| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Francisca Corrà | + 7418 word(s) | 7418 | 2020-11-23 10:42:07 | | | |
| 2 | Rita Xu | -569 word(s) | 6849 | 2020-11-26 07:10:34 | | |
Video Upload Options
The occurrence of peripheral neuropathy (PNP) is often observed in Parkinson's disease (PD) patients with a prevalence up to 55%, leading to more prominent functional deficits. Motor assessment with mobile health technologies allows high sensitivity and accuracy and is widely adopted in PD, but scarcely used for PNP assessments. This entry provides a comprehensive overview of the methodologies and the most relevant features to investigate PNP and PD motor deficits with wearables. Because of the lack of studies investigating motor impairments in this specific subset of PNP-PD patients, Pubmed, Scopus, and Web of Science electronic databases were used to summarize the state of the art on PNP motor assessment with wearable technology and compare it with the existing evidence on PD.
1. Introduction
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disorder, clinically defined by the presence of resting tremor, rigidity, and bradykinesia [1]. These features are collectively referred to as motor symptoms and mostly related to loss of dopaminergic neurons in the pars compacta of midbrain substantia nigra. Alpha-synuclein-positive intracytoplasmatic inclusions, known as Lewy bodies, are the pathological hallmark of the disease [2]. As the disease progresses, motor disturbances represent considerable illness burdens. Deficits in balance and gait are common and disabling features that significantly increase the patient’s risk of falling [3] and the managing of daily living activities [4].
PD is also characterized by strong clinical and neuropathological evidence of systemic involvement. The presence of Lewy bodies in several other nervous structures, such as the nervous fibers in the skin, indicate that peripheral nervous system (PNS) involvement may be an intrinsic part in the PD pathological process [5][6]. Since the PNS is a target of alpha-synuclein deposition, it is plausible that intrinsic pathogenic features of PD may predispose to peripheral neuropathy (PNP).
PNP refers to any disorder of the PNS including single and multiple mononeuropathies, symmetrical involvement of nerves (polyneuropathies), or isolated involvement of sensory ganglia (ganglionopathies) [7]. It usually starts gradually and presents in the most common types a distal-proximal gradient, affecting first the feet and later the hands [8].
The occurrence of PNP in PD (PNP-PD) has been shown to be present in up to 55%, compared to 8% in the general population with comparable age [9][10][11]. Typical features of PNP include postural instability, muscle cramps, and numbness, of which the latter two are more prominent at distal part of the legs. As both PD and PNP pathologies are associated with these symptoms, the concurrence of peripheral involvement could be considered as an additional cause of motor deficits and general worsening in PD [12].
PNP can worsen the global functional mobility of patients, since neuromuscular factors (hip strength, ankle proprioception, and decreased peripheral sensation) have been linked to gait and balance difficulties [13]. It is, therefore, plausible to hypothesize that PD patients with PNP (PNP-PD) may develop more prominent gait and balance deficits and, consequently, be at risk of falling, injuries, and reduced quality of life [14].
Wearables are constituted of all mobile devices worn on the body (also called on-body sensors), such as inertial measurement units (IMUs), smartwatches, or Holter electrocardiogram monitors [15]. They provide objective and quantitative measures from controlled and unsupervised environments, allowing the development of accurate treatment plans and disease monitoring. In particular, data obtained from IMUs can successfully estimate spatial-temporal parameters and provide sensitive and objective information about motor deficits of various neurological pathologies, which nontechnological motor assessments often cannot identify. Mobility assessment with wearable health technologies are widely investigated in a variety of illnesses, particularly in PD, and allows high sensitivity, accuracy, and reproducibility [16]. However, these methodologies are scarcely studied and have yet to be explored in PNP [17], although a small number of previous works using wearable sensors have successfully demonstrated motor and physical activity characteristics in PNP compared to controls [18][19]. Since the presence of PNP has only recently been considered related to PD, we were interested in understanding whether PNP-PD patients showed specific motor deficits, which can be measured with the use of wearable health technology. For such purpose, a preliminary review of literature performed by the authors showed no studies evaluating the functional impact of PNP in PD on mobility using wearables. Identifying specific gait and balance patterns in this specific subset of PNP-PD patients could provide additional information about gait and balance problems, which can be used to monitor and stratify patients, optimize treatment, prevent falls, and increase quality of life.
2. Sensor Type and Placement
2.1. PNP
Multiple wearable sensor types were used within the included articles to assess measures of gait and postural stability in PNP patients. Among the 24 included articles, the most commonly used inertial sensors included a tri-axial accelerometer and a tri-axial gyroscope (83.3% of the studies): LegSys™ and BalanSens™ (BioSensics), used, respectively, for gait and balance assessment; the Opal v1 (APDM) and the Physilog® (BioAGM) for balance assessment; the GaitMeter™ for gait assessment; and the mHT (mHealth Tecnologies) for both gait and balance assessment. Accelerometers only were used in two studies: PAMSys™ (BioSensics) and DynaPort Mini-Mod (McRoberts BV). One study used a gyroscope-based sensor (SwayStar device, Balance International Innovations GmbH) for balance assessment [20]. Sampling frequencies between 50 and 200 Hz were used to acquire the signals. The most commonly used sampling frequency was 100 Hz.
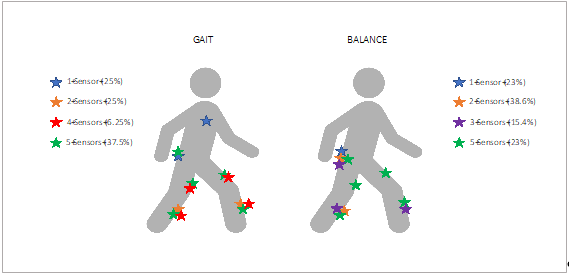
Several sensor placements and numbers of wearable sensors were used, depending on the task and on the type of assessment. Among the 16 included studies analyzing gait in PNP, four papers (25%) used one sensor, four studies (25%) analyzed gait with sensors on both shanks (two sensors), one paper (6.25%) used four sensors, and six studies (37.5%) assessed gait with five wearable sensors placed on thighs, shanks, and lower back. One study did not report sensor placement (6.25%).
Postural stability was assessed in 13 studies: Three studies (23%) used one sensor on the lower back, five studies (38.6%) used two sensors, and two studies (15.4%) used three sensors on both shanks and lower back. The remaining three studies (23%) utilized five sensors (Figure 1, Table 1).
Figure 1. Anatomical representation of sensor placement for gait and balance assessment in patients with polyneuropathy (PNP).
2.2. PD
There is currently no consensus available on the optimum number and placement of sensors to measure PD symptoms. All reviews included that evaluated sensor number and placement showed that the majority of the studies used one sensor placed on the lower back (at lumbar vertebrae level L3, L4–L5, sacrum, or waist) or on the dominant lower limb (thigh, shank, ankle, or foot). Single sensors seemed sufficiently robust for all applications: For gait assessment at home, one sensor was used in 28% to 47% of the studies [21][22][23], while for gait evaluation in the laboratory it ranged from 44% to 69% [24][25]. Not surprisingly, for balance assessment the use of one sensor, and specifically on the lower back, was preferred in 77% to 100% of the studies included in the reviews [25][26][27]. Other most commonly used sensor placements for PD were on both wrists or lower limbs (in 30% of studies) or on lower back and both lower limbs (in 14% of studies) for the home assessment and at both lower limbs (8% of the studies) for laboratory assessment (Table 2).
