Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qihui Wu | -- | 1239 | 2022-11-02 03:15:18 | | | |
| 2 | Conner Chen | + 4 word(s) | 1243 | 2022-11-02 04:39:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, C.; Lu, B.; Xiong, H.; Lin, C.; Fang, L.; Fu, J.; Deng, D.; Fan, X.; Li, Y.; Wu, Q. Crystal and Electronic Structure of Perovskite Oxides. Encyclopedia. Available online: https://encyclopedia.pub/entry/32382 (accessed on 05 March 2026).
Zhang C, Lu B, Xiong H, Lin C, Fang L, Fu J, et al. Crystal and Electronic Structure of Perovskite Oxides. Encyclopedia. Available at: https://encyclopedia.pub/entry/32382. Accessed March 05, 2026.
Zhang, Chi, Bin Lu, Haiji Xiong, Chengjun Lin, Lin Fang, Jile Fu, Dingrong Deng, Xiaohong Fan, Yi Li, Qi-Hui Wu. "Crystal and Electronic Structure of Perovskite Oxides" Encyclopedia, https://encyclopedia.pub/entry/32382 (accessed March 05, 2026).
Zhang, C., Lu, B., Xiong, H., Lin, C., Fang, L., Fu, J., Deng, D., Fan, X., Li, Y., & Wu, Q. (2022, November 02). Crystal and Electronic Structure of Perovskite Oxides. In Encyclopedia. https://encyclopedia.pub/entry/32382
Zhang, Chi, et al. "Crystal and Electronic Structure of Perovskite Oxides." Encyclopedia. Web. 02 November, 2022.
Copy Citation
Perovskites have been proven to be the one of best cathodes for the solid oxide electrolyte cell (SOEC) devices, in particular, Co-based ones usually exhibit extremely high catalytic performances due to the multivalent properties of Co ions. Thorough understanding of the crystal and electronic structure of perovskite oxides are important.
cobalt-based perovskite
oxygen evolution reactions
catalyst
1. Introduction
In recent years, the methods for hydrogen gas production have attracted much attentions due to the increasing concerns of environmental protection. As the reverse process of solid oxide fuel cells (SOFCs), solid oxide electrolysis cells (SOECs) have been considered as effective devices for hydrogen production with low cost, high efficiency, and environmental friendly [1]. High working temperatures could accelerate the reaction speed in SOECs, thus improving their electrocatalytic capacity [2]. However, even if SOECs take the advantages from elevated temperatures, it is still essential to decrease the working temperatures over long device duration times [3]. However, unfortunately, low working temperatures would also decrease the electrocatalytic activity of the oxygen electrodes and thus result in the degradation of the catalytic performance. Therefore, the development of low-temperature oxygen electrodes with high electrochemical activities is essential research concerning SOECs [4]
Oxygen electrodes applied in SOECs are mainly reference the extensively studied cathodes for SOFCs [5][6][7][8]. In SOEC processes, the oxygen evolution reactions (OER) are the most important reactions during water splitting into oxygen and hydrogen gases, in this case, the study of OER, therefore, have become a main topic in the study of hydrogen production in SOECs. Trasatti et al. [9] investigated the OER activities of different perovskite oxides and emphasized a volcano-shaped relationship combining the enthalpy and the corresponding OER overpotential. At the same time, Bockris et al. [10] clarified that the current density at a constant overpotential was contrarily dependent on the formation enthalpy of M(OH)3 hydroxide. The previous activity and mechanism study became the basis of the subsequent design of efficient oxide electrocatalysts [11]. The perovskite structure, possessing various types of rare/alkaline earth metal cations (A site) and 3D transition-metal cations (M sites), has been reported to exhibit different architectures, some of them possessing diffusion channels for oxygen ions that would be advantageous in the OER process [12]. Partial metal substitution either at A or M sites would significantly influence not only the charge state and distribution of ions in the perovskite structure, but also its electric conductivity, and finally the OER performance [13].
