
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Akinari Sawada | + 3027 word(s) | 3027 | 2020-11-11 05:13:47 | | | |
| 2 | Nicole Yin | -19 word(s) | 3008 | 2020-11-26 04:58:23 | | |
Video Upload Options
Belching is defined as “an audible escape of air from the esophagus or the stomach into the pharynx”.
1. Introduction
Although everyone belches, it can be bothersome when excessive and/or triggering reflux symptoms. If patients cannot control belching in public, they often feel embarrassed, which profoundly disturbs their social lives. Such patients understandably seek medical care for their belching symptom. On the other hand, some patients predominantly complain of typical reflux symptoms rather than belching, even though excessive belching itself causes reflux symptoms[1][2][3]. Due to poor response to proton pump inhibitors (PPIs) therapy, those patients are often referred to specialists as PPI-refractory gastroesophageal reflux disease (GERD) [1][3][4][5].
2. Two Types of Belching: Gastric Belching and Supragastric Belching
2.1. Gastric Belching (GB)
GB is a physiological mechanism to vent swallowed air from the stomach. Air-induced distension of the proximal stomach triggers transient lower esophageal relaxation (TLESR), which allows the gas to be released retrogradely to the pharynx (Figures 1 and 2). TLESR is a vagal reflex via the brainstem, not a local reflex[6]. In detail, the vagal afferent conveys the stretch of cardiac stomach to the brainstem via nodose ganglion and solitary nucleus. Subsequently, vagal efferent projecting to LES from the brainstem releases nitric oxide and vasoactive intestinal peptide, which relaxes the LES[7]. During TLESR, esophageal shortening often occurs by much stronger contraction of the longitudinal muscle than circular muscle. The discordance of these two muscle layers stretches myenteric neurons to release nitric oxide, which is possibly involved with the mechanism of TLESR[8].
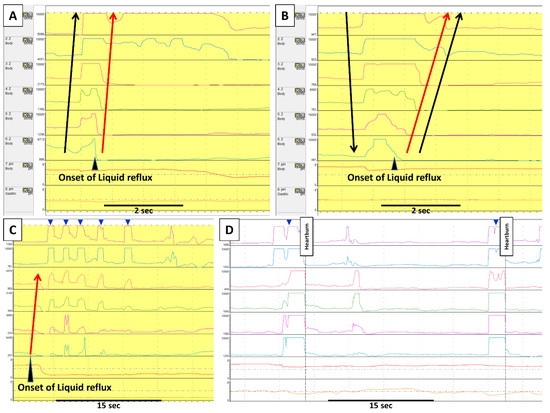
Figure 1. Gastric belching and supragastric belching in impedance–pH monitoring. Each row in impedance–pH tracings measures, from the bottom, gastric pH; esophageal pH at 5 cm above the lower esophageal sphincter (LES); and impedance at 3, 5, 7, 9, 15, and 17cm above the LES. In GB (A), an increase of impedance, indicating air from the stomach, moves retrogradely from the distal to the proximal esophagus. This GB is immediately followed by a liquid reflux episode (i.e., retrograde impedance decrease). On the other hand, SGB (B) starts with antegrade movement of impedance increase by sucking or swallowing air from the pharynx; subsequently, such increase returns to baseline retrogradely by abdominal straining. This SGB induces a liquid reflux episode. Apart from the SGB inducing reflux, there are two other patterns of the relationship between SGB and reflux. (C) shows that several SGBs (blue arrowheads) occur during liquid reflux in which an uncomfortable sensation by reflux might lead a patient to perform SGBs. (D) shows SGBs (blue arrowheads) without reflux. In this case, a patient constantly marked heartburn immediately after SGBs, which suggests the SGBs caused reflux symptoms by esophageal distension. Black arrows indicate the movement of air, whereas red arrows indicate liquid reflux.
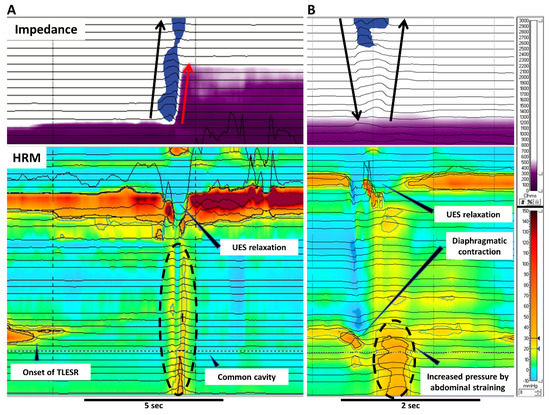
Figure 2. Gastric belching and supragastric belching in HRM combined with impedance. These pictures illustrate GB (A) and SGB (B) in simultaneous recording of impedance and HRM. (A) A GB occurs with liquid reflux during TLESR. GB can be recognized as a common cavity in HRM. The air evacuates from the stomach to the pharynx through the relaxed UES. (B) In SGB, the contraction of the diaphragm generates negative pressure in the esophagus. UES relaxation lets air into the esophagus followed by expelling the air by abdominal straining. No peristalsis is involved with the air movement in SGB. Black arrows indicate the movement of air whereas red arrows indicate liquid reflux. HRM; high resolution manometry, TLESR; transient lower esophageal sphincter relaxation, UES; upper esophageal sphincter.