3. Parameters and Main Outcomes
3.1. PNP
We included 24 original full-text manuscripts: Eleven studies (45.8%) investigated gait, eight (33.4%) analyzed balance, and five (20.8%) evaluated both gait and balance in PNP patients.
Gait was assessed mainly during a straight walking task at preferred gait speed, with a distance varying from 7 to 50 m. In two studies patients were asked to perform a 90° turn during walking [28][29]. Several parameters were calculated from the signals acquired through the wearable sensors. The most commonly reported parameters computed from the filtered signals were spatiotemporal gait parameters: gait speed (m/s), stride and step length (m), stride and step time (sec), number of steps, double limb support time (%), and cadence (steps/min). Coefficient of variation (CV) of gait speed and stride length and time (%) was calculated in eight studies [28][29][30][31][32][33][34][35]. Gait speed initiation, number of steps, and total distance required to reach steady-state walking were studied in four papers [33][34][36][37]. Duration (%) and number of walking bouts were extracted in one study [18].
Clinical trials among the included papers did not show any statistically significant changes in the gait parameters when comparing pre- and post-intervention. Najafi [38] analyzed gait differences between intervention and control groups after plantar electrical stimulation in DPN patients and Schwenk et al. [32] evaluated gait after a new interactive training in CIPN subjects. Nevertheless, the effect size of these studies suggested the presence of a moderate to large improvement of cadence and gait speed post-treatment. In contrast, Caronni [39] compared the responsiveness to rehabilitation in a group of PNP patients and found a statistically significant difference in gait speed between groups (p = 0.001, Table 1). Spatiotemporal parameters were significantly different between PNP patients and healthy controls only in studies investigating gait under more challenging conditions. Kang et al. [31] described a statistically significant difference between DPN and healthy participants in the coefficient of variation of gait speed and stride length during dual-task gait. De Bruin et al. [40] found significant differences in speed, step length, and cadence when comparing DPN patients during dual-task walking on paved trajectories compared to single-task. Another study by Kang [41] showed improvement in stride velocity, stride length, and double limb support (%) during dual-task and fast walking, compared to single-task, after plantar mechanical stimulation. Differences from controls were found in step time, cadence, and gait speed but not in stride length in a study by Esser et al. [17], and gait speed was also 10% decreased in DPN group compared to controls in a study by Ling et al. [30]. Another important result was pointed out by Najafi et al. [33], who found differences in spatiotemporal parameters only during long distances, especially in gait variability and in double support time, when comparing DPN patients with controls. These differences were more pronounced during barefoot walking.
Balance and postural stability were investigated through numerous tasks. The most frequently used task in all 13 studies was the double leg stance performed in different conditions:
- Position of feet: Standing balance was assessed with feet together in eight (61.5%) studies, feet apart (spaced shoulder width) in two studies (15.3%), and both feet positions in one paper (7.6%), while two papers (15.3%) did not specify the position of the feet. In two studies patients were also asked to perform a semi-tandem position [32][42], while one other study introduced a detailed balance test protocol with single leg stance [20].
- Open and closed eyes: Twelve studies (92.3%) analyzed balance with both open and closed eyes, and one study only used eyes-open condition [43].
- Foam: Two studies used a foam surface (height 10 cm, density 25 kg/m3) to analyze balance [20][42]. The other papers only performed balance tasks on firm surfaces.
Other tools to assess postural stability were clinical tests such as the functional reach test [44]. Functional tests (to investigate functional mobility, addressing both gait and balance characteristics) were performed in three selected studies [39][41][44]. They applied the timed up-and-go (TUG) test. This test was split by Caronni et al. [39] into five subphases, and the duration of each phase was measured, as well as the total TUG test duration.
The included studies reported multiple outcomes of standing balance and postural stability that were calculated from the signals provided by the wearable sensors (Table 1). Of these outcomes, the most commonly reported measures included center of mass (COM) sway (cm2), defined as total sway (in seven studies, 53.8%), and related parameters (anterior-posterior (AP) and medio-lateral (ML) sway (cm)). These parameters were also reported in three studies analyzing gait to investigate balance control during walking and gait initiation [33][34][37]. In addition, ankle sway (deg2), hip sway (deg2), and COM sway area (m2) were calculated in six papers (46.1%). Center of gravity (COG) sway (cm2), COG AP, and COG ML (expressed in cm) were calculated in one paper [45]. Other parameters were root mean square (RMS, m/s2), trunk acceleration, and trunk jerk (m2/s3) [39][46]; postural coordination of upper and lower body (defined as the reciprocal coordination between hip and ankle motions) [36]; roll and pitch velocity (deg/sec) and roll and pitch angle (deg) [20]. Further parameters were local (in short time intervals, sec) and central (in long time intervals) control balance strategies [45], and cross-correlation function (CCF) of angular velocity to investigate the coordination of human movements [46].
A significant reduction in COM sway area (a parameter of postural sway) was shown by Schwenk et al. [32] and Grewal et al. [47] after an interactive sensor-based balance training and by Yalla et al. [44] after an intervention on postural stability with an ankle foot orthosis. These results were found during balance tasks with open eyes, while, interestingly, no significant reduction was found during closed-eyes condition. In contrast, changes of the parameters COM sway area and ML sway area were significant after a virtual reality intervention with eyes-closed and -open conditions [35].
3.2. PD
In PD, a multiplicity of parameters derived from inertial sensors could be described. For the purpose of this review, parameters from the upper part of the body (upper limb) were not considered. The included reviews listed a series of most relevant spatiotemporal parameters representative of five domains (pace, variability, rhythm, asymmetry, and postural control), which included stride length, stride velocity, cadence, double support time [48][49], and turning velocity [50] followed by step time variability [25][48] and step height, reaction time, and gait cycle duration [51]. Frequency-based measures were dynamics in trunk movement during gait, turning and smoothness [52], harmonic ratio, amplitude, slope and width of dominant frequency, peak trunk horizontal velocity, and phase coordination index of gait cycle [25]. Number of steps, single versus multiple step response, turning duration, turn-to-sit duration, and sit-to-stand and stand-to-sit time- and amplitude-based measures were reported to be important features to determine gait impairment [51]. In more detail, PD patients have been shown to have slower gait, less foot clearance, smaller step lengths, lower turning velocity, lower cadence, and lower peak trunk rotation compared to controls [48][50]. Turning velocity, cadence, and peak trunk rotation were associated with disease progression [53]. Another important parameter in PD is gait variability, also referred to as unsteadiness and arrhythmicity of stepping [54]. Increased gait variability can be seen throughout the disease, and the magnitude of the variability tends to increase with disease severity [48].
Home assessment may have greater ecological validity and gives a true picture of the burden of disease [15]. Parameters that may be particularly relevant for this assessment type are walking bouts (total number of walking bouts, median number of steps per bout, bout duration), turns per hour during the day, duration of each turn, number of steps per turn, peak and average rotational turning rate, and variability of these measures throughout the day and week [22][23].
Regarding standing balance and postural stability, often used parameters were postural sway velocity, RMS accelerations, and jerk [27]. Parameters that may discriminate most effectively between PD and controls are sway area, sway velocity, jerk index, sway amplitude and range of acceleration signals (time domain), and frequency dispersion and centroidal frequency [26][48] (Table 2).
