
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eleftherios Panteris | -- | 1656 | 2022-11-01 09:20:24 | | | |
| 2 | Lindsay Dong | Meta information modification | 1656 | 2022-11-02 08:34:37 | | | | |
| 3 | Lindsay Dong | + 4 word(s) | 1660 | 2022-11-02 08:41:09 | | |
Video Upload Options
Micro-computed tomography (micro-CT) is a promising novel medical imaging modality that allows for non-destructive volumetric imaging of surgical tissue specimens at high spatial resolution.
1. Introduction
2. Micro-CT in Pulmonary Diseases Diagnosis
Micro-CT has been used for the quantitative assessment of the pulmonary microanatomy, and in the context of a wide range of lung diseases. The imaging modality has been successfully utilized for high-resolution 3D visualization and morphometric analysis of lung lobules, capillary alveoli, and terminal bronchioles, providing the ability to measure pathological alterations in restrictive and obstructive lung diseases. Determination of positive surgical margins in lung cancer and understanding transplantation-related disorders are two further common clinical applications of micro-CT imaging [1][13][14] (Figure 1).
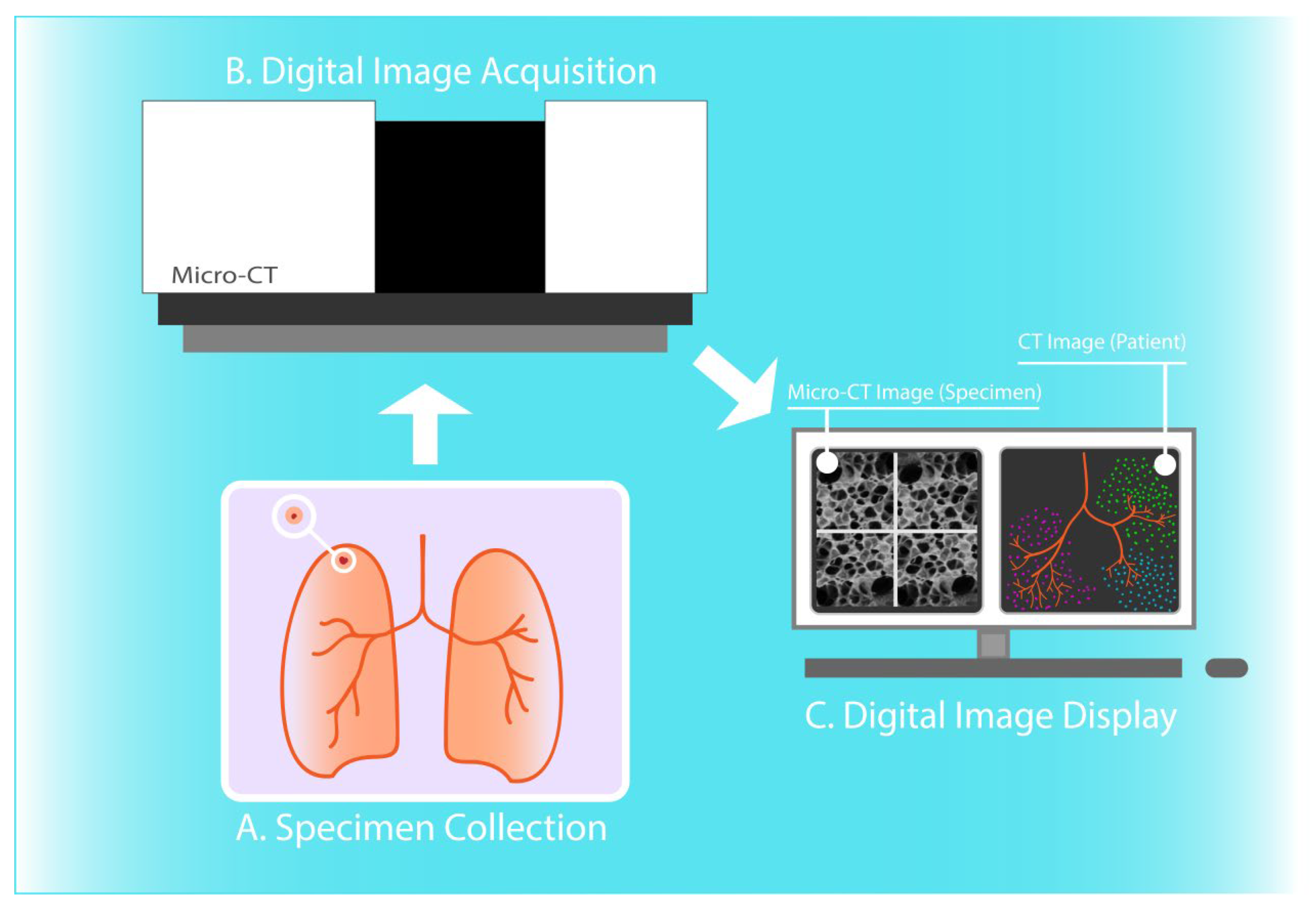
2.1. Visualization and Quantitative Analysis of the Lung Microanatomy in Chronic Obstructive and Restrictive Pulmonary Disease
2.2. Non-Destructive Imaging of Surgical Specimens for Lung Cancer Diagnosis
2.3. Evaluation of Lung Allograft Specimens upon Transplant Rejection or Prior to Transplantation
2.4. Assessing COVID-19-Related Alterations of Lung Tissue
2.5. Example of a Potential Future Micro-CT Application in Lung Cancer Management
References
- Hutchinson, J.C.; Shelmerdine, S.C.; Simcock, I.C.; Sebire, N.J.; Arthurs, O.J. Early clinical applications for imaging at microscopic detail: Microfocus computed tomography (micro-CT). Br. J. Radiol. 2017, 90, 20170113.