2.2. Supragastric Belching (SGB)
SGB is a behavioral disorder where a patient sucks or swallows air from the mouth into the esophagus, immediately followed by expelling it through the pharynx (Figures 1 and 2). Regarding its physiological mechanism, the diaphragm contracts to generate negative pressure in the esophagus (thoracic cavity). The upper esophageal sphincter (UES) relaxes, letting air into the esophagus (i.e., suck) from the pharynx due to the pressure gradient. Afterwards, the air is emitted retrogradely into the pharynx, increasing gastric and esophageal pressure by abdominal straining[9]. Apart from air sucker type, a minority of patients use the pharyngeal pump (i.e., swallow) instead for the intake of air.
A small number of SGB and GB can be seen in healthy asymptomatic subjects[10][11]. SGB becomes bothersome when excessive as it can impair quality of life[12] and induce pathological gastroesophageal reflux[2][5][13]. We previously proposed ≤13 episodes/24 h as the normal value of SGB[10]. However, it should be taken into consideration that personal factors can alter the threshold for feeling belching as irritating. In many cases, patients can identify warning symptoms such as throat/chest/abdominal discomfort to trigger SGB[2]. Presumably, patients learn the behavior to try to ease such an unpleasant sensation in some way, and with time it turns into a subconscious act. We sometimes encounter patients who remember the exact moment or time when they began having excessive SGB. In such cases, stressful life events might precipitate SBG. In animal experiments, Lang et al. found that esophageal distension can trigger a reflex of inhaling air followed by belching via vagal nerves and esophageal tension/mucosal mechanoreceptors[14]. In light of this finding, a limited part of SGB might be a reflex rather than a behavior, especially when gas reflux causes the initial esophageal distension.
Psychological factors can influence the frequency of SGB. Bredenoord et al. showed that patients have more SGBs when aware of impedance recording, whilst distraction by filling in questionnaires or speaking can reduce the number of SBGs[15]. Additionally, SGB is hardly observed during sleeping[16].
Regarding personality, our previous study found that patients with excessive SGB showed lower levels of neuroticism (i.e., tend to less worry about symptoms) and the same level of hypervigilance (i.e., tend not to be too alert for bodily sensation) compared to healthy subjects[2].
3. Conceptual Change of Belching Disorders and Aerophagia in Rome Diagnostic Criteria
Excessive belching was initially thought to be a consequence of aerophagia (frequent air swallowing)[17]. Therefore, Rome II defined excessive belching as a part of “Aerophagia”. The advent of intraluminal impedance monitoring enabled us to assess both the type and movement of bolus in the esophagus (i.e., gas or liquid, and antegrade or retrograde movement)[18][19]. With this technique, Bredenoord et al. found a novel type of belching named supragastric belching (SGB)[20]. This finding brought research about belching into the next level because, until this study, all the belching had been considered as gastric belching. Consequently, Rome III expanded the disease concept by adding “unspecified excessive belching (i.e., excessive belching without air swallowing)” as the other subcategory apart from “aerophagia”, and renamed the disorder as “Belching Disorders”[21]. At that time, the two terms, “aerophagia” and “SGB”, were used in a bewildering way since SGB was thought to be found only in “aerophagia”[20]. However, the two disorders have been segregated from each other recently because they have distinctive features or physiology. In aerophagia, esophageal peristalsis propels swallowed air, which ends up accumulating in the intestine and colon. It mainly manifests itself as bloating, abdominal distention and constipation, although some patients complain of PPI refractory GERD symptoms[3]. On the other hand, SGB does not involve any esophageal peristalsis, and swallowed air does not reach the stomach[22][23]. Nevertheless, aerophagia still lacks clear diagnostic criteria to distinguish from normal air swallowing. The latest Rome IV removed the term “Aerophagia” from “Belching disorders” in which belching was simply classified into excessive GB and SGB on the basis of the origin of the gas[24] (Figure 3).
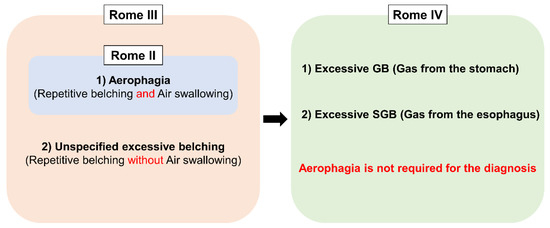
Figure 3. Conceptual change of Belching disorders and Aerophagia in Rome diagnostic criteria. GB; gastric belching, SGB; supragastric belching.