All these features are able to differentiate between PD and healthy controls (HC) at early stage [25][48], different PD stages [27], different medication states in advanced PD, and PD progression (in particular sway dispersion and sway velocity) [48]. Postural sway is also a good measure of balance control to be used as a primary outcome for interventions [48].
Table 1. Summary of the major characteristics of the research design, analyses, and outcomes for the studies on PNP that met the inclusion criteria.
|
REFERENCE |
POPULATION (Mean Age ± SD) |
SENSORS (Number and Type) |
SENSOR PLACEMENT |
ASSESSMENT PROTOCOL |
PARAMETERS EXTRACTED/INVESTIGATED/OUTCOMES |
MAIN FINDINGS |
|
Ling et al., 2020 [30] |
· 12 DPN + DFU (55.6 ± 3) · 27 DPN (64.3 ± 1) · 47 Healthy controls (62.9 ± 2) |
5 Inertial sensors (ACC, GYR and MAG) (LegSys™, BioSensics) Freq: 100 Hz |
· Thighs · Shanks · Lower back |
Straight walking test at preferred speed for 10 m on a flat floor |
· Gait speed and gait speed unsteadiness, stride length and stride length unsteadiness, gait cycle time, double support and double support limp, step length limp, gait symmetry |
People with DPN and DFUs wearing offloading devices have poorer gait function compared to controls. DFUs and offloading devices further deteriorate gait beyond DPN, specifically for performance in gait speed, stride length and gait cycle time. Compared to controls, DPN showed 10% decreased in gait speed and increased stride length of 48%. |
|
Kang et al., 2020 [36] |
· 38 DPN (72.6 ±5) · 33 Healthy controls (77.9 ± 8) |
5 Inertial sensors (ACC, GYR and MAG) (LegSys™, BioSensics) Freq: 100 Hz |
· Thighs · Shanks · Lower back |
Straight walking test at preferred speed for 12 m on a flat floor at two conditions: during single and dual (cognitive) task |
· Number of steps and distance to reach steady-state gait · Gait speed and body sway in the mediolateral direction in the gait initiation phase and steady-state gait speed. |
For both single-task and dual-task gait conditions, number of steps, distance, and mediolateral body sway were significantly greater for the DPN group than for the CON group. Gait initiation steps and dynamic balance may be more sensitive than gait speed for detecting gait deterioration due to DPN. |
|
Kang et al., 2020 [31] |
44 DPN + CIPN: · 25 PNP without cognitive impairment (66.5 ± 9) · 19 PNP with cognitive impairment (68.5 ± 9) |
2 Inertial sensors (ACC, GYR and MAG) (LegSys™, BioSensics) Freq: 100 Hz |
· Shanks |
Straight walking test at preferred speed for 12 m on a flat floor at two conditions: during single and dual (cognitive) task |
· Coefficient of variation (CV) of gait speed, stride length and stride time · Spatio-temporal gait parameters: gait speed, stride length and stride time |
During dual-task walking, between-group differences were significant for gait variability for gait speed and stride length (51.4% and 71.1%, respectively; p = 0.014 and 0.011, respectively). The presence of cognitive impairment exacerbates the risk of falls in people with PN. |
|
Kang and Najafi, 2020 [18] |
· 49 PNP (DPN+ CIPN) (68.5 ± 7) |
1 accelerometer (ACC) (PAMSys™, BioSensics LLC) Freq: 50 Hz |
· Chest |
48-h period recording |
· Durations of standing posture · Sedentary posture · Total number of walking bouts · Number of total steps |
People with PN and low concern about falling tended to have more activity, but people with PN and high concern about falling tended to have less activity. Furthermore, the duration and amount of being active (i.e., walking bout and total step counts) may predict the level of concern about falling, and thus may be used as eHealth targets and strategies for fall risk assessment among people with PN. |
|
Zahiri et al., 2019 [55] |
· 84 subjects with cancer (CIPN+ and CIPN-) (71.1 ± 9) · 57 Healthy controls (69.5 ± 9) |
5 Inertial sensors (ACC, GYR and MAG) (LEGSys™and BalanSens™; Biosensics LLC) Freq: not reported |
· Shanks · Thighs · Lower back |
· Gait assessment: single-task (no cognitive distraction) over 15 m at a self-selected speed. · Balance: double leg stance 30 s with feet close together during eyes-open and eyes-closed situations. |
· Gait parameters: stride velocity, stride length, stride time and double support time. · Balance parameters: area of ankle sway, area of hip sway, area of center of mass (CoM) sway, and CoM sway in the medial- lateral (ML) direction. |
The deterioration in gait parameters was more pronounced in the CIPN+ than the CIPN- subgroup, when compared to the control group. CIPN+ on average had 8% and 18% slower stride velocity compared to the CIPN- and control groups, respectively. Stride velocity was also on average 11% slower in CIPN, when compared to control. Similar trends were observed for other gait parameters of interest. Results also suggest high visual dependency in the CIPN+ subgroup. The negative impact of CIPN on motor-performance is confirmed with the largest effects on ankle stability and stride time. Vibration perception threshold (VPT) is a predictor of motor deterioration and may be used to determine the severity of CIPN symptom |
|
Kang et al., 2019 [41] |
• 30 DPN (68.1 ± 9) |
• Gait assessment: 5 Inertial sensors (ACC, GYR and MAG) (LEGSys™, Biosensics) • Balance assessment: 2 Inertial sensors (ACC and GYR) (BalanSens™, Biosensics) Freq: not reported |
Gait assessment: · Shanks · Thighs · Lower back Balance assessment: · Dominant leg · Lower back |
• Gait: 10 m walking test at normal and fast pace, and at two conditions: single and dual tasks. • Static balance: (i) double leg stance for 30 s with feet together with eyes open and eyes closed (EC). (ii) semi-tandem stance for 30 s • Global functional mobility: TUG test |
• Gait parameters: stride velocity, stride length, stride time and double support time. • Balance parameters: area of ankle sway, area of hip sway, area of center of mass (CoM) sway, and CoM sway in the medial- lateral (ML) direction. |
Daily use of plantar mechanical stimulation through a micro-mobile foot compression device installed in a shoe insole is effective for improving vibration perception, which likely results in improvements in some balance outcomes and gait parameters. Key findings were improvements in plantar sensation in the foot, CoM sway in the ML direction during quiet standing and stride velocity and other spatiotemporal gait parameters in dual task condition after using the wearable foot compression device for four weeks. |
|
Fino et al., 2019 [43] |
• 216 CIPN+ (63.0 ± 6) • 218 CIPN- (62.2 ± 6) •49 Healthy controls (63.3 ± 6) |
1 Inertial sensor (ACC and GYR) (Opal v1, APDM) Freq: 128 Hz. |
• Lower back |
Double leg stance test with eyes open for 30 feet apart |
AP-sway, ML-sway, or resultant sway |
Cancer survivors had worse sway than healthy control subjects in components related to sway magnitude and mediolateral frequency of sway, but no difference in the component related to resultant/AP sway jerk and frequency. Cancer survivors who reported neuropathy were more likely to have higher resultant/AP sway frequencies and jerk than asymptomatic survivors, while survivors who reported a fall were more likely to have lower frequencies of mediolateral sway than non-fallers. Neuropathy influenced the associations between specific characteristics of sway and falls, which may have implications for fall prevention interventions. |
|
Caronni et al., 2019 [39] |
• 25 PNP-LL (76.5 ± 6) |
1 Inertial sensor (ACC and GYR) (mHT, mHealth Technologies) Freq: 100 Hz |
• Lower back |
• Gait: 10 m walking test and TUG test repeated five times each. • Static balance: double leg stance for 30 s with (i) feet apart (FA) and eyes open (EO), (ii) feet apart and eyes closed (EC), (iii) feet together (FT) and eyes open an (iv) feet together and eyes closed. |
• Gait: 5 subsequent phases of TUG test: sit to stand (STS), walk 1 (W1), turn 1 (T1), walk 2 (W2) and turn and sit (TAS); duration of each phase and total TUG duration (TTD); mean vertical angular velocity during turn 1 and during TAS • Root mean square (RMS), trunk acceleration (Trunk acc) and trunk jerk (Trunk jerk). |