- Ritman, E.L. Current status of developments and applications of Micro-CT. Annu. Rev. Biomed. Eng. 2011, 13, 531–552.
- Papazoglou, A.S.; Karagiannidis, E.; Moysidis, D.V.; Sofidis, G.; Bompoti, A.; Stalikas, N.; Panteris, E.; Arvanitidis, C.; Herrman, M.D.; Michaelson, J.S.; et al. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hell. J. Cardiol. 2021.
- Du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S. Laboratory x-ray micro-computed tomography: A user guideline for biological samples. GigaScience 2017, 6, 1–11.
- Cecchini, M.J.; Tarmey, T.; Ferreira, A.; Mangaonkar, A.A.; Ferrer, A.; Patnaik, M.M.; Wylam, M.E.; Jenkins, S.M.; Spears, G.M.; Yi, E.S.; et al. Pathology, radiology, and genetics of interstitial lung disease in patients with shortened telomeres. Am. J. Surg. Pathol. 2021, 47, 871–884.
- Ritman, E.L. Micro-Computed tomography of the lungs and pulmonary-vascular system. Proc. Am. Thorac. Soc. 2005, 2, 477–480.
- Wells, W.A.; Thrall, M.; Sorokina, A.; Fine, J.; Krishnamurthy, S.; Haroon, A.; Rao, B.; Shevchuk, M.M.; Wolfsen, H.C.; Tearney, G.J.; et al. In vivo and ex vivo microscopy: Moving toward the integration of optical imaging technologies into pathology practice. Arch. Pathol. Lab. Med. 2018, 143, 288–298.
- Liu, J.T.C.; Glaser, A.K.; Bera, K.; True, L.D.; Reder, N.P.; Eliceiri, K.W.; Madabhushi, A. Harnessing non-destructive 3D pathology. Nat. Biomed. Eng. 2021, 5, 203–218.
- DiCorpo, D.; Tiwari, A.; Tang, R.; Griffin, M.; Aftreth, O.; Bautista, P.; Hughes, K.; Gershenfeld, N.; Michaelson, J. The role of Micro-CT in imaging breast cancer specimens. Breast Cancer Res. Treat. 2020, 180, 343–357.
- Mori, K. From macro-scale to micro-scale computational anatomy: A perspective on the next 20 years. Med. Image Anal. 2016, 33, 159–164.
- Hochhegger, B.; Langer, F.W.; Irion, K.; Souza, A.; Moreira, J.; Baldisserotto, M.; Pallaoro, Y.; Muller, E.; Medeiros, T.M.; Altmayer, S.; et al. Pulmonary acinus: Understanding the computed tomography findings from an acinar perspective. Lung 2019, 197, 259–265.
- Zhou, Y.; Chen, H.; Ambalavanan, N.; Liu, G.; Antony, V.B.; Ding, Q.; Nath, H.; Eary, J.F.; Thannickal, V.J. Noninvasive imaging of experimental lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 8–13.
- Kayı Cangır, A.; Dizbay Sak, S.; Güneş, G.; Orhan, K. Differentiation of benign and malignant regions in paraffin embedded tissue blocks of pulmonary adenocarcinoma using micro CT scanning of paraffin tissue blocks: A pilot study for method validation. Surg. Today 2021, 51, 1594–1601.
- Verleden, S.E.; Martens, A.; Ordies, S.; Heigl, T.; Bellon, H.; Vandermeulen, E.; Van Herck, A.; Sacreas, A.; Verschakelen, J.; Coudyzer, W.; et al. Radiological analysis of unused donor lungs: A tool to improve donor acceptance for transplantation? Arab. Archaeol. Epigr. 2017, 17, 1912–1921.