4. Epidemiology
There are several studies reporting the prevalence of belching symptom in the general population, which ranges from 6.7% to 28.8%[25][26][27][28]. Subjects are more likely to complain of belching symptom when having concomitant typical reflux symptoms (i.e., heartburn and/or regurgitation) (Table 1). In GERD patients, the prevalence of belching ranges widely from 4.1–75.6% [13][29][30][31][32][33]. Interestingly, Klauser et al. [29] showed no difference in the prevalence of belching between normal and pathological acid exposure in GERD. It is important to note that these epidemiological studies include both types of belching (i.e., GB and SGB) due to interview-based survey. Besides, heterogeneous definitions of excessive belching and GERD can influence the results significantly.
Table 1. Prevalence of belching symptom.
|
Author |
Country |
Study subjects |
Number of people surveyed (n) |
Overall prevalence of belching |
Prevalence of belching symptom without GERD |
Prevalence of belching symptom with GERD |
Definition of GERD |
|
Westbrook et al.[25] |
Australia |
General population |
2300 |
6.7% |
|
|
|
|
Bor et al. [26] |
Turkey |
General population |
630 |
15.9% |
11.5% (n = 393) |
23.2% (n = 237) |
Heartburn and/or regurgitation |
|
Rey et al.[27] |
Spain |
General population |
2500 |
20.5% |
12.6% (n = 1709) |
37.5% (n = 791) |
Heartburn and/or regurgitation |
|
Li et al.[28] |
China |
Outpatients |
15283 |
28.8% (n = 13282) |
26.3% (n = 12257) |
58.5% (n = 1025) |
RDQ score > 12 |
|
Kessing et al.[13] |
Netherland |
GERD |
90 |
- |
- |
75.6% |
Reflux symptoms |
|
Klauser et al.[29] |
Germany |
GERD |
304 |
|
|
44.7% |
Esophageal symptom |
|
Dore et al. [30] |
Italy |
GERD |
266 |
- |
- |
26.3% |
Heartburn, regurgitation, dysphagia or odynophagia |
|
Yarandi et al. [31] |
Iran |
GERD |
1522 |
- |
- |
4.1% |
Heartburn, regurgitation and dysphagia |
|
Ribolsi et al. [32] |
Italy |
GERD |
573 |
- |
- |
62.7% |
Heartburn, regurgitation, or noncardiac chest pain |
|
Lin et al. [33] |
USA |
GERD |
180 |
|
|
70% |
Positive DeMeester score ( > 14.2), endoscopic esophagitis, or Barrett’s esophagus |
RDQ; Reflux Disease Questionnaire.
As for the prevalence of SGB, our previous studies found that 3.4% of patients referred to a tertiary GI physiology unit had excessive SGB[10], and the prevalence in PPI-refractory GERD patients differs between regions as the Japanese (18.5%) had lower prevalence of excessive SGB compared to the British (36.1%)[11].
5. Belching and Other Relevant Conditions
5.1. Functional Dyspepsia
Up to 80% of functional dyspepsia (FD) patients complain of belching symptom[33][34][35][36]. According to a study by Conchillo et al., a high prevalence of belching symptom in FD is probably attributed to more frequent air swallowing compared to healthy subjects[37]. Belching symptom was associated with lower quality of life and more weight loss in patients with FD[34]. It could be explained by higher sensitivity to gastric dilatation in FD patients with concomitant belching symptom than without[35]. However, it remains uncertain if epigastric discomfort causes air swallowing or vice versa. The two factors might interact mutually to increase distressing symptoms. Interestingly, FD shows less correlation between belching symptom and acid reflux than GERD, and PPIs do not improve belching in FD unlike that in GERD[33].
Although little is known about the association between FD and SGB, patients with excessive SGB often identify abdominal discomfort as a warning signal[2].
5.2. Globus
Globus is a functional gastrointestinal disorder consisting of a persistent or intermittent lump sensation in the throat[38]. Patients with globus had higher prevalence of pathological SGB and aerophagia than GERD patients[39]. There might be a causal link between SGB and globus as some patients recognize throat discomfort as a warning signal for SGB[40].
5.3. Bariatric Surgery (Sleeve Gastrectomy)
Sleeve gastrectomy is one of the bariatric surgeries, which removes a generous portion of the stomach on the greater curvature side to create a tubular gastric pouch. Burgerhart et al. showed laparoscopic sleeve gastrectomy (LSG) almost doubled the number of GBs (from 29.7 to 59.5/24 h) despite the decrease of liquid and air swallows, whereas the number of SGBs did not alter[41]. As LSG is known to increase esophageal acid exposure with the decrease of LES pressure[42], LSG might increase GB by the anatomical change of the stomach (i.e., lack of gastric fundus and small accommodation capacity) and the weakened LES.
5.4. Miscellaneous Conditions
Various disorders causing distressing symptoms around the chest and abdomen (i.e., peptic ulcer disease, pancreatitis, angina pectoris and symptomatic cholelithiasis) are reported to manifest in belching symptom[43]. However, their causal link remains vague.
