumption is required.
2. Cadmium Ingestion and Accumulation in Humans
Depending on the exact dose and nutritional composition of a food, the human gastrointestinal tract can take up 3–5% of ingested cadmium [12][31]. Various factors can affect cadmium uptake in humans, such as low intakes of calcium, vitamin D, zinc, and copper [1]. One possible mechanism of high cadmium resorption is related to the assumption that cadmium shares molecular homology with zinc and calcium; as a result, low levels of these minerals are compensated by higher cadmium resorption [32]. This observation was closely replicated in competitive resorption studies in rats against other polyvalent cations such as Cr3+, Mg, Ni, Pb, and Sr [33]. Notably, a low zinc/iron status in individuals who subsist on diets characterised by high rice intake may cause high absorption of cadmium in contrast to other staple diets [34]. Other factors that affect cadmium uptake include gender, nutritional status, diet, and smoking status can also affect the bioavailability of cadmium in humans [35].
Indeed, various human studies show that cadmium intake can be increased by dietary fibre intake [6]. Animal experiments have shown that diets with high concentrations of protein and lipids can also increase net intestinal uptake of cadmium and that diets high in wheat bran may reduce cadmium intake [5]. The exact mechanisms of these effects on cadmium intake are yet to be fully elucidated. On the other hand, cadmium can bind to low-molecular-weight proteins rich in cysteine such as metallothionein, which may increase its bioavailability [36]. This has been demonstrated naturally in various marine organisms where cadmium seems to be bound to small, soluble cytoplasmic proteins, including in oysters, mussels, scallops [36], and green crab (Carcinus maenas) [37][38][39]. In rat studies, cadmium binds to amino acids and peptides in the intestinal tract [40], which undoubtedly has implications for its bioavailability. What these studies suggest is that these effects may be the result of a food matrix effect in a similar way to dairy products, where nutrients are more or less bioavailable depending on the food’s structure and composition [41]. This implies that the foods or ingredients that people mix with foods containing high cadmium levels may affect the overall bioavailability of cadmium. Therefore, it may be possible to mitigate cadmium’s bioavailability when preparing foods that may have higher levels of cadmium by altering the food matrix. However, research is very limited in this area, and further studies are required to confirm such associations.
Evidence from animal studies shows that marginal deficiencies in zinc, iron, and calcium can enhance the absorption, organ accumulation, and retention of dietary cadmium [34]. Moreover, marginal deficiencies can enhance cadmium absorption as much as 10-fold in diets containing low cadmium concentrations similar to those consumed by some human populations, indicating that people who are nutritionally marginal with respect to zinc, iron, and calcium are at higher risk of cadmium-related diseases than those who are nutritionally adequate [42][43][44]. Indeed, similar studies in humans show that an individual’s iron levels may be a metabolic factor of concern in the resorption of cadmium. It has been demonstrated that a lack of iron leads to a 6% higher uptake of cadmium in individuals with normal iron levels [45]. A study of iron-deficient children in the United States found elevated blood cadmium levels [46]. This accounts for higher cadmium absorption in individuals with a habitual iron deficit (e.g., children or menstruating women) or people with anaemia [1]. It seems that these observations are the result of the expression of DCT-1 and MTP1—metal ion transporters in the gastrointestinal tract that act as a gate for cadmium resorption when low iron levels occur [47][48]. Overall, the evidence presented supports the notion that dietary components and trace element status can affect the fractional intestinal uptake of cadmium, as reviewed by Andersen et al. [5].
These findings hint at the possibility of ensuring that individuals who may be at high risk of exposure to cadmium have a healthy nutritional status. Those who may be deficient in some minerals may consider dietary alterations or dietary supplements to ameliorate mineral deficiencies.
3. Cadmium’s Transport, Bioavailability, and Excretion in Humans
Cadmium is well-known for its toxicity to humans, as evidenced by decades of observational studies and research. Like many heavy metals, bioaccumulation of cadmium in mammals can differentially affect certain tissues, including bone, the liver, muscle, and the kidneys. Indeed, Cd2+ is dangerous in that it can substitute for Zn in enzyme structures. Likewise, calcium and cadmium have similar ionic radii (109 pm and 114 pm, respectively), meaning that cadmium can accumulate in the bone along with calcium [49].
Once taken up by the gastrointestinal tract and deposited into the bloodstream, cadmium binds to proteins such as albumin and metallothionein. From there, it is transported to the liver, where cadmium can induce the production of metallothionein. Following the necrosis and apoptosis of hepatocytes, cadmium–metallothionein (Cd–M) complexes form, which are washed from sinusoidal blood. Some cadmium then enters the enterohepatic cycle via secretion into the biliary tract in the form of cadmium–glutathione conjugates. Cadmium can then be enzymatically degraded to cadmium–cysteine complexes in the biliary tree, where it can re-enter the small intestine [1][50]. Cadmium accumulates in the renal tubular cells in the cortex of the kidneys via the transport of metallothionein. It resides there, where it can have a half-life of 10–30 years [51]. Lifelong exposure to and consumption of foods containing cadmium can lead to the accumulation of cadmium, and as it is very slowly excreted from the body, it causes irreversible tubular cell necrosis in the kidneys [1]. Unfortunately, the kidneys are the organs most susceptible to damage from cadmium accumulation [52], although chronic and prolonged exposure to cadmium can have devastating effects on various tissues of the human body and can even cause bone demineralisation [53]. When cadmium arrives at the kidneys in the form of Cd–M, it is filtered in the glomerulus and reabsorbed in the proximal convoluted tubules, where it tends to remain [1].