At high temperatures, the defective structure of oxides and their related chemical activities have been extensively detected. Ln2NiO4-δ (Ln = Pr, La, Nd) series have been investigated as oxygen electrodes for SOECs [14]. Former data has indicated that La2NiO4-δ (LNO) exhibited obvious superiority electrocatalytic activities [15] and performance of surface oxygen exchange [16]. Liu and coworkers confirmed that LNO applied as an SOEC oxygen electrode showed excellent electrochemical catalytic properties [17]. However, former research has also indicated that LNO electrodes would decompose in SOEC operating situations if CexGd1-xO2-δ (GDC) was used as the oxide electrolyte [18]. This resulted in a rapid degeneracy in performance [16]. Flua’s group further proved that the decomposition of LNO on GDC was induced by element diffusion at the LNO–GDC interphase [19]. This decomposition phenomenon was also found at the La0.5Sr0.5MnO3 (LSMO) electrode [20]. The LSMO gradually cracked into nanoparticles due to oxygen ions migrating through the electrolyte during anodic polarization, which led to an alteration in valence of manganese ions and thereafter resulted in lattice shrinkage and consequent breakdown into particles [21][22]. Due to this concern, improved stability of perovskite oxides is a critical point in SOEC research. In addition, although LNO and LSMO have superior performance in oxygen surface exchange [16], they have weakness in electronic conductivity. With the aim to overcome this shortcoming, a La2NiO4-δ-coated PrBa0.5Sr0.5Co1.5Fe0.5O5-δ electrode was prepared which showed good electrochemical performances [23][24]. At the same time, La1-xSrxCo1-yNiyO3-δ is a common cathode for SOFCs [25][26], moreover, La1-xSrxCo1-yNiyO3-δ (LSCNO), La1-xSrxCo1-yFeyO3-δ (LSCFO) [27], and BaxSr1-xCoyFe1-yO3-δ (BSCFO) [28] have also been widely investigated as electrodes for SOECs [29][30]. High electronic conductivity was found for Co-based electrodes [31][32], however this was accompanied by an increasing thermal expansion coefficient [33]. In this case, preparing Co-based perovskites with high thermal stability is a challenge.
2. Crystal and Electronic Structure of Perovskite Oxides
The crystal structure of perovskite oxide (AMO3), taking LaCoO3 (LCO) as example, is shown in Figure 1a, which easily describes the prototypical ion-covalent, and the CoO6 octahedra connected by the corners [34][35]. It features good divisibility in that the A site can be located in any rare-earth or alkaline earth metals, while the M site can be any transition metal, as shown in Figure 1b for the Sr-substituted LaCoO3. In addition, the oxygen stoichiometry can also be varied based on the ratio of different kinds of metal ions. This structural adaptability has been successfully applied to tune the electronic structure of M site cations, specifically recognized as the OER active center, and therefore a target to manipulate the electrocatalytic activities. For example, Cheng and coworkers partially doped Sr2+ at the A site in LCO and then obtained increasing activity in OERs, which was attributed to the enhancement in overlap between the unoccupied Co 3d conduction bands and the occupied O 2p valence bands [36]. Zhang et al. replaced Ni with Fe in LaNiO3 (LNO) to promote its OER activity, resulting from the influence of the Fe doping in strengthening the Ni–O bonding and thus restraining the generation of Ni2+ ions on the LaNi1-xFexO3 surface [37]. By employing a reductive heating treatment, Yang et al. produced high oxygen-deficient CaMnO2.5, which exhibited great enhancement in OER performance when compared with unreduced CaMnO3. The increase in OER activity was assigned primarily to the propitious electronic configuration of high-spin Mn3+ ions induced by sufficient oxygen vacancies in CaMnO2.5 [38].
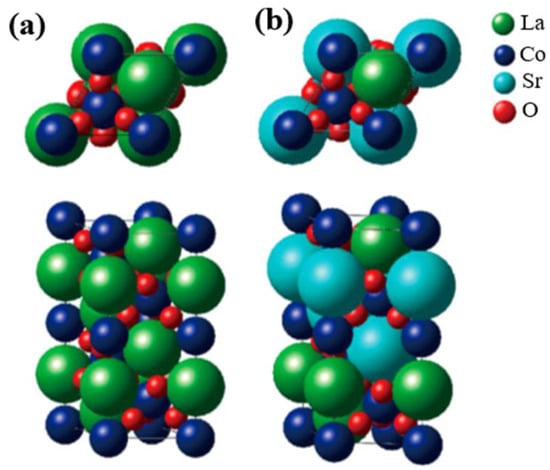
Figure 1. Crystal structure of (a) LaCoO3 and (b) Sr-doped LaCoO3 (La0.5Sr0.5CoO3). The bulk structure corresponds to the space group rhombohedral R3ch. Derived from [35].