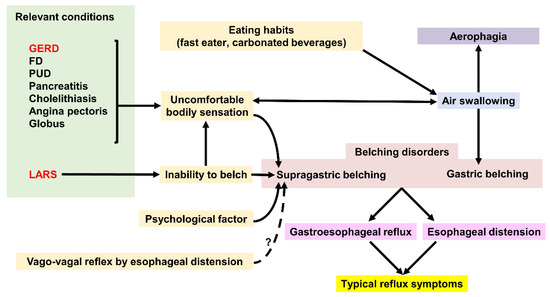
Figure 4. Factors affecting Gastric belching and Supragastric belching. GERD; gastroesophageal reflux disease, FD; functional dyspepsia, PUD; peptic ulcer disease, LARS; laparoscopic anti-reflux surgery.
6. Clinical Approach and Treatment for Belching
6.1. Clinical Approach to Excessive Belching
Patients with troublesome belching can be divided into two groups: GB- or SGB-predominant type. Li et al. found that both groups have similar reflux profile and air swallow, but the SGB-predominant type showed more belching episodes than GB-predominant type[44]. With respect to the symptom, Kessing et al. found the severity of belching symptom depends on the number of SGBs, not GBs[13]. The number or severity of belching could provide clinicians with a hint on the type of belching (i.e., SGB tends to be repetitive whereas GB tend to be single isolated events). However, impedance–pH monitoring, a gold standard modality, is often necessary for precise diagnosis.
6.2. Clinical Approach to Hidden SGB in PPI-Refractory GERD
In PPI-refractory GERD, many patients do not report belching symptom even when SGB is a culprit of reflux symptoms. Therefore, clinicians have to pay attention to impedance tracing so that hidden SGB is not overlooked. As approximately 50–60% of the SGBs exist close to reflux episodes[1][5], manual editing of automatic analysis of all reflux episodes can identify pathologic SGB. It is important to evaluate to what extent SGB causes acid exposure or reflux symptom.
6.3. Treatment for Gastric Belching
Treatment for GB should target air swallowing and/or TLESR.
To suppress air swallowing, it is worth trying to provide instruction to eat slowly and avoid carbonated drinks. Several studies showed that PPI therapy improves belching symptom[30][45][46][47], except for one study reporting no benefit[48]. The effect of PPIs on reflux symptoms might stop air swallowing as a response to chest/abdominal discomfort. Anti-reflux surgery reduces the number of TLESRs[49]. Besides, gamma aminobutyric acid receptor type B (GABAB) receptor agonist, baclofen, can alleviate belching symptom by reducing the number of TLESRs[50] and increasing LES pressure[51]. However, baclofen has a side effect of drowsiness, and thus, is not widely used. Lesogaberan, peripherally acting as a GABAB receptor agonist, showed its ability to reduce TLESR with little central nervous system effect[52]. Nevertheless, it has never been launched.
In most cases, patients suffer from both excessive GB and reflux symptom. In addition to the eating habit modification, the therapeutic options can be optimized on the basis of reflux phenotype. For patients with severe erosive reflux disease or NERD, laparoscopic fundoplication or a maximum dose of PPI plus baclofen would be the first choice. For RH and FH patients (normal acid exposure), adding baclofen to their mainstream therapy, which is pain modulators, is likely to be beneficial as long as patients can tolerate its side effect.
In functional dyspepsia, several drugs shows a suppressive effect on belching symptom, such as rifaximin[53], acotiamide (acetylcholinesterase inhibitor)[54][55], rebamipide (mucosal protective agents), and famotidine (H2 receptor antagonist)[56].
6.4. Treatment for Supragastric Belching
SGB benefits from psychological therapy to unlearn or interrupt the acquired behavioral. Dedicated cognitive behavior therapy[2][57][58] or speech therapy[59][60] have established its efficacy on SGB. Speech therapy improves the severity of belching symptom in 83% of SGB patients, whereas CBT reduces the number of SGBs objectively by > 50% in half of patients[2]. These two treatments share the common concept consisting of cognitive and behavioral components.
For instance, our CBT has five sessions. In the cognitive part, firstly therapists explain the mechanism of SGB so that patients can understand SGB is a subconscious but deliberate behavior. We should be careful not to make patients feel blamed in this process. Otherwise, it will impact adherence to the treatment negatively. Secondly, a warning signal (premonitory sensations) should be identified, which causes patients to commence SGB to deal with the sensation. In most cases, throat, chest, or abdominal discomfort are identified as a warning signal, which can be used for a cue to start exercise to stop SGB as below. Additionally, the thought that SGB is useful to ease that discomfort needs to be corrected.
In the behavioral part, therapists instruct patients in the use of 1) diaphragmatic breathing, and 2) mouth opening/tongue position to make it physically impossible to perform SGB. Diaphragmatic breathing inhales and exhales, spending 3 s in each phase by moving the abdominal wall, not the thoracic wall. Patients need to breathe through the mouth with opening moderately and place the tongue behind the top front teeth. These procedures should be trained at least twice per day for 3–5 min in a supine or sitting position. Once patients get used to the breathing technique, they are encouraged to use it as often as possible, especially when noticing a warning signal or commencing a bunch of SGBs. Our previous study found a lower number of SGBs, lower hypervigilance, and higher CBT proficiency (i.e., possible to identify a warning signal, better acceptance and adherence to CBT), which are predictive factors for better outcome, and the therapeutic effect of CBT sustains for at least 12 months[40].