Cadmium concentrations can be measured in urine, hair, blood, nail, and saliva samples. Cadmium-induced kidney damage correlates with urinary cadmium excretion. Indeed, proteinuria characterised by the excretion of low-molecular-weight proteins such as retinol-binding protein or ß2-microglobulin [54] is likely to occur with a 10% response rate when the concentration of cadmium in the cortex exceeds approximately 200 μg/g wet weight (200 ppm) [51]. Moreover, urinary cadmium has been used as a non-invasive detection method of the accumulation of cadmium in the kidneys, and as a marker of tubular dysfunction in industrial workers and those who have had low environmental exposure. This is due to the curvilinear relationship between urinary cadmium and cadmium accumulation in the kidneys [31][55]. This allows for the urinary cadmium value corresponding to the critical kidney cadmium level of 200 ppm to be estimated at 10 μg/g creatinine, which is estimated in concordance with the relationship between urinary cadmium and proteinuria [54][56][57]. These measurements are now well-established and, in populations with excessive exposure to cadmium, urinary cadmium is correlated with the renal cadmium levels or body burden. Worryingly, these levels remain elevated many years after cessation of exposure [58].
While measuring ß2-microglobulin was previously thought to be the most reliable and accepted method of measuring cadmium burden and levels in humans, there are several other urinary biomarkers for the assessment of the renal effects of cadmium. A significant debate about the utility of these various biomarkers is ongoing [24]. These markers are outlined in Table 2 as per the publication of Satarug [24]. The associated renal biological effects are also enclosed in Table 2. These biomarkers are currently being used to assess the impacts of seafood and crab consumption on human health [59][60].
Table 2. Urinary biomarkers for the assessment of cadmium burden on the kidneys. Adapted from Satarug [24].
| Biomarkers | Abnormal Values | Interpretations and Associations |
|---|---|---|
| NAG | >4 U/g creatinine | Tubular injury, mortality |
| Lysozyme | >4 mg/g creatinine | Tubular injury |
| Total protein | >100 mg/g creatinine | Glomerular dysfunction, CKD |
| Albumin | >30 mg/g creatinine | Glomerular dysfunction, CKD |
| ß2MG | ≥1000 µg/g creatinine | Irreversible tubular dysfunction |
| ß2-MG | ≥300 µg/g creatinine | Mild tubular dysfunction, rapid GFR decline |
| ß2-MG | ≥145 µg/g creatinine | Increased hypertension risk |
| α1-MG | ≥400 µg/g creatinine | Mild tubular dysfunction |
| α1-MG | ≥1500 µg/g creatinine | Irreversible tubular dysfunction |
| KIM-1 | ≥1.6 mg/g creatinine in men ≥2.4 mg/g creatinine in women |
Kidney injury, urinary KIM-1 levels correlated with blood cadmium levels |
Abbreviations: NAG = N-acetyl-β-D-glucosaminidinase; ß2-MG = beta-2 microglobulin; α1-MG = α1-microglobulin; KIM-1 = kidney injury molecule-1; CKD = chronic kidney disease; GFR = glomerular filtration rate.
An interesting in vivo study assessed the bioavailability of cadmium from boiled crab hepatopancreas, inorganic cadmium, or dried wild mushroom fed to mice [61]. The study design included a control group of mice that received low levels of cadmium (<0.007 ppm) in their feed, which did not lead to detectable levels of cadmium over a 9-week exposure period. The authors used cadmium accumulation in the kidneys and liver as a measure of absorption. Notably, the bioavailability of cadmium from boiled crab hepatopancreas was lower than that of cadmium from mushroom or even inorganic cadmium. Cadmium in the crab hepatopancreas is mainly associated with denatured proteins with low solubility, whereas a large proportion of cadmium in dried mushroom is associated with soluble ligands. Therefore, there was an indication that the difference in cadmium speciation might account for the lower bioavailability of cadmium from crab than from mushroom. However, the authors commented that the difference in bioavailability was low, and that restricting intake was recommended if the products were high in cadmium. This may be evidence of cadmium speciation or, indeed, a food matrix effect. A similar study in which rats consumed a diet consisting of high crab intake (4 mg/kg organic-bound cadmium), a low-crab diet (0.2 mg/kg organic-bound cadmium), or a casein-based cadmium diet (4 mg/kg as cadmium chloride) for 6 months showed that cadmium intake from the high-crab diet was only half that of the diet consisting of cadmium chloride [62]. These findings also appear to indicate that there may be a food matrix effect at play. Other studies in humans have shown that cadmium is more bioaccessible from fish (84%) than from shellfish (73%) [63]. Worryingly, individuals who smoke cigarettes and have a high consumption of seafood can experience exacerbated adverse effects of cadmium exposure [63]. This is particularly dangerous for populations such as the PRC, where many of the people smoke frequently. There are still many questions regarding cadmium’s bioavailability that require further investigation, particularly regarding the food matrix effect and how it may be leveraged to mitigate dietary cadmium intake.