A heterogeneous catalysis greatly relied on the surface electronic structure models associated with the catalytic properties. In this case, the surface oxygen reactivity is thus an important factor for oxidation chemical reactions, such as OERs [39][40]. Due to their excellent flexibility in accepting a high variability of cations in A and/or M locations, the electronic conductivity of perovskites can be manipulated from insulating to metallic. Through careful material design, electronic structures in perovskites can be tailored to the thermodynamic energies of a variety of reactions to minimize electrochemical reaction barriers. Before describing the role of the active sites in OERs, common ideas on the electronic structure of perovskites should be given. The charge transfer energy gained from density functional theory (DFT) calculations has been proven to be the most proper descriptor, which leads to a model of the charge transfer energy between the adsorbates and transition metals (assigned as Δ′ in Figure 2). A recently proposed descriptor, charge transfer energy (Δ), displays a linear relationship with the polarization potential (η). This is because that the Δ values are obtained from both theoretical calculations and spectroscopic data. This finding proposes that Δ is the most helpful descriptor in the design of OER perovskite oxide catalysts, as Δ decreases, the Δ′ may also decrease and lower the energy. The O 2p band center relative to the Fermi energy and eg electron number of the transition metal ions at the Msites is not necessarily adequate for the most part of perovskite oxides [41].
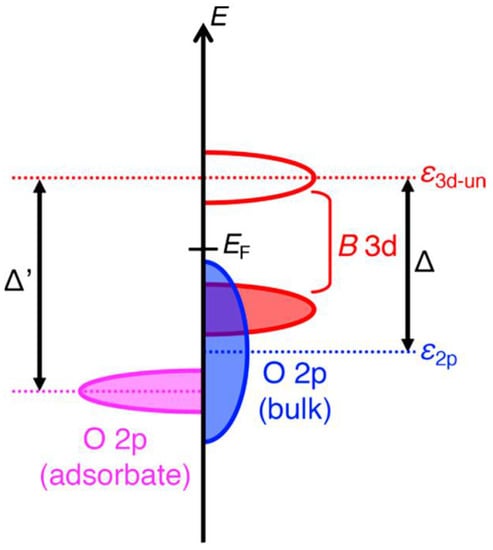
Figure 2. Schematic diagram of the charge transfer energy (Δ) between the catalyst and the adsorbates (Δ′) [41].
References
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218.
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354.
- Gaudillere, C.; Navarrete, L.; Serra, J.M. Syngas production at intermediate temperature through H2O and CO2 electrolysis with a Cu-based solid oxide electrolyzer cell. Int. J. Hydrogen Energy 2014, 39, 3047–3054.
- Buttler, A.; Hartmut, S. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454.
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463.
- Ritucci, H.; Agersted, K.; Zielke, P.; Wulff, A.C.; Khajavi, P.; Smeacetto, F.; Sabato, A.G.; Kiebach, R. A Ba-free sealing glass with a high coefficient of thermal expansion and excellent interface stability optimized for SOFC/SOEC stack applications. Int. J. Appl. Ceram. Technol. 2018, 15, 1011–1022.
- Hou, Y.; Wang, L.; Bian, L.; Wang, Y.; Chou, K.C. Excellent electrochemical performance of La0.3Sr0.7Fe0.9Ti0.1O3-δ as a symmetric electrode for solid oxide cells. ACS Appl. Mater. Interfaces 2021, 13, 22381–22390.
- Li, P.; Xuan, Y.; Jiang, B.; Zhang, S.; Xia, C. Hollow La0.6Sr0.4Ni0.2Fe0.75Mo0.05O3-δ electrodes with exsolved FeNi3 in quasi-symmetrical solid oxide electrolysis cells for direct CO2 electrolysis. Electrochem. Commun. 2022, 134, 107188.
- Trasatti, S. Electrocatalysis by oxides-Attempt at a unifying approach. Electroanal. Chem. Interfacial Electrochem. 1980, 111, 125–131.
- Otagawa, T.; Bockris, J.O. Oxygen evolution on perovskites. J. Phys. Chem. 1983, 87, 2960–2971.
- Gupta, S.; Kellogg, W.; Xu, H.; Liu, X.; Cho, J.; Wu, G. Bifunctional perovskite oxide catalysts for oxygen reduction and evolution in alkaline media. Chem. Asian J. 2016, 11, 10–21.