Concerning medications, a case has been reported that a combination of baclofen and gabapentin alleviates SGB[61]. On the other hand, a randomized controlled trial shows baclofen does not decrease the number of SGBs[62].
6.5. Treatment Implication for SGB With PPI-Refractory GERD
In this setting, SGB is possibly required for improving typical reflux symptoms along with the current mainstream therapy for PPI refractoriness[1][4]. On the basis of relevant literatures, we here propose a possible therapeutic approach for each reflux phenotype with excessive SGB.
6.5.1. Excessive SGB With Severe Erosive Esophagitis or NERD
Sole CBT probably cannot reduce acid exposure to the physiological range in this scenario, although it depends to what extent SGB-induced reflux accounts for acid exposure time. In fact, a previous study showed CBT decreases acid exposure time significantly, however, it was inadequate to normalize it[2]. Therefore, it would be reasonable to perform ARS first to protect damaged esophageal mucosa and subsequently add CBT for SGB, as ARS might further increase the number of SGBs[49].
6.5.2. Excessive SGB With RH
Excessive SGB possibly accounts for approximately 40% of typical reflux symptoms[1]. Considering SGB hardly responds to pain modulators or PPIs, dual therapy (i.e., pain modulators and CBT) might be beneficial for easing reflux symptoms. However, further study is warranted to see which treatment is the best, sole CBT, pain modulators or dual therapy.
6.5.3. Excessive SGB With FH
FH has a lower prevalence of excessive SGB than RH[1], however, there is a possibility that SGB-induced distension causes reflux symptoms in FH like RH. However, there is no study to assess the association between SGB and reflux symptoms in FH. If that is the case, dual therapy of CBT and pain modulators might be effective.
References
- Sawada, A.; Guzman, M.; Nikaki, K.; Sonmez, S.; Yazaki, E.; Aziz, Q.; Woodland, P.; Rogers, B.; Gyawali, C.P.; Sifrim, D. Identification of Different Phenotypes of Esophageal Reflux Hypersensitivity and Implications for Treatment. Clin. Gastroenterol. Hepatol. 2020.
- Glasinovic, E.; Wynter, E.; Arguero, J.; Ooi, J.; Nakagawa, K.; Yazaki, E.; Hajek, P.; Psych, C.C.; Woodland, P.; Sifrim, D. Treatment of supragastric belching with cognitive behavioral therapy improves quality of life and reduces acid gastroesophageal reflux. Am. J. Gastroenterol. 2018, 113, 539–547.
- Bravi, I.; Woodland, P.; Gill, R.S.; Al-Zinaty, M.; Bredenoord, A.J.; Sifrim, D. Increased prandial air swallowing and postprandial gas-liquid reflux among patients refractory to proton pump inhibitor therapy. Clin. Gastroenterol. Hepatol. 2013, 11, 784–789.
- Yadlapati, R.; Tye, M.; Roman, S.; Kahrilas, P.J.; Ritter, K.; Pandolfino, J.E. Postprandial High-Resolution Impedance Manometry Identifies Mechanisms of Nonresponse to Proton Pump Inhibitors. Clin. Gastroenterol. Hepatol. 2018, 16, 211–218.
- Hemmink, G.J.; Bredenoord, A.J.; Weusten, B.L.; Timmer, R.; Smout, A.J. Supragastric belching in patients with reflux symptoms. Am. J. Gastroenterol. 2009, 104, 1992–1997.
- Martin, C.J.; Patrikios, J.; Dent, J. Abolition of gas reflux and transient lower esophageal sphincter relaxation by vagal blockade in the dog. Gastroenterology 1986, 91, 890–896.
- Mittal, R.K.; Balaban, D.H. The esophagogastric junction. N. Engl. J. Med. 1997, 336, 924–932.
- Mittal, R.K. Regulation and dysregulation of esophageal peristalsis by the integrated function of circular and longitudinal muscle layers in health and disease. Am. J. Phys. Gastrointest. Liver Physiol. 2016, 311, G431–G443.
- Kessing, B.F.; Bredenoord, A.J.; Smout, A.J. Mechanisms of gastric and supragastric belching: A study using concurrent high-resolution manometry and impedance monitoring. Neurogastroenterol. Motil. 2012, 24, 573–579.
- Koukias, N.; Woodland, P.; Yazaki, E.; Sifrim, D. Supragastric Belching: Prevalence and Association With Gastroesophageal Reflux Disease and Esophageal Hypomotility. J. Neurogastroenterol. Motil. 2015, 21, 398–403.
- Akinari Sawada; Hideaki Itami; Kenichiro Nakagawa; Shinji Hirano; Hiroyuki Kitamura; Rieko Nakata; Shingo Takashima; Yasuaki Abe; Masahiro Saito; Etsuro Yazaki; et al.Osamu KawamuraFumio TanakaToshihisa TakeuchiTomoyuki KoikeAtsushi MasamuneYasuhiro FujiwaraKazuhide HiguchiDaniel Sifrim Supragastric belching in Japan: lower prevalence and relevance for management of gastroesophageal reflux disease compared to United Kingdom. Journal of Gastroenterology 2020, 55, 1046-1053, 10.1007/s00535-020-01720-9.