- Vasilescu, D.M.; Martinez, F.J.; Marchetti, N.; Galbán, C.J.; Hatt, C.; Meldrum, C.A.; Dass, C.; Tanabe, N.; Reddy, R.M.; Lagstein, A.; et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2019, 200, 575–581.
- Suzuki, M.; Sze, M.A.; Campbell, J.D.; Ii, J.F.B.; Lenburg, M.E.; McDonough, J.; Elliott, W.M.; Cooper, J.D.; Spira, A.; Hogg, J.C. The cellular and molecular determinants of emphysematous destruction in COPD. Sci. Rep. 2017, 7, 9562.
- Tanabe, N.; Vasilescu, D.M.; McDonough, J.; Kinose, D.; Suzuki, M.; Cooper, J.D.; Paré, P.D.; Hogg, J.C. Micro–Computed tomography comparison of preterminal bronchioles in centrilobular and panlobular emphysema. Am. J. Respir. Crit. Care Med. 2017, 195, 630–638.
- Watz, H.; Breithecker, A.; Rau, W.S.; Kriete, A. Micro-CT of the human lung: Imaging of alveoli and virtual endoscopy of an alveolar duct in a normal lung and in a lung with centrilobular Emphysema—Initial observations. Radiology 2005, 236, 1053–1058.
- Dame Carroll, J.R.; Chandra, A.; Jones, A.S.; Berend, N.; Magnussen, J.S.; King, G.G. Airway dimensions measured from micro-computed tomography and high-resolution computed tomography. Eur. Respir. J. 2006, 28, 712–720.
- Kirby, M.; Tanabe, N.; Vasilescu, D.M.; Cooper, J.D.; McDonough, J.; Verleden, S.; Vanaudenaerde, B.; Sin, D.D.; Tan, W.C.; Coxson, H.O.; et al. Computed tomography total airway count is associated with the number of micro–computed tomography terminal bronchioles. Am. J. Respir. Crit. Care Med. 2020, 201, 613–615.
- Tanabe, N.; Vasilescu, D.M.; Kirby, M.; Coxson, H.O.; Verleden, S.; Vanaudenaerde, B.; Kinose, D.; Nakano, Y.; Paré, P.D.; Hogg, J.C. Analysis of airway pathology in COPD using a combination of computed tomography, micro-computed tomography and histology. Eur. Respir. J. 2018, 51, 1701245.
- Tanabe, N.; McDonough, J.E.; Vasilescu, D.M.; Ikezoe, K.; Verleden, S.; Xu, F.; Wuyts, W.A.; Vanaudenaerde, B.; Colby, T.V.; Hogg, J.C. Pathology of idiopathic pulmonary fibrosis assessed by a combination of microcomputed tomography, histology, and immunohistochemistry. Am. J. Pathol. 2020, 190, 2427–2435.
- Hariri, L.P.; Mino-Kenudson, M.; Mark, E.J.; Suter, M.J. In vivo optical coherence tomography: The role of the pathologist. Arch. Pathol. Lab. Med. 2012, 136, 1492–1501.
- Adams, D.C.; Hariri, L.P.; Miller, A.J.; Wang, Y.; Cho, J.L.; Villiger, M.; Holz, J.A.; Szabari, M.V.; Hamilos, D.L.; Harris, R.S.; et al. Birefringence microscopy platform for assessing airway smooth muscle structure and function in vivo. Sci. Transl. Med. 2016, 8, 359ra131.
- Mai, C.; Verleden, S.; McDonough, J.; Willems, S.; De Wever, W.; Coolen, J.; Dubbeldam, A.; Van Raemdonck, D.; Verbeken, E.K.; Verleden, G.M.; et al. Thin-Section CT features of idiopathic pulmonary fibrosis correlated with Micro-CT and histologic analysis. Radiology 2017, 283, 252–263.
- Verleden, S.; Tanabe, N.; McDonough, J.; Vasilescu, D.M.; Xu, F.; Wuyts, W.A.; Piloni, D.; De Sadeleer, L.; Willems, S.; Mai, C.; et al. Small airways pathology in idiopathic pulmonary fibrosis: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 573–584.
- Boon, M.; Verleden, S.; Bosch, B.; Lammertyn, M.E.J.; McDonough, J.E.; Mai, C.; Verschakelen, J.; De Corput, M.K.-V.; Tiddens, H.A.W.; Proesmans, M.; et al. Morphometric analysis of explant lungs in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2016, 193, 516–526.
- Jones, M.G.; Fabre, A.; Schneider, P.; Cinetto, F.; Sgalla, G.; Mavrogordato, M.; Jogai, S.; Alzetani, A.; Marshall, B.; O’Reilly, K.M.; et al. Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. JCI Insight 2016, 1, e86375.
