Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Perovskites have been proven to be the one of best cathodes for the SOEC devices, in particular, Co-based ones usually exhibit extremely high catalytic performances due to the multivalent properties of Co ions. Thorough understanding of the crystal and electronic structure of perovskite oxides are important.
- cobalt-based perovskite
- oxygen evolution reactions
- catalyst
1. Introduction
In recent years, the methods for hydrogen gas production have attracted much attentions due to the increasing concerns of environmental protection. As the reverse process of solid oxide fuel cells (SOFCs), solid oxide electrolysis cells (SOECs) have been considered as effective devices for hydrogen production with low cost, high efficiency, and environmental friendly [1]. High working temperatures could accelerate the reaction speed in SOECs, thus improving their electrocatalytic capacity [2]. However, even if SOECs take the advantages from elevated temperatures, it is still essential to decrease the working temperatures over long device duration times [3]. However, unfortunately, low working temperatures would also decrease the electrocatalytic activity of the oxygen electrodes and thus result in the degradation of the catalytic performance. Therefore, the development of low-temperature oxygen electrodes with high electrochemical activities is essential research concerning SOECs [4]
Oxygen electrodes applied in SOECs are mainly reference the extensively studied cathodes for SOFCs [5,6,7,8]. In SOEC processes, the oxygen evolution reactions (OER) are the most important reactions during water splitting into oxygen and hydrogen gases, in this case, the study of OER, therefore, have become a main topic in the study of hydrogen production in SOECs. Trasatti et al. [9] investigated the OER activities of different perovskite oxides and emphasized a volcano-shaped relationship combining the enthalpy and the corresponding OER overpotential. At the same time, Bockris et al. [10] clarified that the current density at a constant overpotential was contrarily dependent on the formation enthalpy of M(OH)3 hydroxide. The previous activity and mechanism study became the basis of the subsequent design of efficient oxide electrocatalysts [11]. The perovskite structure, possessing various types of rare/alkaline earth metal cations (A site) and 3D transition-metal cations (M sites), has been reported to exhibit different architectures, some of them possessing diffusion channels for oxygen ions that would be advantageous in the OER process [12]. Partial metal substitution either at A or M sites would significantly influence not only the charge state and distribution of ions in the perovskite structure, but also its electric conductivity, and finally the OER performance [13].
At high temperatures, the defective structure of oxides and their related chemical activities have been extensively detected. Ln2NiO4-δ (Ln = Pr, La, Nd) series have been investigated as oxygen electrodes for SOECs [14]. Former data has indicated that La2NiO4-δ (LNO) exhibited obvious superiority electrocatalytic activities [15] and performance of surface oxygen exchange [16]. Liu and coworkers confirmed that LNO applied as an SOEC oxygen electrode showed excellent electrochemical catalytic properties [17]. However, former research has also indicated that LNO electrodes would decompose in SOEC operating situations if CexGd1-xO2-δ (GDC) was used as the oxide electrolyte [18]. This resulted in a rapid degeneracy in performance [16]. Flua’s group further proved that the decomposition of LNO on GDC was induced by element diffusion at the LNO–GDC interphase [19]. This decomposition phenomenon was also found at the La0.5Sr0.5MnO3 (LSMO) electrode [20]. The LSMO gradually cracked into nanoparticles due to oxygen ions migrating through the electrolyte during anodic polarization, which led to an alteration in valence of manganese ions and thereafter resulted in lattice shrinkage and consequent breakdown into particles [21,22]. Due to this concern, improved stability of perovskite oxides is a critical point in SOEC research. In addition, although LNO and LSMO have superior performance in oxygen surface exchange [16], they have weakness in electronic conductivity. With the aim to overcome this shortcoming, a La2NiO4-δ-coated PrBa0.5Sr0.5Co1.5Fe0.5O5-δ electrode was prepared which showed good electrochemical performances [23,24]. At the same time, La1-xSrxCo1-yNiyO3-δ is a common cathode for SOFCs [25,26], moreover, La1-xSrxCo1-yNiyO3-δ (LSCNO), La1-xSrxCo1-yFeyO3-δ (LSCFO) [27], and BaxSr1-xCoyFe1-yO3-δ (BSCFO) [28] have also been widely investigated as electrodes for SOECs [29,30]. High electronic conductivity was found for Co-based electrodes [31,32], however this was accompanied by an increasing thermal expansion coefficient [33]. In this case, preparing Co-based perovskites with high thermal stability is a challenge.