After rehabilitation, patients with PN-LL consistently improved straight walking, walking along curved trajectories and transfers, with no apparent modification of static balance. Four gait measures (i.e., gait speed, angular velocities during TUG) and the TTD showed a large improvement after rehabilitation. The improvement was medium for the walking phases of the TUG test (i.e., W1, T1 and W2) and TUG transfers (i.e., STS and TAS). |
|
Findling et al., 2018 [20] |
• 11 CIDN (chronic inflammatory demyelinating polyneuropathy) (61.1 ± 11) • 10 not inflammatory PNP (68.5 ± 11) |
1 gyroscope SwayStar device (GYR) (Balance International Innovations GmbH) Freq: 100 Hz |
• Lower back |
12 stance tasks: • 4 double leg tests with the feet spaced shoulder width apart; • 4 tasks with eyes open on a normal surface and on a foam surface (height 10 cm, density 25 kg/m3)± and eyes closed. • 3 single leg stance tasks with eyes open, 2 on a normal surface (right and left leg) and 1 on the foam surface. • 1 task with single leg standing. 5 tasks for dynamic balance: • 8 steps tandem gait • 3 m walking on heels • 3 m walking pitching the head up and down • 3 m walking with eyes closed and 8 m walking with eyes open |
Global balance control index (BCI); trunk sway and trunk velocity |
CIDP patients have reduced ability to decrease trunk sway with lower gait speed. A similar effect was noted for pitch velocity walking eyes closed. This is possibly associated with an increased risk of falls |
|
Esser et al., 2018 [17] |
• 17 DPN (63 ± 9) • 42 Healthy controls (61 ± 4) |
1 inertial sensor (ACC and GYR). Freq: 100 HZ |
• Lower back |
• Gait: 10 m at normal and fast pace |
Step time, cadence, stride length, walking speed |
A single IMU used in clinical setting has the potential to discriminate patients with DPN compared to healthy controls. Walking speed was the most sensitive parameter, while no significant differences were found in stride length compared to controls. |
|
Najafi et al., 2017 [38] |
• 28 DPN: 17 intervention group (56 ± 5) • 11 Healthy controls (64 ± 10) |
• Gait assessment: 2 Inertial sensors (ACC, GYR and MAG) (LEGSys™, Biosensics) • Balance assessment: 2 Inertial sensors (ACC and GYR) (BalanSens™, Biosensics) Freq: not reported |
Gait assessment: • Shanks Balance assessment: • Dominant leg • Lower back |
• Gait: 10 m at normal and fast pace • Balance: double stance for 30 s with feet close together (without touching), with eyes open (EO), and eyes closed (EC). |
• Gait: Stride velocity, stride time, stride length and cadence. • Balance: COM anterior-posterior (AP) sway, medial-lateral (ML) sway, and total sway area |
No differences were observed between the groups for baseline characteristics or for motor performance including postural sway and spatiotemporal parameters of gait. However, the majorities of measurable metrics were improved post-treatment in the intervention group with no significant changes in the control group. This study suggests that daily home use of plantar electrical-stimulation may be a practical means to enhance motor-performance and plantar-sensation in people with DPN. |
|
Schwenk et al., 2016 [32] |
• 22 CIPN (70.3 ± 8) |
• Gait assessment: 4 Inertial sensors (ACC, GYR and MAG) (LEGSys™, Biosensics) • Balance assessment: 3 Inertial sensors (ACC and GYR) (BalanSens™, Biosensics) Freq: not reported |
Gait assessment: • Shanks •Thighs Balance assessment: • Shanks • Lower back |
• Gait: 10 m at normal pace • Balance: double stance 30 s with feet close together (without touching), with eyes open (EO), and eyes closed (EC), and semi-tandem position with EO. |
• Gait: gait speed and variability • Balance: COM AP sway and ML sway; hip sway and ankle sway |
ML CoM sway, hip sway, and ankle sway were reduced in the intervention group compared to control group during balance assessment with feet close together and EO. Significant reductions in postural sway parameters were also found during the more challenging semi-tandem position, except for ankle sway. Older cancer patients with CIPN can significantly improve their postural balance with specifically tailored, sensor-based exercise training. |
|
Toosizadeh et al., 2015 [45] |
• 18 DPN (65 ± 8) • 18 Healthy controls (69 ± 3) |
2 Inertial sensors (ACC and GYR) (BalanSens™, Biosensics) Freq: not reported |
• Ankle • Hip |
2 Romberg balance trials (with open and closed eyes) for 15 s |
Center of gravity (COG) sway (total sway) and COG (AP) sway, COG (ML) sway; local- (in short time-intervals) and central- (in long time intervals) control balance strategies. |
The rate of sway within local-control was significantly higher in the DPN group by 49%, which suggests a compromised local-control balance behavior in DPN patients. Unlike local-control, the rate of sway within central-control was 60% smaller in the DPN group, which suggests an adaptation mechanism to reduce the overall body sway in DPN patients. In the lack of sensory feedback cueing, DPN participants were highly unstable compared to controls. However, as soon as they perceived the magnitude of sway using sensory feedback, they chose a high rigid postural control strategy, probably due to high concerns for fall, which may increase the energy cost during extended period of standing. |
|
Grewal et al., 2015 [47] |
• 35 DPN: 19 intervention group (62.5 ± 7) • 16 Healthy controls (64.9 ± 8) |
5 Inertial sensors (ACC, GYR and MAG) (LEGSys™, Biosensics LLC) Freq: 100 HZ |
•Shanks •Thighs • Lower back |
Double leg stance for 30 s with open and closed eyes and feet together |
COM sway, COM AP, COM ML sway, Hip sway. |
On average, the CoM sway area for the intervention group (IG) was reduced significantly by 58.31% compared to a reduction of 7.8% in the control group (CG). The IG showed a significant reduction in the ML CoM sway; similarly, significant reductions were observed for the hip and ankle sway in the IG compared to the CG. During balance assessment with closed eyes, the IG achieved a reduction in CoM sway of 62.68%; however, none of the sway components (AP, ML or CoM area) reached significance. People with DPN can significantly improve their postural balance with diabetes specific, tailored, sensor-based exercise training |
|
Yalla et al., 2014 [44] |
• 30 DPN (73 ± 6) |
5 Inertial sensors (ACC and GYR) (BalanSens™, BioSensics LLC) Freq:100 Hz |
•Shanks •Thighs • Lower back |
• 6 double stance of 30 s trials (2 for each footwear condition during eyes-open and eyes-closed) with their arms crossed, feet positioned closeto each other without being in contact. • Dynamic balance: Functional reach task • Global functional mobility: TUG test |
Ankle, hip, and COM sway |
The orthoses reduced center of mass sway on average by 49.0% and 40.7% during eyes-open balance trials. The reduction was amplified during the eyes-closed trials with average reductions of 65.9% and 47.8%, compared to barefoot and ‘shoes alone’ conditions. Ankle foot orthoses reduced postural sway and improved lower extremity coordination in the elderly participants without limiting their ability to perform a standard activity of daily living. |
|
Karmakar et al., 2014 [28] |
• 19 NeP-DPN (65.7 ± 10) |
2 Inertial sensors (ACC and GYR) (GaitMeter™) Freq: not reported |
•Shanks |
Straight walking test at preferred speed for 50 m on a flat floor and a 90° turn without rest time. |
Step length, step velocity, gait variability |
DPN subjects with neuropathic pain receiving pregabalin treatment had increasing variance for both step length and step velocity. No significant differences in durations of time required to walk, step length and step velocity measures were found between timepoints and interventions. The degree of variability in both step length and step velocity significantly increased for subjects receiving pregabalin for comparison of baseline and final visits. The potential relief of NeP using pharmacotherapy may not improve gait dysfunction. |
|
Najafi et al., 2013 [33] |
• 12 DPN (60 ± 12) • 8 Healthy controls (60 ± 6) |