- Han, P.; Jin, K.J.; Lu, H.B.; Jia, J.F.; Qiu, J.; Hu, C.L.; Zhen, G.Z. Influence of oxygen vacancy on transport property of perovskite oxide heterostructures. Chin. Phys. Lett. 2009, 26, 027301.
- Lu, C.H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and ion substitution in colloidal metal halide perovskite nanoscrystals. Chem. Soc. Rev. 2020, 49, 4953–5007.
- Laguna-Bercero, M.A.; Monzon, H.; Larrea, A.; Orera, V.M. Improved stability of reversible solid oxide cells with a nickelate-based oxygen electrode. J. Mater. Chem. 2016, 4, 1446–1453.
- Lee, Y.; Kim, H. Electrochemical performance of La2NiO4-δ cathode for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2015, 41, 5984–5991.
- Tong, X.; Zhou, F.; Yang, S.; Zhong, S.; Wei, M.; Liu, Y. Performance and stability of Ruddlesden-Popper La2NiO4-δ oxygen electrodes under solid oxide electrolysis cell operation conditions. Ceram. Int. 2017, 43, 10927–10933.
- Kim, S.J.; Kim, K.J.; Dayaghi, A.M.; Choi, G.M. Polarization and stability of La2NiO4-δ in comparison with La0.6Sr0.4Co0.2Fe0.8O3-δ as air electrode of solid oxide electrolysis cell. Int. J. Hydrogen Energy 2016, 41, 14498–14506.
- Spiridigliozzi, L.; Di Bartolomeo, E.; Dell’Agli, G. Zurlo, F. GDC-based infiltrated electrodes for solid oxide electrolyzer cells (SOECs). Appl. Sci. 2020, 10, 3882.
- Flura, A.; Nicollet, C.; Vibhu, V.; Zeimetz, B.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. Application of the Adler-Lane-Steele model to porous La2NiO4-δ SOFCcathode: Influence of interfaces with gadolinia doped ceria. J. Electrochem. Soc. 2016, 163, F523–F532.
- Liu, H.; Qi, J.; Feng, M.; Xu, H.; Liu, R.; Li, N.; Wang, C.; Zhang, Y.; Zhang, Y.; Lu, W. The tunability of oxygen evolution reaction in flexible van der Waals manganite membrane. Adv. Sustain. Sys. 2021, 5, 2100073.
- Huang, M.; Jiang, H.; Liu, X.; Xiao, Y.; Kong, J.; Zhou, T. LSCM-GDC as composite cathodes for high temperature steam electrolysis: Performance optimization by composition and microstructure tailoring. Int. J. Hydrogen Energy 2022, 47, 34784–34793.
- Busse, P.; Yin, Z.; Mierwaldt, D.; Scholz, J.; Kressdorf, B.; Glaser, L.; Miedema, P.S.; Rothkirch, A.; Viefhaus, J.; Jooss, C.; et al. Probing the surface of La0.6Sr0.4MnO3 in water vapor by in situ photo-in/photon-out spectroscopy. J. Phys. Chem. C 2020, 124, 7893–7902.
- Li, J.; Qiu, P.; Xia, M.; Jia, L.; Chi, B.; Pu, J.; Li, J. Microstructure optimization for high performance PrBa0.5Sr0.5Co1.5Fe0.5O5-δ-La2NiO4-δ core-shell cathode of solid oxide fuel cells. J. Power Sources 2018, 379, 206–211.
- Li, J.; Zhang, Q.; Qiu, P.; Jia, L.; Chi, B.; Pu, J.; Li, J. A CO2-tolerant La2NiO4-δ-coated PrBa0.5Sr0.5Co1.5Fe0.5O5-δ cathode for intermediate temperature solid oxide fuel cells. J. Power Sources 2017, 342, 623–628.
- Hjalmarsson, P.; Sogaard, M.; Mogensen, M. Electrochemical behaviour of (La1-xSrx)sCo1-yNiyO3-δ as porous SOFC cathodes. Solid State Ionics 2009, 180, 1395–1405.
- Chen, J.; Liang, F.L.; Liu, L.; Jiang, S.P.; Li, J. Characterization and evaluation of La0.8Sr0.2Co0.8Ni0.2O3-δ prepared by a polymer-assisted combustion synthesis as a cathode material for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2009, 34, 6845–6851.