Another point to note is that current health risk assessments relating to cadmium exposure in humans rely heavily on the evaluation of the toxicity to the kidneys alone. In 2010, the Joint Food and Agriculture Organisation (FAO) and World Health Organisation (WHO) Expert Committee on Food Additives and Contaminants (JECFA) deemed the kidneys to be a suitable target for evaluating cadmium toxicity, as measurements of ß2-microglobulin could be used as a surrogate biomarker for the effects of dietary cadmium intake [64]. The JECFA established a tolerable monthly intake of 25 µg/kg/bodyweight per month, with a urinary cadmium excretion rate of 5.24 µg/g creatinine or 0.8 µg/kg/day as a nephrotoxicity threshold [64][65][66]. While the EFSA and JECFA share the same critical ß2-microglobulin endpoint of 300 µg/g creatinine, the EFSA adopted a different cadmium excretion rate of 1 µg/g creatinine as the nephrotoxicity threshold, along with an uncertainty factor of 0.36 µg/kg bodyweight per day for 50 years as a benchmark dose [67]. While these values are important references to monitor to stay within safe levels of cadmium exposure, relying on one biomarker (ß2-microglobulin) is insufficient. In 2019, Satarug et al. [68] showed that ß2-microglobulin excretion levels as low as 100–299 µg/g creatinine were associated with a 4.7-fold increase in eGFR to ≤60 mL/min/1.73 m2—a measurement consistent with chronic kidney disease. Therefore, a ß2-microglobulin endpoint of 300 µg/g creatinine may not be a low enough threshold to detect early nephrotoxicity [65].
Considering the emerging evidence that many organ systems are affected by cadmium exposure, other toxicity endpoints may be informative for risk assessment. As reviewed by Satarug et al. [65], other biomarkers of chronic low-dose cadmium exposure may contribute to risk assessments. For example, reductions in estimated glomerular filtration rate (eGFR) and lower fecundity have been observed at cadmium excretion levels as low as 0.5 µg/g creatinine, with worsening outcomes noted in a dose-dependent manner [65]. In men, sperm cadmium levels are inversely associated with sperm motility [69][70] and appear to be associated with other measures of sperm quality, viability, and acrosome reactions [65]. In females, high blood cadmium levels have been associated with infertility [71]. High urinary cadmium levels (~0.70 µg/L) have been associated with ovarian reserve depletion and ovarian insufficiency, with serum follicle-stimulating hormone (FSH) levels ≥ 10 IU/L [72] and ≥25 IU/L [73], respectively. However, sampling and monitoring of reproductive health is intrusive and inconvenient; therefore, surrogate markers such as serum FSH or anti-Mullerian hormone (AMH) in females may be useful. Blood biomarkers are preferred because of the convenience of analysing a blood sample. Therefore, alternative approaches have been sought, including monitoring of epigenetic factors [74]. Preliminary research indicates that cadmium exposure induces epigenetic changes in micro ribonucleic acids (miRNAs) that may lead to the development of novel blood-borne biomarkers [75][76]. Collectively, these findings indicate that additional novel biomarkers of human cadmium exposure are necessary to determine one’s risk of toxicity and disease, as opposed to the reliance on monitoring kidney function alone.
4. Cadmium Toxicity in Humans
Cadmium can affect important cellular functions such as cell differentiation, proliferation, and apoptosis, which is of concern considering that these processes overlap with the important processes of the generation of reactive oxygen species (ROS) and DNA repair mechanisms [77]. Cadmium at low concentrations even has the capacity to bind to mitochondria and can inhibit cellular oxidative phosphorylation and cellular respiration [78]. Cadmium exposure results in chromosomal aberrations, DNA strand breaks, sister chromatid exchange, and DNA–protein crosslinks. Cadmium can potentially cause mutations and chromosomal deletions [79]. Cadmium toxicity encompasses the depletion of reduced glutathione (GSH), binds sulfhydryl groups with proteins, and causes the enhanced production of ROS, resulting in oxidative stress, which may promote organ toxicity, apoptotic cell death, and carcinogenicity [77]. Cadmium can also inhibit the capacity of the natural antioxidant enzymes, such as catalase, manganese superoxide dismutase, and copper/zinc-dismutase [80]. Metallothionein is also involved in these processes and can act as a free-radical scavenger of hydroxyl and superoxide radicals [81]. Largely, the cells that contain metallothioneins are resilient to the effects of cadmium toxicity. However, it has been observed that cells that do not synthesise metallothioneins are sensitive to cadmium [77].