5 Inertial sensors (ACC and GYR) (LEGSys™, Biosensics LLC) Freq: not reported |
•Shanks •Thighs • Lower back |
Straight walking test at preferred speed for 7 m (short distance) and 20 m (long distance) at two conditions: barefoot and with regular shoes. |
Gait initiation velocity, stride velocity, gait variability, average range of motion of ML- and AP- CoM during each stride, double support time, stride time, stride length, number of steps. |
Most gait parameters showed alterations in patients with DPN during the barefoot and shoe conditions compared with those in the control group. However, the effect size was usually larger in the long walking distance trials, and none of the observed differences were statistically significant in the short walking distance trials. Gait speed during the gait initiation and gait steady state phases was reduced on average by 15%. Variability was 84% higher in the DPN group. Double support time was more than 20% during the barefoot and shod conditions in those with DPN, suggesting a more altered gait while walking barefoot. The benefit of footwear was significant only during the long walking distance trials. |
|
Lalli et al., 2013 [29] |
• 20 DM (60.2 ± 13) • 20 DPN (62.6 ± 9) • 22 NeP-DPN (63.9 ± 9) • 24 Healthy controls (58.8 ± 11) |
2 Inertial sensors (ACC and GYR) (GaitMeter™) Freq: not reported |
• Shanks |
Straight walking test at preferred speed for 50 m on a flat floor and a 90° turn without rest time. |
Gait variability, cadence, step length, step velocity and total duration of walk |
No differences were observed among groups in the total duration of walk, step length and step velocity. The degree of variability in both step length and velocity were both significant in participants with NeP-DPN compared to DPN. Participants with NeP-DPN had greater variance in gait when compared to DPN and controls. Also, patients with DPN or DM only were not significantly different from controls with respect to most gait measures utilized. NeP contributes to gait variability, potentially contributing to the risk of falling in DM patients. |
|
Kelly et al., 2013 [34] |
• 16 DPN (73 ± 8) • 18 DM (62 ± 8) |
5 Inertial sensors (ACC, GYR and MAG) (LEGSys™, Biosensics LLC) Freq: not reported |
•Shanks •Thighs • Lower back |
Straight walking test at preferred speed for 20 m on a flat floor |
• Gait: stride velocity, stride length, stride time, double support time, gait speed variability, steps required to reach steady-state walking, AP and ML COM sway during walking |
Gait performance was relatively worse in participants with DPN compared with DM individuals. However, only steps taken during gait initiation and double-support percentage achieved statistical significance. The DPN and non-DPN groups had almost the same level of concern about falling, suggesting a prevalence in older adults with DM but not a relation with DPN. |
|
Grewal et al., 2013 [35] |
• 29 DPN (57 ± 10) |
2 Inertial sensors (ACC and GYR) (BalanSens™, BioSensics LLC) Freq: 100 Hz |
• 1 Shank • Lower back |
Double stance position for 30 s at open and closed eyes (width not specified) |
COM sway (AP and ML) and sway area. Postural coordination between the upper and lower body (in the mediolateral and anteroposterior directions) |
Significant reduction in center of mass sway after training. A higher postural stability deficit (high body sway) at baseline was associated with higher training gains in postural balance (reduction in center of mass sway). In addition, significant improvement was observed in postural coordination between the ankle and hip joints. |
|
Grewal et al., 2013 [37] |
• 16 DPN + DFU (58.3 ± 4) • 15 DPN (54.2 ± 11) • 8 Healthy controls (59.6 ± 6) |
A set of Inertial sensors (LEGSys™, Biosensics LLC) (ACC and GYR) Freq: not reported |
Not reported |
Not reported |
Stride velocity, stride length, gait cycle time, double support time, AP- and ML- COM sway area, knee range of motion, gait variability, number of steps and total distance required to achieve gait steady state |
During gait initiation, number of steps, knee range of motion and CV stride velocity revealed significant differences among groups. The presence of PNP increases the number of steps required to reach steady state gait by nealry 90% compared to healthy individuals. During steady state gait, double support, COM sway area anf CV stride velocity were significantly different between groups. The reuslts demonstrates that neuropathy deteriorates gait, but the presence of foot ulcers does not alter gait parameters further than neuropathy. In addition, patients with foot ulcers demonstrated a better gait compared with DPN patients without ulcers. |
|
Turcot et al., 2012 [46] |
• 25 DPN (63.5 ± 7) |
3 Inertial sensors (ACC and GYR) (Physilog®, BioAGM). Freq: 200 Hz |
• Shanks • Lower back |
Double leg stance for 30 s with open and closed eyes (width not specified) |
Angular velocity at trunk and ankle levels in two terms: RMS and with cross-correlation function (CCF), to investigate the coordination of human movements in motor control. CFF was calculated between trunk and right ankle, trunk and left ankle, right and left ankle. |
The analyses of anterior-posterior angular velocities between the trunk and both ankles showed positive CCFs in the eyes open condition in 23/25 patients and in all patients in the eyes closed condition. It has been demonstrated that the level of PNP was linked to postural strategies and instability during different standing tasks. RMS of the angular velocities at the trunk and ankle levels increases as the task complexity increases. These results highlighted the relation of the level of PNP with postural strategies and instability. |
|
de Bruin et al., 2012 [40] |
• 29 DPN (with and without PNP) (61.9 ± 5) |
1 accelerometer (DynaPort Mini-Mod, McRoberts BV) (ACC) Freq: not reported |
• Lower back |
Walking at preferred velocity under two conditions. Single task: walking on the walkway; dual task: walking on the walkway with a counting task. The walkway contained a paved trajectory, cobble stones, and gravel rocks |
Step time, step length, velocity, cadence |
Significant differences between single versus dual task walking at baseline were identified for all gait parameters. Gait speed, step length, and cadence were significantly decreased under dual tasking, and step duration was significantly increased compared to normal walking. Gait speed, cadence, step duration, and step length under more challenging conditions can be reliably measured in adults with diabetes |
|
Najafi et al., 2010 [42] |
• 17 DPN (59.2 ± 8) • 21 Healthy controls (24.4 ± 1) |
2 Inertial sensors (ACC, GYR and MAG) (BalanSens™, Biosensics) Freq: not reported |
• 1 Shank • Lower back |
Double leg stance for 30 s with open (EO) and closed eyes (EC) and feet together, with firm and foam surfaces. |
COM sway area, hip and ankle motions |
DPN individuals exhibit significantly greater COM sway than healthy subjects during both EO and EC conditions. Sway area was significantly higher than healthy subjects on average by 98%. No significant difference was observed for both ankle and hip sways during EO. At EC, both ankle and hip sways were significantly higher in DPN subjects. Results suggest that postural compensatory strategies during EO condition is significantly better in healthy subjects compared to DPN subjects. During EC condition, although postural control strategy was better in healthy subjects, the observed difference was not significant. It has been shown that PNP significantly affects postural compensantory strategies. |
ACC: accelerometer; AP: anterior-posterior; CIDN: chronic inflammatory demyelinating polyneuropathy; CIPN: chemotherapy-induced peripheral neuropathy; COG: center of gravity; COM: center of mass; DFU: diabetic foot ulcer; DM: diabetes mellitus; DPN: diabetic peripheral neuropathy; Freq: sample frequency; GYR: gyroscope; MAG: magnetometer; ML: medio-lateral; NeP-DPN: neuropathic pain diabetic neuropathy; PNP-LL: peripheral neuropathy of the lower limbs; TUG: timed up-and-go test.