- Albert J. Bredenoord; Andre J. Smout; Impaired health-related quality of life in patients with excessive supragastric belching. European Journal of Gastroenterology & Hepatology 2010, 22, 1420-1423, 10.1097/meg.0b013e328340887c.
- B. F. Kessing; A. J. Bredenoord; M. Velosa; A. J. P. M. Smout; Supragastric belches are the main determinants of troublesome belching symptoms in patients with gastro-oesophageal reflux disease. Alimentary Pharmacology & Therapeutics 2012, 35, 1073-1079, 10.1111/j.1365-2036.2012.05070.x.
- Lang, I.M.; Medda, B.K.; Shaker, R. Characterization and mechanisms of the supragastric belch in the cat. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G220–G229.
- Bredenoord, A.J.; Weusten, B.L.; Timmer, R.; Smout, A.J. Psychological factors affect the frequency of belching in patients with aerophagia. Am. J. Gastroenterol. 2006, 101, 2777–2781.
- Karamanolis, G.; Triantafyllou, K.; Tsiamoulos, Z.; Polymeros, D.; Kalli, T.; Misailidis, N.; Liakakos, T.; Ladas, S.D. Effect of sleep on excessive belching: A 24-hour impedance-pH study. J. Clin. Gastroenterol. 2010, 44, 332–334.
- Talley, N.J.; Stanghellini, V.; Heading, R.C.; Koch, K.L.; Malagelada, J.R.; Tytgat, G.N. Functional gastroduodenal disorders. Gut 1999, 45, 37–42.
- Fass, J.; Silny, J.; Braun, J.; Heindrichs, U.; Dreuw, B.; Schumpelick, V.; Rau, G. Measuring esophageal motility with a new intraluminal impedance device. First clinical results in reflux patients. Scand. J. Gastroenterol. 1994, 29, 693–702.
- Sifrim, D.; Silny, J.; Holloway, R.H.; Janssens, J.J. Patterns of gas and liquid reflux during transient lower oesophageal sphincter relaxation: A study using intraluminal electrical impedance. Gut 1999, 44, 47–54.
- Bredenoord, A.J.; Weusten, B.L.; Sifrim, D.; Timmer, R.; Smout, A.J. Aerophagia, gastric, and supragastric belching: A study using intraluminal electrical impedance monitoring. Gut 2004, 53, 1561–1565.
- Tack, J.; Talley, N.J.; Camilleri, M.; Holtmann, G.; Hu, P.; Malagelada, J.R.; Stanghellini, V. Functional gastroduodenal disorders. Gastroenterology 2006, 130, 1466–1479.
- Bredenoord, A.J. Excessive belching and aerophagia: Two different disorders. Dis. Esophagus 2010, 23, 347–352.
- Hemmink, G.J.; Weusten, B.L.; Bredenoord, A.J.; Timmer, R.; Smout, A.J. Aerophagia: Excessive air swallowing demonstrated by esophageal impedance monitoring. Clin. Gastroenterol. Hepatol. 2009, 7, 1127–1129.
- Vincenzo Stanghellini; Francis K. L. Chan; William L. Hasler; Juan R. Malagelada; Hidekazu Suzuki; Jan Tack; Nicholas J. Talley; Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380-1392, 10.1053/j.gastro.2016.02.011.
- Westbrook, J.I.; Talley, N.J. Empiric clustering of dyspepsia into symptom subgroups: A population-based study. Scand. J. Gastroenterol. 2002, 37, 917–923.
- Bor, S.; Mandiracioglu, A.; Kitapcioglu, G.; Caymaz-Bor, C.; Gilbert, R.J. Gastroesophageal reflux disease in a low-income region in Turkey. Am. J. Gastroenterol. 2005, 100, 759–765.
- Rey, E.; Elola-Olaso, C.M.; Rodríguez-Artalejo, F.; Locke, G.R.; Díaz-Rubio, M. Prevalence of atypical symptoms and their association with typical symptoms of gastroesophageal reflux in Spain. Eur. J. Gastroenterol. Hepatol. 2006, 18, 969–975.
- Li, Y.M.; Du, J.; Zhang, H.; Yu, C.H. Epidemiological investigation in outpatients with symptomatic gastroesophageal reflux from the Department of Medicine in Zhejiang Province, east China. J. Gastroenterol. Hepatol. 2008, 23, 283–289.
- Klauser, A.G.; Schindlbeck, N.E.; Müller-Lissner, S.A. Symptoms in gastro-oesophageal reflux disease. Lancet 1990, 335, 205–208.
- Dore, M.P.; Pedroni, A.; Pes, G.M.; Maragkoudakis, E.; Tadeu, V.; Pirina, P.; Realdi, G.; Delitala, G.; Malaty, H.M. Effect of antisecretory therapy on atypical symptoms in gastroesophageal reflux disease. Dig. Dis. Sci. 2007, 52, 463–468.