Cadmium has also been shown to be an endocrine disruptor. Cadmium may affect thyroid function, as demonstrated in both animal and human studies [82], where tissue damage in the thyroid led to hyperplasia and hypertrophy [83][84][85]. Moreover, cadmium has been linked with changes in hormone function [86][87], and there is suspicion that chronic cadmium exposure may lead to thyroid cancer, but further research is required [88]. Cadmium may also act as a metalloestrogen, as it can bind to the oestrogen receptor [89], which has led to a concern that chronic cadmium exposure may be associated with breast cancer [90][91][92]. There have also been links drawn between cadmium and the inhibition of progesterone synthesis, ovarian and reproductive tract morphological alterations, disruption to menstrual cycles, and issues with pregnancy and birth [93]. Likewise, cadmium may mimic some of the effects of androgens and may play a role in prostate cancer [94][95] and reduce male fertility by affecting spermatogenesis and motility [96].
The vast and various effects of cadmium exposure on the human body that have been explored in the above contents lead to various clinical manifestations. As such, it is known that different forms of cadmium compounds lead to different clinical manifestations. However, the details of this require further investigation. While cadmium poisoning is very rare, it can happen. Itai-itai disease is the most severe form of chronic cadmium toxicity in humans, caused by the prolonged ingestion of cadmium. Areas severely polluted by cadmium, such as the Jinzu River Basin in Toyama, Japan, have high incidences of cadmium-related pathologies. In that example, the river was polluted with slag from a mine upstream. The cadmium-polluted water was subsequently used to irrigate crops and rice between the 1910s and 1960s. The water from this river was used as potable water and for cooking, bathing, etc. [97]. This was significant, as cadmium is a food-chain contaminant that has high rates of soil-to-plant transference [24] and, thus, a high risk of ingestion. Itai-itai disease is characterised by renal tubular disorder and renal osteomalacia [98]. Even if people did not get itai-itai disease in the Jinzu Basin, they were at serious risk of cancer [99]. Some of the main effects of cadmium on the human body are presented in Figure 1. Patients with cadmium toxicity require significant treatment, including gastrointestinal tract irrigation, supportive care, and chemical decontamination via traditional chelation therapy with novel chelating agents and nanoparticle-based antidotes [77].
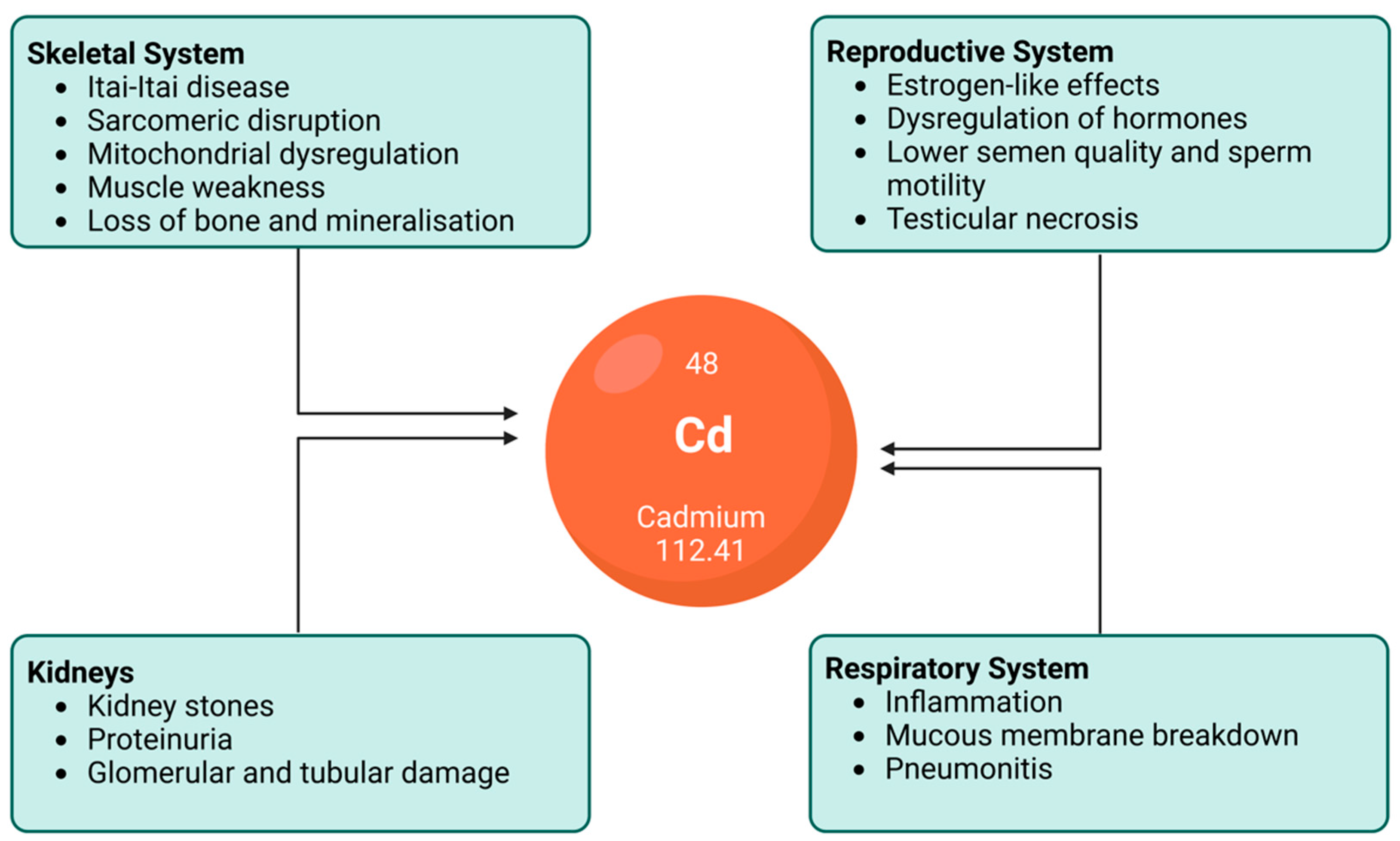
Figure 1. An illustration depicting the effects of chronic cadmium exposure on various human organs and systems. While not an exclusive list, this figure demonstrates the importance of mitigating excess cadmium ingestion.