Table 2. Summary of the major characteristics of the PD reviews that met the inclusion criteria.
|
REFERENCE |
REVIEW CHARACTERISTICS |
NUMBER OF STUDIES INVESTIGATING PD |
SAMPLE SIZE (H&Y Stage) |
SENSORS (Number and Type) |
EXTRACTED PARAMETERS |
|
Morgan et al., 2020 [21] |
Analysis of gait during home assessment |
65 papers |
Almost half of the studies used between 10 and 49 PD participants. 12 studies used fewer than 10 and 8 more than 100 participants. |
45.5% of the studies used 1 sensor at the lower back; 2 studies used 3 sensors at lower back and feet; 1 paper used 1 sensor on the chest, 1 used 1 sensor on the wrist. 2 papers do not discribe the position |
Features not specified. |
|
Ghislieri et al., 2019 [26] |
Analysis of standing balance |
14 papers |
From 10 to 58 PD patients (and one study with 104 patients) |
The 93% of studies used 1 sensors on the lower back. 1 study used 3 sensors: 1 on the lower back and 2 on lower limbs |
Jerk index, sway amplitude, range of acceleration signals, frequency dispersion and centroidal frequency. |
|
Rovini et al., 2018 [22] |
Analysis of gait during home assessment |
30 papers |
Ranging from 1 to 75 PD patients |
6 papers (28.2%) used 1 sensor: 4 on the waist and 2 on the lower back. 10 (33.3%) papers used 2 sensors: 5 on the wrists, 1 on the feet, 3 on the ankles, one on ankle and dominant leg. 6 studies used 3 sensors on the waist and feet. 2 papers used 5 sensors (on wrists, ankles and trunk; on shanks, wrists and sternum). The last 3 papers used more than 6 sensors. |
Average time and distance walked, cadence, gait speed, step length, swing time, double support time; stride time and stride time variability. Inter-trial variability, inter-subject variability; inter-task variability. Number of turns per hour, turn angle amplitude, turn duration, turn mean velocity, number of steps per turn, hourly frequency of turning, duration of each turn, number of steps per turn, peak and average rotational turning rate, jerk, variability of these measures throughout the day and week. |
|
Merola et al., 2018 [51] |
Analysis of gait and balance |
6 papers |
From 6 to 40 (and 2 studies with 190 and 139 PD patients) |
Not reported |
Gait: temporal (reaction time, gait cycle duration), spatial (step length, step height) and biomechanical (ankle torque, vertical landing force) variables, and gait strategies (i.e., number of steps, single versus multiple step response). Balance and postural instability: trajectory of the center of pressure (COP) and center of mass (COM) misplacement, trunk acceleration and postural sway |
|
Vienne et al., 2017 [24] |
General analysis of gait |
16 papers |
Not reported |
11 studies (68.7%) described the assessment of PD with 1 sensor at the lower back. one paper used one sensor at one ankle, one at one shank and one at one foot. One paper used 2 sensors (upper and lower back), and one paper utilized 3 sensors at lower back and shanks |
Features not specified. |
|
Rovini et al., 2017 [52] |
Analysis of wearable sensors on support of PD treatment and diagnosis |
80 papers |
From 5 to 47 (and 1 study of 75 PD patients) |
Not reported |
Statistical (e.g., mean, variance, skewness, kurtosis), frequency (e.g., energy, power spectral density, fundamental frequency), and spatiotemporal/kinematic (e.g., stride length, TUG time, stride velocity) features; step or stride segmentation. |
|
Godinho et al., 2016 [16] |
Mobile health technology characteristics |
76 papers |
Not reported |
Not reported |
ISway measures (jerk, RMS amplitude and mean velocity from the time-domain measures, and centroidal frequency); gait parameters with a high degree of accuracy; total number of walking bouts, the percent of time spent walking, the total number of steps, median walking bout duration, median number of steps, and median cadence per bout. Quality-related sensor derived measures included: frequency measures, regularity measures and the harmonic ratio. |
|
Del Din et al., 2016 [23] |
Analysis of gait during home assessment |
19 papers |
From 2 to 169 PD participants (and one study of 467 patients) |
9 studies (47.3%) used 1 sensor on lower back; 3 used 2 sensors on thighs; 2 papers used 2 sensors on feet; 1 on both shanks and 1 used 1 sensor on the chest; the other papers used more than 4 sensors. |
Number of walking bouts, walking duration, total number of steps, median number of steps per bout, bout duration, cadence, step and stride regularity, frequency domain measures (harmonic ratio, amplitude, slope and width of dominant frequency), step duration, step symmetry, acceleration range and dynamic stability |
|
Oung et al., 2015 [49] |
Assessment of motor disorders in PD |
Not reported |
Not reported |
Not reported |
Step frequency, stride length, entropy and arm swing |
|
Hubble et al., 2015 [27] |
Analysis of standing balance and walking stability |
26 papers |
From 5 to 67 PD patients |
20 studies (76.9%) used 1 sensor on the lower back (sacrum/L3/L4/L5); 2 studies used 2 sensors on the shanks; 2 studies used 1 sensor on sternum/chest; 1 study utilized one sensor on the wrist; and another one on the lateral side of the pelvis. |
Sway velocity (23% of studies), RMS accelerations (19% of studies) and jerk (19% of studies). Harmonic ratio (31% of studies) and stride time variability (27% of studies). |
|
Steins et al., 2014 [50] |
Assessment of functional activities with wearable devices |
6 papers |
Not reported |
Not reported |
Stride length, stride velocity, cadence, and turning velocity |
|
Maetzler et al., 2013 [25] |
Quantitative objective assessment of gait and balance |
16 papers |
Not reported |
Gait: 4 papers used one sensor on the lower back (44.4%). 2 papers utilized 1 sensor on the shank and 2 papers 2 sensors on both feet. 1 paper used 1 sensor on the forearm and two studies used more than 5 sensors. Balance: 5 papers used 1 sensor on lower back (100%). |
Gait: Phase coordination index of gait cycle; stride length; frequency-based measures of gait (harmonic ratio, amplitude, slope and width of dominant frequency); cadence, step time variability; peak trunk horizontal velocity, turning duration, turn-to-sit duration; time- and amplitude-based measures of sit-to-stand and stand-to-sit; peak trunk rotation velocity and rotation range of motion, turning velocity; Walk peak roll velocity, total turning duration, turn peak yaw and roll velocity. Balance: Velocity, jerk, acceleration, frequency-based measures; displacement, velocity; Peak trunk acceleration during anticipatory postural adjustments towards the stance leg; Hilbert-Huang transformation of postural parameters |
|
Horak et al., 2013 [48] |
Biomarkers of gait and balance |
Not reported |
Not reported |
Not reported |
Gait: Stride Time Variability, double support time, peak arm velocity, trunk rotation, gait velocity, cadence, stride length. Balance: Postural sway (area, velocity, frequency) and jerk. |
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Rev. Dis. Primers 2017, 3, 17013.