2. Crystal and Electronic Structure of Perovskite Oxides
The crystal structure of perovskite oxide (AMO3), taking LaCoO3 (LCO) as example, is shown in Figure 1a, which easily describes the prototypical ion-covalent, and the CoO6 octahedra connected by the corners [35,36]. It features good divisibility in that the A site can be located in any rare-earth or alkaline earth metals, while the M site can be any transition metal, as shown in Figure 1b for the Sr-substituted LaCoO3. In addition, the oxygen stoichiometry can also be varied based on the ratio of different kinds of metal ions. This structural adaptability has been successfully applied to tune the electronic structure of M site cations, specifically recognized as the OER active center, and therefore a target to manipulate the electrocatalytic activities. For example, Cheng and coworkers partially doped Sr2+ at the A site in LCO and then obtained increasing activity in OERs, which was attributed to the enhancement in overlap between the unoccupied Co 3d conduction bands and the occupied O 2p valence bands [37]. Zhang et al. replaced Ni with Fe in LaNiO3 (LNO) to promote its OER activity, resulting from the influence of the Fe doping in strengthening the Ni–O bonding and thus restraining the generation of Ni2+ ions on the LaNi1-xFexO3 surface [38]. By employing a reductive heating treatment, Yang et al. produced high oxygen-deficient CaMnO2.5, which exhibited great enhancement in OER performance when compared with unreduced CaMnO3. The increase in OER activity was assigned primarily to the propitious electronic configuration of high-spin Mn3+ ions induced by sufficient oxygen vacancies in CaMnO2.5 [39].
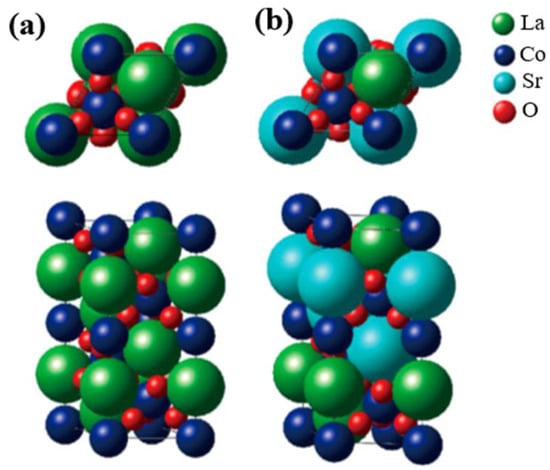
Figure 1. Crystal structure of (a) LaCoO3 and (b) Sr-doped LaCoO3 (La0.5Sr0.5CoO3). The bulk structure corresponds to the space group rhombohedral R3ch. Derived from [36].
A heterogeneous catalysis greatly relied on the surface electronic structure models associated with the catalytic properties. In this case, the surface oxygen reactivity is thus an important factor for oxidation chemical reactions, such as OERs [40,41]. Due to their excellent flexibility in accepting a high variability of cations in A and/or M locations, the electronic conductivity of perovskites can be manipulated from insulating to metallic. Through careful material design, electronic structures in perovskites can be tailored to the thermodynamic energies of a variety of reactions to minimize electrochemical reaction barriers. Before describing the role of the active sites in OERs, common ideas on the electronic structure of perovskites should be given. The charge transfer energy gained from density functional theory (DFT) calculations has been proven to be the most proper descriptor, which leads to a model of the charge transfer energy between the adsorbates and transition metals (assigned as Δ′ in Figure 2). A recently proposed descriptor, charge transfer energy (Δ), displays a linear relationship with the polarization potential (η). This is because that the Δ values are obtained from both theoretical calculations and spectroscopic data. This finding proposes that Δ is the most helpful descriptor in the design of OER perovskite oxide catalysts, as Δ decreases, the Δ′ may also decrease and lower the energy. The O 2p band center relative to the Fermi energy and eg electron number of the transition metal ions at the Msites is not necessarily adequate for the most part of perovskite oxides [42].
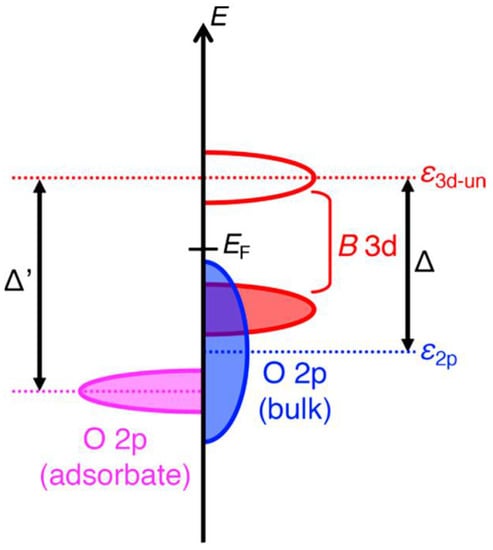
Figure 2. Schematic diagram of the charge transfer energy (Δ) between the catalyst and the adsorbates (Δ′) [42].
This entry is adapted from the peer-reviewed paper 10.3390/inorganics10110187
This entry is offline, you can click here to edit this entry!