References
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22.
- Ahn, J.; Kim, N.-S.; Lee, B.-K.; Oh, I.; Kim, Y. Changes of Atmospheric and Blood Concentrations of Lead and Cadmium in the General Population of South Korea from 2008 to 2017. Int. J. Environ. Res. Public Health 2019, 16, 2096.
- Lanceleur, L.; Schäfer, J.; Chiffoleau, J.-F.; Blanc, G.; Auger, D.; Renault, S.; Baudrimont, M.; Audry, S. Long-term records of cadmium and silver contamination in sediments and oysters from the Gironde fluvial–estuarine continuum–Evidence of changing silver sources. Chemosphere 2011, 85, 1299–1305.
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96.
- Andersen, O.; Nielsen, J.B.; Nordberg, G.F. Nutritional interactions in intestinal cadmium uptake–Possibilities for risk reduction. Biometals 2004, 17, 543–547.
- Järup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vanter, M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24, 1–51.
- Ashraf, M.W. Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Sci. World J. 2012, 2012, 729430.
- Keil, D.E.; Berger-Ritchie, J.; McMillin, G.A. Testing for Toxic Elements: A Focus on Arsenic, Cadmium, Lead, and Mercury. Lab. Med. 2011, 42, 735–742.
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 2004, 112, 1099–1103.
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445.
- European Food Safety Authority. Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551.
- Authority, E.F.S. Cadmium in food-Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2009, 7, 980.
- Food Safety Authority of Ireland. Metals of Toxicological Importance in the Irish Diet. Available online: https://www.lenus.ie/handle/10147/609836 (accessed on 29 August 2020).
- Irish Universities Nutrition Alliance. National Adult Nutrition Survey: Physical Measurements, Physical Activity Patterns and Food Choice Motives. Available online: https://www.iuna.net/surveyreports (accessed on 29 August 2022).
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2019, 11, 2.
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Chapter 1-The Origin of Chronic Diseases with Respect to Cardiovascular Disease. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–21.
- Awata, H.; Linder, S.; Mitchell, L.E.; Delclos, G.L. Association of Dietary Intake and Biomarker Levels of Arsenic, Cadmium, Lead, and Mercury among Asian Populations in the United States: NHANES 2011–2012. Environ. Health Perspect. 2017, 125, 314–323.
- Hecht, E.M.; Landy, D.C.; Ahn, S.; Hlaing, W.M.; Hennekens, C.H. Hypothesis: Cadmium explains, in part, why smoking increases the risk of cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 550–554.
- Zeng, L.; Ruan, M.; Liu, J.; Wilde, P.; Naumova, E.N.; Mozaffarian, D.; Zhang, F.F. Trends in Processed Meat, Unprocessed Red Meat, Poultry, and Fish Consumption in the United States, 1999–2016. J. Acad. Nutr. Diet. 2019, 119, 1085–1098.e1012.
- Kim, H.; Lee, J.; Woo, H.D.; Kim, D.W.; Choi, I.J.; Kim, Y.-I.; Kim, J. Association between dietary cadmium intake and early gastric cancer risk in a Korean population: A case–control study. Eur. J. Nutr. 2019, 58, 3255–3266.
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978.
- He, P.; Lu, Y.; Liang, Y.; Chen, B.; Wu, M.; Li, S.; He, G.; Jin, T. Exposure assessment of dietary cadmium: Findings from shanghainese over 40 years, China. BMC Public Health 2013, 13, 590.
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759.
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15.
- Callan, A.; Hinwood, A.; Devine, A. Metals in commonly eaten groceries in Western Australia: A market basket survey and dietary assessment. Food Addit. Contam. Part A 2014, 31, 1968–1981.