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840.
- Dennison, A.C.; Noorigian, J.V.; Robinson, K.M.; Fisman, D.N.; Cianci, H.J.; Moberg, P.; Bunting-Perry, L.; Martine, R.; Duda, J.; Stern, M.B. Falling in Parkinson Disease: Identifying and prioritizing risk factors in recurrent fallers. J. Phys. Med. Rehabil. 2007, 86, 621–632.
- Hammarlund, C.S.; Westergren, A.; Åström, I.; Edberg, A.-K.; Hagell, P. The Impact of Living with Parkinson’s Disease: Balancing within a Web of Needs and Demands. Parkinson Dis. 2018, 2018, 4598651.
- Rodríguez-Leyva, I.; Calderón-Garcidueñas, A.L.; Jiménez-Capdeville, M.E.; Rentería-Palomo, A.A.; Hernandez-Rodriguez, H.G.; Valdés-Rodríguez, R.; Fuentes-Ahumada, C.; Torres-Álvarez, B.; Sepúlveda-Saavedra, J.; Soto-Domínguez, A.; et al. α-Synuclein inclusions in the skin of Parkinson's disease and parkinsonism. Clin. Transl. Neurol. 2014, 1, 471–478.
- Doppler, K.; Ebert, S.; Üçeyler, N.; Trenkwalder, C.; Ebentheuer, J.; Volkmann, J.; Sommer, C. Cutaneous neuropathy in Parkinson’s disease: A window into brain pathology. Acta Neuropathol. 2014, 128, 99–109.
- Zis, P.; Sarrigiannis, P.G.; Rao, D.; Hewamadduma, C.; Hadjivassiliou, M. Chronic idiopathic axonal polyneuropathy: A systematic review. Neurol. 2016, 263, 1903–1910.
- Karceski, S. Patient page. Parkinson disease and polyneuropathy. About Parkinson disease. Neurology 2011, 77, e132–e134.
- Zis, P.; Grünewald, R.A.; Chaudhuri, R.K.; Hadjivassiliou, M. Peripheral neuropathy in idiopathic Parkinson's disease: A systematic review. Neurol. Sci. 2017, 378, 204–209.
- Toth, C.; Breithaupt, K.; Ge, S.; Duan, Y.; Terris, J.M.; Thiessen, A.; Wiebe, S.; Zochodne, D.W.; Suchowersky, O. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Neurol. 2010, 68, 28–36.
- Toth, C.; Brown, M.S.; Furtado, S.; Suchowersky, O.; Zochodne, D. Neuropathy as a potential complication of levodopa use in Parkinson's disease. Disord. 2008, 23, 1850–1859.
- Ceravolo, R.; Cossu, G.; Bandettini di Poggio, M.; Santoro, L.; Barone, P.; Zibetti, M.; Frosini, D.; Nicoletti, V.; Manganelli, F.; Iodice, R.; et al. Neuropathy and levodopa in Parkinson's disease: Evidence from a multicenter study. Disord. 2013, 28, 1391–1397.
- DeMott, T.K.; Richardson, J.K.; Thies, S.B.; Ashton-Miller, J.A. Falls and Gait Characteristics Among Older Persons with Peripheral Neuropathy. J. Phys. Med. Rehabil. 2007, 86, 125–132.
- Beaulieu, M.L.; Müller, M.; Bohnen, N.I. Peripheral neuropathy is associated with more frequent falls in Parkinson's disease. Parkinsonism Relat. Disord. 2018, 54, 46–50.
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020, 19, 462–470.
- Godinho, C.; Domingos, J.; Cunha, G.V.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Gonçalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. Neuroeng. Rehabil. 2016, 13, 24.
- Esser, P.; Collett, J.; Maynard, K.; Steins, D.; Hillier, A.; Buckingham, J.; Tan, G.D.; King, L.; Dawes, H. Single Sensor Gait Analysis to Detect Diabetic Peripheral Neuropathy: A Proof of Principle Study. Diabetes Metab. J. 2018, 42, 82–86.
- Kang, G.E.; Najafi, B. Sensor-Based Daily Physical Activity: Towards Prediction of the Level of Concern about Falling in Peripheral Neuropathy. Sensors 2020, 20, 505.
- Loprinzi, P.D.; Crush, E. Sensory Impairment, Functional Balance and Physical Activity with All-Cause Mortality. Phys. Act. Health 2016, 13, 980–987.
- Findling, O.; Van Der Logt, R.; Nedeltchev, K.; Achtnichts, L.; Allum, J.H.J. A comparison of balance control during stance and gait in patients with inflammatory and non-inflammatory polyneuropathy. PLoS ONE 2018, 13, e0191957.
- Morgan, C.; Rolinski, M.; McNaney, R.; Jones, B.; Rochester, L.; Maetzler, W.; Craddock, I.; Whone, A.L. Systematic Review Looking at the Use of Technology to Measure Free-Living Symptom and Activity Outcomes in Parkinson’s Disease in the Home or a Home-like Environment. J. Parkinson Dis. 2020, 10, 429–454.
- Rovini, E.; Maremmani, C.; Cavallo, F. Automated Systems Based on Wearable Sensors for the Management of Parkinson’s Disease at Home: A Systematic Review. Telemed. J. E Health 2018, 25, 167–183.
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313.
- Vienne-Jumeau, A.; Barrois, R.P.; Buffat, S.; Ricard, D.; Vidal, P.-P. Inertial Sensors to Assess Gait Quality in Patients with Neurological Disorders: A Systematic Review of Technical and Analytical Challenges. Front. Psychol. 2017, 8, 817.
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov. Disord. 2013, 28, 1628–1637.
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075.
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Wearable Sensor Use for Assessing Standing Balance and Walking Stability in People with Parkinson’s Disease: A Systematic Review. PLoS ONE 2015, 10, e0123705.