- Yarandi, S.S.; Nasseri-Moghaddam, S.; Mostajabi, P.; Malekzadeh, R. Overlapping gastroesophageal reflux disease and irritable bowel syndrome: Increased dysfunctional symptoms. World J. Gastroenterol. 2010, 16, 1232–1238.
- Ribolsi, M.; Cicala, M.; Zentilin, P.; Neri, M.; Mauro, A.; Efthymakis, K.; Petitti, T.; Savarino, V.; Penagini, R. Prevalence and clinical characteristics of refractoriness to optimal proton pump inhibitor therapy in non-erosive reflux disease. Aliment. Pharmacol. Ther. 2018, 48, 1074–1081.
- Lin, M.; Triadafilopoulos, G. Belching: Dyspepsia or gastroesophageal reflux disease? Am. J. Gastroenterol. 2003, 98, 2139–2145.
- Piessevaux, H.; De Winter, B.; Louis, E.; Muls, V.; De Looze, D.; Pelckmans, P.; Deltenre, M.; Urbain, D.; Tack, J. Dyspeptic symptoms in the general population: A factor and cluster analysis of symptom groupings. Neurogastroenterol. Motil. 2009, 21, 378–388.
- Tack, J.; Caenepeel, P.; Fischler, B.; Piessevaux, H.; Janssens, J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology 2001, 121, 526–535.
- Tack, J.; Caenepeel, P.; Arts, J.; Lee, K.J.; Sifrim, D.; Janssens, J. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut 2005, 54, 1370–1376.
- J. M. Conchillo; M. Selimah; A. J. Bredenoord; M. Samsom; A. J. P. M. Smout; Air swallowing, belching, acid and non-acid reflux in patients with functional dyspepsia. Alimentary Pharmacology & Therapeutics 2007, 25, 965-971, 10.1111/j.1365-2036.2007.03279.x.
- Qasim Aziz; Ronnie Fass; C. Prakash Gyawali; Hiroto Miwa; John E. Pandolfino; Frank Zerbib; Esophageal Disorders. Gastroenterology 2016, 150, 1368-1379, 10.1053/j.gastro.2016.02.012.
- Paivi Nevalainen; M A Walamies; Olli Kruuna; Perttu E T Arkkila; L.-M. Aaltonen; Supragastric belch may be related to globus symptom - a prospective clinical study. Neurogastroenterology & Motility 2016, 28, 680-686, 10.1111/nmo.12764.
- Akinari Sawada; Natasha Anastasi; Alicia Green; Esteban Glasinovic; Emily Wynter; Ahmed Albusoda; Peter Hajek; Daniel Sifrim; Management of supragastric belching with cognitive behavioural therapy: factors determining success and follow-up outcomes at 6-12 months post-therapy. Alimentary Pharmacology & Therapeutics 2019, 50, 530-537, 10.1111/apt.15417.
- Jan S. Burgerhart; Paul C. Van De Meeberg; Femke A. Mauritz; Erik J. Schoon; Johannes F. Smulders; Peter D. Siersema; André J. P. M. Smout; Increased Belching After Sleeve Gastrectomy. Obesity Surgery 2015, 26, 132-137, 10.1007/s11695-015-1718-3.
- Jan S. Burgerhart; Charlotte A. I. Schotborgh; Erik J. Schoon; Johannes F. Smulders; Paul C. Van De Meeberg; Peter D. Siersema; André J. P. M. Smout; Effect of Sleeve Gastrectomy on Gastroesophageal Reflux. Obesity Surgery 2014, 24, 1436-1441, 10.1007/s11695-014-1222-1.
- A. J. Bredenoord; Management of Belching, Hiccups, and Aerophagia. Clinical Gastroenterology and Hepatology 2013, 11, 6-12, 10.1016/j.cgh.2012.09.006.
- Jianbo Li; Yinglian Xiao; Sui Peng; Jinkun Lin; Minhu Chen; Characteristics of belching, swallowing, and gastroesophageal reflux in belching patients based on Rome III criteria. Journal of Gastroenterology and Hepatology 2013, 28, 1282-1287, 10.1111/jgh.12187.
- Kahrilas, P.J.; Miner, P.; Johanson, J.; Mao, L.; Jokubaitis, L.; Sloan, S. Efficacy of rabeprazole in the treatment of symptomatic gastroesophageal reflux disease. Dig. Dis. Sci. 2005, 50, 2009–2018.
- Miner, P.; Orr, W.; Filippone, J.; Jokubaitis, L.; Sloan, S. Rabeprazole in nonerosive gastroesophageal reflux disease: A randomized placebo-controlled trial. Am. J. Gastroenterol. 2002, 97, 1332–1339.
- Gerson, L.B.; Kahrilas, P.J.; Fass, R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin. Gastroenterol. Hepatol. 2011, 9, 824–833.