- Calafat, A.M. The U.S. National Health and Nutrition Examination Survey and human exposure to environmental chemicals. Int. J. Hyg. Environ. Health 2012, 215, 99–101.
- Adams, S.V.; Quraishi, S.M.; Shafer, M.M.; Passarelli, M.N.; Freney, E.P.; Chlebowski, R.T.; Luo, J.; Meliker, J.R.; Mu, L.; Neuhouser, M.L.; et al. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women’s Health Initiative. Environ. Health Perspect. 2014, 122, 594–600.
- Puerto-Parejo, L.M.; Aliaga, I.; Canal-Macias, M.L.; Leal-Hernandez, O.; Roncero-Martín, R.; Rico-Martín, S.; Moran, J.M. Evaluation of the Dietary Intake of Cadmium, Lead and Mercury and Its Relationship with Bone Health among Postmenopausal Women in Spain. Int. J. Environ. Res. Public Health 2017, 14, 564.
- Lavado-García, J.M.; Puerto-Parejo, L.M.; Roncero-Martín, R.; Moran, J.M.; Pedrera-Zamorano, J.D.; Aliaga, I.J.; Leal-Hernández, O.; Canal-Macias, M.L. Dietary Intake of Cadmium, Lead and Mercury and Its Association with Bone Health in Healthy Premenopausal Women. Int. J. Environ. Res. Public Health 2017, 14, 1437.
- Lin, J.; Zhang, F.; Lei, Y. Dietary intake and urinary level of cadmium and breast cancer risk: A meta-analysis. Cancer Epidemiol. 2016, 42, 101–107.
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.-t.; Kong, Q.; Wang, Z.; Li, P.; Lundström, N.-G.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 2002, 15, 397–410.
- Taylor, W.R. Permeation of barium and cadmium through slowly inactivating calcium channels in cat sensory neurones. J. Physiol. 1988, 407, 433–452.
- Foulkes, E.C. Interactions between metals in rat jejunum: Implications on the nature of cadmium uptake. Toxicology 1985, 37, 117–125.
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19.
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Fox, M.R.S. Nutritional Factors that May Influence Bioavailability of Cadmium. J. Environ. Qual. 1988, 17, 175–180.
- Bondgaard, M.; Nørum, U.; Bjerregaard, P. Cadmium accumulation in the female shore crab Carcinus maenas during the moult cycle and ovarian maturation. Mar. Biol. 2000, 137, 995–1004.
- Bjerregaard, P.; Bjørn, L.; Nørum, U.; Pedersen, K.L. Cadmium in the shore crab Carcinus maenas: Seasonal variation in cadmium content and uptake and elimination of cadmium after administration via food. Aquat. Toxicol. 2005, 72, 5–15.
- Bjerregaard, P.; Jensen, L.B.E.; Pedersen, K.L. Effect of size on concentrations and cadmium inducibility of metallothionein in the shore crab Carcinus maenas. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109146.
- Siewicki, T.; Balthrop, J. Comparison of the digestion of oyster tissue containing intrinsically or extrinsically labeled cadmium . Nutr. Rep. Int. 1983, 27, 899–909.
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29.
- Reeves, P.G.; Chaney, R.L. Nutritional status affects the absorption and whole-body and organ retention of cadmium in rats fed rice-based diets. Environ. Sci. Technol. 2002, 36, 2684–2692.
- Reeves, P.G.; Chaney, R.L. Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ. Res. 2004, 96, 311–322.
- Reeves, P.G.; Chaney, R.L.; Simmons, R.W.; Cherian, M.G. Metallothionein induction is not involved in cadmium accumulation in the duodenum of mice and rats fed diets containing high-cadmium rice or sunflower kernels and a marginal supply of zinc, iron, and calcium. J. Nutr. 2005, 135, 99–108.
- Flanagan, P.R.; McLellan, J.S.; Haist, J.; Cherian, M.G.; Chamberlain, M.J.; Valberg, L.S. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology 1978, 74, 841–846.
- Silver, M.K.; Lozoff, B.; Meeker, J.D. Blood cadmium is elevated in iron deficient U.S. children: A cross-sectional study. Environ. Health 2013, 12, 117.
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488.
- Kim, D.-W.; Kim, K.-Y.; Choi, B.-S.; Youn, P.; Ryu, D.-Y.; Klaassen, C.D.; Park, J.-D. Regulation of metal transporters by dietary iron, and the relationship between body iron levels and cadmium uptake. Arch. Toxicol. 2007, 81, 327–334.
- Richens, D.T. The Chemistry of Aqua Ions; Wiley Chichester: Chichester, UK, 1997.
- Zalups, R.K.; Ahmad, S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003, 186, 163–188.
- Bernard, A. Confusion about Cadmium Risks: The Unrecognized Limitations of an Extrapolated Paradigm. Environ. Health Perspect. 2016, 124, 1–5.
- Barbier, O.; Jacquillet, G.; Tauc, M.; Cougnon, M.; Poujeol, P. Effect of Heavy Metals on, and Handling by, the Kidney. Nephron Physiol. 2005, 99, 105–110.