- Karmakar, S.; Rashidian, H.; Chan, C.; Liu, C.; Toth, C. Investigating the role of neuropathic pain relief in decreasing gait variability in diabetes mellitus patients with neuropathic pain: A randomized, double-blind crossover trial. J. Neuroeng. Rehabil. 2014, 11, 125.
- Lalli, P.; Chan, A.; Garven, A.; Midha, N.; Chan, C.; Brady, S.; Block, E.; Hu, B.; Toth, C. Increased gait variability in diabetes mellitus patients with neuropathic pain. J. Diabetes Complicat. 2013, 27, 248–254.
- Ling, E.; Lepow, B.; Zhou, H.; Enriquez, A.; Mullen, A.; Najafi, B. The impact of diabetic foot ulcers and unilateral offloading footwear on gait in people with diabetes. Clin. Biomech. 2020, 73, 157–161.
- Kang, G.E.; Yang, J.; Najafi, B. Does the Presence of Cognitive Impairment Exacerbate the Risk of Falls in People with Peripheral Neuropathy? An Application of Body-Worn Inertial Sensors to Measure Gait Variability. Sensors 2020, 20, 1328.
- Schwenk, M.; Grewal, G.S.; Holloway, D.T.; Muchna, A.; Garland, L.; Najafi, B. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2016, 62, 553–563.
- Najafi, B.; Khan, T.; Fleischer, A.E.; Wrobel, J. The Impact of Footwear and Walking Distance on Gait Stability in Diabetic Patients with Peripheral Neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 165–173.
- Kelly, C.; Fleischer, A.E.; Yalla, S.; Grewal, G.S.; Albright, R.; Berns, D.; Crews, R.T.; Najafi, B. Fear of Falling Is Prevalent in Older Adults with Diabetes Mellitus But Is Unrelated to Level of Neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 480–488.
- Grewal, G.; Sayeed, R.; Schwenk, M.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Balance rehabilitation: Promoting the role of virtual reality in patients with diabetic peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 498–507.
- Kang, G.E.; Zhou, H.; Varghese, V.; Najafi, B. Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults. Clin. Biomech. 2020, 72, 155–160.
- Grewal, G.S.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.; Najafi, B. Diabetic Peripheral Neuropathy and Gait: Does Footwear Modify This Association? J. Diabetes Sci. Technol. 2013, 7, 1138–1146.
- Najafi, B.; Talal, T.K.; Grewal, G.S.; Menzies, R.; Armstrong, D.G.; Lavery, L.A. Using Plantar Electrical Stimulation to Improve Postural Balance and Plantar Sensation Among Patients with Diabetic Peripheral Neuropathy: A Randomized Double Blinded Study. J. Diabetes Sci. Technol. 2017, 11, 693–701.
- Caronni, A.; Picardi, M.; Pintavalle, G.; Aristidou, E.; Redaelli, V.; Antoniotti, P.; Sterpi, I.; Tropea, P.; Corbo, M. Responsiveness to rehabilitation of balance and gait impairment in elderly with peripheral neuropathy. J. Biomech. 2019, 94, 31–38.
- De Bruin, E.D.; Hubli, M.; Hofer, P.; Wolf, P.; Murer, K.; Zijlstra, W. Validity and Reliability of Accelerometer-Based Gait Assessment in Patients with Diabetes on Challenging Surfaces. J. Aging Res. 2012, 2012, 954378.
- Kang, G.E.; Zahiri, M.; Lepow, B.; Saleem, N.; Najafi, B. The Effect of Daily Use of Plantar Mechanical Stimulation Through Micro-Mobile Foot Compression Device Installed in Shoe Insoles on Vibration Perception, Gait, and Balance in People with Diabetic Peripheral Neuropathy. J. Diabetes Sci. Technol. 2019, 13, 847–856.
- Najafi, B.; Horn, D.; Marclay, S.; Crews, R.T.; Wu, S.; Wrobel, J.S. Assessing Postural Control and Postural Control Strategy in Diabetes Patients Using Innovative and Wearable Technology. J. Diabetes Sci. Technol. 2010, 4, 780–791.
- Fino, P.C.; Horak, F.B.; El-Gohary, M.; Guidarelli, C.; Medysky, M.E.; Nagle, S.J.; Winters-Stone, K.M. Postural sway, falls, and self-reported neuropathy in aging female cancer survivors. Gait Posture 2019, 69, 136–142.
- Yalla, S.V.; Crews, R.T.; Fleischer, A.E.; Grewal, G.; Ortiz, J.; Najafi, B. An immediate effect of custom-made ankle foot orthoses on postural stability in older adults. Clin. Biomech. 2014, 29, 1081–1088.
- Toosizadeh, N.; Mohler, J.; Armstrong, D.G.; Talal, T.K.; Najafi, B. The Influence of Diabetic Peripheral Neuropathy on Local Postural Muscle and Central Sensory Feedback Balance Control. PLoS ONE 2015, 10, e0135255.
- Turcot, K.; Allet, L.; Golay, A.; Hoffmeyer, P.; Armand, S. Postural Strategies in Diabetes Patients with Peripheral Neuropathy Determined Using Cross-Correlation Functions. Diabetes Technol. Ther. 2012, 14, 403–410.
- Grewal, G.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-Based Interactive Balance Training with Visual Joint Movement Feedback for Improving Postural Stability in Diabetics with Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2015, 61, 567–574.
- Horak, F.B.; Mancini, M. Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov. Disord. 2013, 28, 1544–1551.
- Oung, Q.W.; Hariharan, M.; Lee, H.L.; Basah, S.N.; Yaacob, S.; Sarillee, M.; Lee, C.H. Technologies for Assessment of Motor Disorders in Parkinson’s Disease: A Review. Sensors 2015, 15, 21710–21745.
- Steins, D.; Dawes, H.; Esser, P.; Collett, J. Wearable accelerometry-based technology capable of assessing functional activities in neurological populations in community settings: A systematic review. J. Neuroeng. Rehabil. 2014, 11, 36.
- Merola, A.; Sturchio, A.; Hacker, S.; Serna, S.; Vizcarra, J.A.; Marsili, L.; Fasano, A.; Espay, A.J. Technology-based assessment of motor and nonmotor phenomena in Parkinson disease. Expert Rev. Neurother. 2018, 18, 825–845.
- Rovini, E.; Maremmani, C.; Cavallo, F. How Wearable Sensors Can Support Parkinson’s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555.
- Micó-Amigo, M.E.; Kingma, I.; Heinzel, S.; Rispens, S.M.; Heger, T.; Nussbaum, S.; Van Lummel, R.C.; Berg, D.; Maetzler, W.; Van Dieen, J.H. Potential Markers of Progression in Idiopathic Parkinson’s Disease Derived From Assessment of Circular Gait With a Single Body-Fixed-Sensor: A 5 Year Longitudinal Study. Front. Hum. Neurosci. 2019, 13, 59.
- Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 2009, 19, 026113.
- Zahiri, M.; Chen, K.M.; Zhou, H.; Nguyen, H.; Workeneh, B.T.; Yellapragada, S.V.; Sada, Y.H.; Schwenk, M.; Najafi, B. Using wearables to screen motor performance deterioration because of cancer and chemotherapy-induced peripheral neuropathy (CIPN) in adults—Toward an early diagnosis of CIPN. J. Geriatr. Oncol. 2019, 10, 960–967.