- Chatchai Kriengkirakul; Tanisa Patcharatrakul; Sutep Gonlachanvit; The Therapeutic and Diagnostic Value of 2-week High Dose Proton Pump Inhibitor Treatment in Overlapping Non-erosive Gastroesophageal Reflux Disease and Functional Dyspepsia Patients. Journal of Neurogastroenterology and Motility 2012, 18, 174-180, 10.5056/jnm.2012.18.2.174.
- J. A. J. L. Broeders; A. J. Bredenoord; E. J. Hazebroek; I. A. M. J. Broeders; H. G. Gooszen; A. J. P. M. Smout; Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2010, 60, 435-441, 10.1136/gut.2010.224824.
- M. J. Cossentino; K. Mann; S. P. Armbruster; J. M. Lake; C. Maydonovitch; R. K. H. Wong; Randomised clinical trial: the effect of baclofen in patients with gastro-oesophageal reflux - a randomised prospective study. Alimentary Pharmacology & Therapeutics 2012, 35, 1036-1044, 10.1111/j.1365-2036.2012.05068.x.
- Q Zhang; A Lehmann; R Rigda; J Dent; R H Holloway; Control of transient lower oesophageal sphincter relaxations and reflux by the GABAB agonist baclofen in patients with gastro-oesophageal reflux disease. Gut 2002, 50, 19-24, 10.1136/gut.50.1.19.
- G. E. Boeckxstaens; Hans Rydholm; Aaltje Lei; John Adler; Magnus Ruth; Effect of lesogaberan, a novel GABAB-receptor agonist, on transient lower oesophageal sphincter relaxations in male subjects. Alimentary Pharmacology & Therapeutics 2010, 31, 1208-1217, 10.1111/j.1365-2036.2010.04283.x.
- V. P. Y. Tan; K. S. H. Liu; F. Y. F. Lam; I. F. N. Hung; M. F. Yuen; Wai K Leung; Randomised clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Alimentary Pharmacology & Therapeutics 2017, 45, 767-776, 10.1111/apt.13945.
- Porika, S.K.; Veligandla, K.C.; Muni, S.K.; Acharya, S.; Mehta, S.C.; Sharma, A.D. Real-World, Non-Interventional, Observational Study to Evaluate Effectiveness and Tolerability of Acotiamide Hydrochloride Hydrate in Treatment of Functional Dyspepsia. Adv. Ther. 2018, 35, 1884–1893.
- Amini, M.; Ghamar Chehreh, M.E.; Khedmat, H.; Valizadegan, G.; Babaei, M.; Darvishi, A.; Taheri, S. Famotidine in the treatment of functional dyspepsia: A randomized double-blind, placebo-controlled trial. J. Egypt. Public Health Assoc. 2012, 87, 29–33.
- Hiroto Miwa; Taro Osada; Akihito Nagahara; Toshifumi Ohkusa; Mariko Hojo; Toshihiko Tomita; Kazutoshi Hori; Takayuki Matsumoto; N Sato; Effect of a gastro-protective agent, rebamipide, on symptom improvement in patients with functional dyspepsia: A double-blind placebo-controlled study in Japan. Journal of Gastroenterology and Hepatology 2006, 21, 1826-1831, 10.1111/j.1440-1746.2006.04446.x.
- Ong, A.M.; Chua, L.T.; Khor, C.J.; Asokkumar, R.; S/O Namasivayam, V.; Wang, Y.T. Diaphragmatic Breathing Reduces Belching and Proton Pump Inhibitor Refractory Gastroesophageal Reflux Symptoms. Clin. Gastroenterol. Hepatol. 2018, 16, 407–416.
- Katzka, D.A. Simple office-based behavioral approach to patients with chronic belching. Dis. Esophagus 2013, 26, 570–573.
- Hemmink, G.J.; Ten Cate, L.; Bredenoord, A.J.; Timmer, R.; Weusten, B.L.; Smout, A.J. Speech therapy in patients with excessive supragastric belching—A pilot study. Neurogastroenterol. Motil. 2010, 22, 24-e3.
- Ten Cate, L.; Herregods, T.V.K.; Dejonckere, P.H.; Hemmink, G.J.M.; Smout, A.J.P.M.; Bredenoord, A.J. Speech Therapy as Treatment for Supragastric Belching. Dysphagia 2018, 33, 707–715.
- Hagen Kunte; Golo Kronenberg; Katharina Fink; Lutz Harms; Rainer Hellweg; Successful treatment of excessive supragastric belching by combination of pregabalin and baclofen. Psychiatry and Clinical Neurosciences 2014, 69, 124-125, 10.1111/pcn.12223.
- Kathleen Blondeau; Veerle Boecxstaens; Nathalie Rommel; Richard Farré; Stephanie Depeyper; Lieselot Holvoet; Guy Boeckxstaens; Jan F. Tack; Baclofen Improves Symptoms and Reduces Postprandial Flow Events in Patients With Rumination and Supragastric Belching. Clinical Gastroenterology and Hepatology 2012, 10, 379-384, 10.1016/j.cgh.2011.10.042.