- Aitio, A.; Kiilunen, M.; Santonen, T.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Chapter: Gold and Gold Mining; Elsevier: Amsterdam, The Netherlands, 2015.
- Bernard, A. Renal dysfunction induced by cadmium: Biomarkers of critical effects. Biometals 2004, 17, 519–523.
- Bernard, A.; Roels, H.; Buchet, J.-P.; Cardenas, A.; Lauwerys, R. Cadmium and health: The Belgian experience. IARC Sci. Publ. 1992, 118, 15–33.
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2015.
- Chaumont, A.; De Winter, F.; Dumont, X.; Haufroid, V.; Bernard, A. The threshold level of urinary cadmium associated with increased urinary excretion of retinol-binding protein and β2-microglobulin: A re-assessment in a large cohort of nickel-cadmium battery workers. Occup. Environ. Med. 2011, 68, 257–264.
- Liang, Y.; Lei, L.; Nilsson, J.; Li, H.; Nordberg, M.; Bernard, A.; Nordberg, G.F.; Bergdahl, I.A.; Jin, T. Renal Function after Reduction in Cadmium Exposure: An 8-Year Follow-up of Residents in Cadmium-Polluted Areas. Environ. Health Perspect. 2012, 120, 223–228.
- Dyck, K.N.; Bashir, S.; Horgan, G.W.; Sneddon, A.A. Regular crabmeat consumers do not show increased urinary cadmium or beta-2-microglobulin levels compared to non-crabmeat consumers. J. Trace Elem. Med. Biol. 2019, 52, 22–28.
- Guan, S.; Palermo, T.; Meliker, J. Seafood intake and blood cadmium in a cohort of adult avid seafood consumers. Int. J. Hyg. Environ. Health 2015, 218, 147–152.
- Lind, Y.; Wicklund Glynn, A.; Engman, J.; Jorhem, L. Bioavailability of cadmium from crab hepatopancreas and mushroom in relation to inorganic cadmium: A 9-week feeding study in mice. Food Chem. Toxicol. 1995, 33, 667–673.
- Maage, A.; Julshamn, K. A comparison of dressed crab and a cadmium salt (CdCl2) as cadmium sources in rat diets. Comp Biochem. Physiol. C Comp. Pharm. Toxicol. 1987, 88, 209–211.
- Ju, Y.-R.; Chen, W.-Y.; Liao, C.-M. Assessing human exposure risk to cadmium through inhalation and seafood consumption. J. Hazard. Mater. 2012, 227–228, 353–361.
- FAO/WHO (Food and Agriculture Organisation/World Health Organization). Joint FAO/WHO Expert Committee on Food Additives. In Proceedings of the Seventy-Third Meeting, Geneva, Switzerland,, 8–17 June 2010; Summary and Conclusions. Issued 24 June 2010. Available online: https://www.who.int/publications/i/item/9789241209601 (accessed on 29 September 2022).
- Satarug, S.; Gobe, G.C.; Vesey, D.A. Multiple Targets of Toxicity in Environmental Exposure to Low-Dose Cadmium. Toxics 2022, 10, 472.
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122.
- The Panel on Contaminants in the Food Chain of the European Food Safety Authority (CONTAM Panel). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975.
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019, 173, 40–47.
- Mitra, S.; Varghese, A.C.; Mandal, S.; Bhattacharyya, S.; Nandi, P.; Rahman, S.M.; Kar, K.K.; Saha, R.; Roychoudhury, S.; Murmu, N. Lead and cadmium exposure induces male reproductive dysfunction by modulating the expression profiles of apoptotic and survival signal proteins in tea-garden workers. Reprod. Toxicol. 2020, 98, 134–148.
- Wang, Y.X.; Wang, P.; Feng, W.; Liu, C.; Yang, P.; Chen, Y.J.; Sun, L.; Sun, Y.; Yue, J.; Gu, L.J.; et al. Relationships between seminal plasma metals/metalloids and semen quality, sperm apoptosis and DNA integrity. Environ. Pollut. 2017, 224, 224–234.
- Lee, S.; Min, J.-Y.; Min, K.-B. Female Infertility Associated with Blood Lead and Cadmium Levels. Int. J. Environ. Res. Public Health 2020, 17, 1794.
- Upson, K.; O’Brien, K.M.; Hall, J.E.; Tokar, E.J.; Baird, D.D. Cadmium Exposure and Ovarian Reserve in Women Aged 35-49 Years: The Impact on Results From the Creatinine Adjustment Approach Used to Correct for Urinary Dilution. Am. J. Epidemiol. 2021, 190, 116–124.
- Pan, W.; Ye, X.; Zhu, Z.; Li, C.; Zhou, J.; Liu, J. Urinary cadmium concentrations and risk of primary ovarian insufficiency in women: A case–control study. Environ. Geochem. Health 2021, 43, 2025–2035.
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782.