- Ai, N.; He, S.; Li, N.; Zhang, Q.; Rickard, W.D.A.; Chen, K.; Zhang, T.; Jiang, S.P. Suppressed Sr segregation and performance of directly assembled La0.6Sr0.4Co0.2Fe0.8O3-δ oxygen electrode on Y2O3-ZrO2 electrolyte of solid oxide electrolysis cells. J. Power Sources 2018, 384, 125–135.
- Qiu, P.; Wang, A.; Li, J.; Li, Z.; Jia, L.; Chi, B.; Pu, J.; Li, J. Promoted CO2-poisoningresistance of La0.8Sr0.2MnO3-δ-coated Ba0.5Sr0.5Co0.8Fe0.2O3-δ cathode for intermediate temperature solid oxide fuel cells. J. Power Sources 2016, 327, 408–413.
- Tan, Y.; Duan, N.; Wang, A.; Yan, D.; Chi, B.; Wang, N.; Pu, J.; Li, J. Performance enhancement of solution impregnated nanostructured La0.8Sr0.2Co0.8Ni0.2O3-δ oxygen electrode for intermediate temperature solid oxide electrolysis cells. J. Power Sources 2016, 305, 168–174.
- Zheng, H.; Tian, Y.; Zhang, L.; Chi, B.; Pu, J.; Jian, L. La0.8Sr0.2Co0.8Ni0.2O3-δ impregnated oxygen electrode for H2O/CO2 co-electrolysis in solid oxide electrolysis cells. J. Power Sources 2018, 383, 93–101.
- Liu, Y.; Chen, J.; Liang, F.; Pu, J.; Chi, B.; Jian, L. Thermochemical compatibility and polarization behaviors of La0.8Sr0.2Co0.8Ni0.2O3-δ as a cathode material for solid oxide fuel cell. Int. J. Hydrogen Energy 2013, 38, 6802–6808.
- Sun, C.; Alonso, J.A.; Bian, J. Recent advances in perovskite-type oxides for energy conversion and storage applications. Adv. Energy Mater. 2020, 11, 2000459.
- van Doorn, R.H.E.; Bouwmeester, H.J.M.; Burggraaf, A.J. Kinetic decomposition of La0.3Sr0.7CoO3-δ perovskite membranes during oxygen permeation. Solid State Ionics 1998, 111, 263–272.
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756.
- Seo, M.H.; Park, H.W.; Lee, D.U.; Park, M.G.; Chen, Z. Design of highly active perovskite oxides for oxygen evolution reaction by combining experimental and ab initio studies. ACS Catal. 2015, 5, 4337–4344.
- Cheng, Y.; Raman, A.S.; Paige, J.; Zhang, L.; Sun, D.; Chen, M.U.; Vojvodic, A.; Gorte, R.J.; Vohs, J.M. Enhancing oxygen exchange activity by tailoring perovskite surfaces. J. Phys. Chem. Lett. 2019, 10, 4082–4088.
- Zhang, D.; Song, Y.; Du, Z.; Wang, L.; Li, Y.; Goodenough, J.B. Active LaNi1-xFexO3 bifunctional catalysts for cathodes in alkaline media. J. Mater. Chem. A 2015, 3, 9421–9426.
- Kim, J.; Chen, X.; Pan, Y.; Shih, P.; Yang, H. W-doped CaMnO2.5 and CaMnO3 electrocatalysts for enhanced performance in oxygen evolution and reduction reactions. J. Electrochem. Soc. 2017, 164, F1074–F1080.
- Dickens, C.F.; Montoya, J.H.; Kulkarni, A.R.; Bajdich, M.; Norskov, J.K. An electronic structure descriptor for oxygen reactivity at metal and metal-oxide surfaces. Surf. Sci. 2019, 681, 122–129.
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385.
- Yamada, I.; Takamatsu, A.; Asai, K.; Shirakawa, T.; Ohzuku, H.; Seno, A.; Uchimura, T.; Fujii, H.; Kawaguchi, S.; Wada, K.; et al. Systematic study of descriptors for oxygen evolution reaction catalysis in perovskite oxides. J. Phys. Chem. C 2018, 122, 27885–27892.
More
Information
Subjects:
Crystallography
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