- Awadalla, A.; Mortada, W.I.; Abol-Enein, H.; Shokeir, A.A. Correlation between blood levels of cadmium and lead and the expression of microRNA-21 in Egyptian bladder cancer patients. Heliyon 2020, 6, e05642.
- Wallace, D.R.; Taalab, Y.M.; Heinze, S.; Tariba Lovaković, B.; Pizent, A.; Renieri, E.; Tsatsakis, A.; Farooqi, A.A.; Javorac, D.; Andjelkovic, M.; et al. Toxic-Metal-Induced Alteration in miRNA Expression Profile as a Proposed Mechanism for Disease Development. Cells 2020, 9, 901.
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.-A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145.
- Patrick, L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern. Med. Rev. 2003, 8, 106–128.
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279.
- Filipič, M. Mechanisms of cadmium induced genomic instability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2012, 733, 69–77.
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214.
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A.; Cella, V.; Lenzi, A.; Ferretti, E.; Migliaccio, S. The endocrine disruptor cadmium: A new player in the pathophysiology of metabolic diseases. J. Endocrinol. Investig. 2021, 44, 1363–1377.
- Nie, X.; Chen, Y.; Chen, Y.; Chen, C.; Han, B.; Li, Q.; Zhu, C.; Xia, F.; Zhai, H.; Wang, N.; et al. Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ. Pollut. 2017, 230, 320–328.
- Rezaei, M.; Javadmoosavi, S.Y.; Mansouri, B.; Azadi, N.A.; Mehrpour, O.; Nakhaee, S. Thyroid dysfunction: How concentration of toxic and essential elements contribute to risk of hypothyroidism, hyperthyroidism, and thyroid cancer. Environ. Sci. Pollut. Res. Int. 2019, 26, 35787–35796.
- Yu, Y.; Ma, R.; Yu, L.; Cai, Z.; Li, H.; Zuo, Y.; Wang, Z.; Li, H. Combined effects of cadmium and tetrabromobisphenol a (TBBPA) on development, antioxidant enzymes activity and thyroid hormones in female rats. Chem. Biol. Interact. 2018, 289, 23–31.
- Chen, A.; Kim, S.S.; Chung, E.; Dietrich, K.N. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ. Health Perspect. 2013, 121, 181–186.
- Jain, R.B.; Choi, Y.S. Interacting effects of selected trace and toxic metals on thyroid function. Int. J. Environ. Health Res. 2016, 26, 75–91.
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501.
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Cadmium—A metallohormone? Toxicol. Appl. Pharmacol. 2009, 238, 266–271.
- Brama, M.; Gnessi, L.; Basciani, S.; Cerulli, N.; Politi, L.; Spera, G.; Mariani, S.; Cherubini, S.; d’Abusco, A.S.; Scandurra, R.; et al. Cadmium induces mitogenic signaling in breast cancer cell by an ERα-dependent mechanism. Mol. Cell. Endocrinol. 2007, 264, 102–108.
- Strumylaite, L.; Kregzdyte, R.; Bogusevicius, A.; Poskiene, L.; Baranauskiene, D.; Pranys, D. Cadmium Exposure and Risk of Breast Cancer by Histological and Tumor Receptor Subtype in White Caucasian Women: A Hospital-Based Case-Control Study. Int. J. Mol. Sci. 2019, 20, 3029.
- Wang, Y.; Shi, L.; Li, J.; Li, L.; Wang, H.; Yang, H. Long-term cadmium exposure promoted breast cancer cell migration and invasion by up-regulating TGIF. Ecotoxicol. Environ. Saf. 2019, 175, 110–117.
- Henson, M.C.; Chedrese, P.J. Endocrine Disruption by Cadmium, a Common Environmental Toxicant with Paradoxical Effects on Reproduction. Exp. Biol. Med. 2004, 229, 383–392.
- Dai, C.; Heemers, H.; Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452.
- Chandrasekaran, B.; Dahiya, N.R.; Tyagi, A.; Kolluru, V.; Saran, U.; Baby, B.V.; States, J.C.; Haddad, A.Q.; Ankem, M.K.; Damodaran, C. Chronic exposure to cadmium induces a malignant transformation of benign prostate epithelial cells. Oncogenesis 2020, 9, 23.
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338.
- Aoshima, K. Itai-itai disease: Lessons from the investigations of environmental epidemiology conducted in the 1970’s, with special reference to the studies of the Toyama Institute of Health. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2017, 72, 149–158.
- Imura, J.; Tsuneyama, K.; Ueda, Y. Novel Pathological Study of Cadmium Nephropathy of Itai-itai Disease. In Cadmium Toxicity: New Aspects in Human Disease, Rice Contamination, and Cytotoxicity; Himeno, S., Aoshima, K., Eds.; Springer: Singapore, 2019; pp. 39–50.
- Nishijo, M.; Nakagawa, H.; Suwazono, Y.; Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T. Cancer Mortality in Residents of the Cadmium-Polluted Jinzu River Basin in Toyama, Japan. Toxics 2018, 6, 23.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Revisions:
2 times
(View History)
Update Date:
02 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