- McDonough, J.E.; Ahangari, F.; Li, Q.; Jain, S.; Verleden, S.E.; Herazo-Maya, J.; Vukmirovic, M.; Deiuliis, G.; Tzouvelekis, A.; Tanabe, N.; et al. Transcriptional regulatory model of fibrosis progression in the human lung. JCI Insight 2019, 4, e131597.
- Hariri, L.P.; Roden, A.C.; Chung, J.H.; Danoff, S.K.; Manjarres, D.C.G.; Hartwig, M.; Kheir, F.; King, C.; Kreider, M.; Lynch, D.A.; et al. The role of surgical lung biopsy in the diagnosis of fibrotic interstitial lung disease: Perspective from the pulmonary fibrosis foundation. Ann. Am. Thorac. Soc. 2021, 18, 1601–1609.
- Katsamenis, O.L.; Olding, M.; Warner, J.A.; Chatelet, D.S.; Jones, M.G.; Sgalla, G.; Smit, B.; Larkin, O.J.; Haig, I.; Richeldi, L.; et al. X-ray Micro-Computed tomography for nondestructive three-dimensional (3D) X-ray histology. Am. J. Pathol. 2019, 189, 1608–1620.
- Scott, A.E.; Vasilescu, D.M.; Seal, K.A.D.; Keyes, S.D.; Mavrogordato, M.N.; Hogg, J.C.; Sinclair, I.; Warner, J.A.; Hackett, T.-L.; Lackie, P.M. Three dimensional imaging of paraffin embedded human lung tissue samples by Micro-Computed tomography. PLoS ONE 2015, 10, e0126230.
- Guan, C.-S.; Ma, D.-Q.; Cui, D.; Chen, J.-H.; Chen, B.-D.; Zhang, Y.-S.; Liu, W.-H. Short linear shadows connecting pulmonary segmental arteries to oblique fissures in volumetric thin-section CT images: Comparing CT, micro-CT and histopathology. Eur. Radiol. 2015, 26, 2740–2748.
- Robinson, S.K.; Ramsden, J.J.; Warner, J.; Lackie, P.M.; Roose, T. Correlative 3D imaging and microfluidic modelling of human pulmonary lymphatics using immunohistochemistry and high-resolution μCT. Sci. Rep. 2019, 9, 6415.
- Nakamura, S.; Mori, K.; Iwano, S.; Kawaguchi, K.; Fukui, T.; Hakiri, S.; Ozeki, N.; Oda, M.; Yokoi, K. Micro-computed tomography images of lung adenocarcinoma: Detection of lepidic growth patterns. Nagoya J. Med. Sci. 2020, 82, 25–31.
- Troschel, F.M.; Gottumukkala, R.V.; Dicorpo, D.; Mario, J.; Ott, H.C.; Wright, C.D.; Muniappan, A.; Lanuti, M.; Yang, K.; Shepard, J.O.; et al. Feasibility of perioperative micro–computed tomography of human lung cancer specimens: A pilot study. Arch. Pathol. Lab. Med. 2018, 143, 319–325.
- Verleden, S.E.; Vasilescu, A.M.; McDonough, J.E.; Ruttens, D.; Vos, R.; Vandermeulen, E.; Bellon, H.; Geenens, R.; Verbeken, E.K.; Verschakelen, J.; et al. Linking clinical phenotypes of chronic lung allograft dysfunction to changes in lung structure. Eur. Respir. J. 2015, 46, 1430–1439.
- Verleden, S.E.; Gheysens, O.; Goffin, K.E.; Vanaudenaerde, B.M.; Verbeken, E.K.; Weynand, B.; Van Raemdonck, D.E.; Verleden, G.M.; Vos, R. Role of 18F-FDG PET/CT in restrictive allograft syndrome after lung transplantation. Transplantation 2019, 103, 823–831.
- Taghavi-Farahabadi, M.; Mahmoudi, M.; Soudi, S.; Hashemi, S.M. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med. Hypotheses 2020, 144, 109865.
- Boudewijns, R.; Thibaut, H.J.; Kaptein, S.J.F.; Li, R.; Vergote, V.; Seldeslachts, L.; Van Weyenbergh, J.; De Keyzer, C.; Bervoets, L.; Sharma, S.; et al. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat. Commun. 2020, 11, 5838.
- Kawakami, T.; Nabeshima, K.; Hamasaki, M.; Iwasaki, A.; Shirakusa, T.; Iwasaki, H. Small cluster invasion: A possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Arch. 2008, 454, 61–70.
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260.
- Yokoyama, S.; Murakami, T.; Tao, H.; Onoda, H.; Hara, A.; Miyazaki, R.; Furukawa, M.; Hayashi, M.; Inokawa, H.; Okabe, K.; et al. Tumor spread through air spaces identifies a distinct subgroup with poor prognosis in surgically resected lung pleomorphic carcinoma. Chest 2018, 154, 838–847.
- Kadota, K.; Kushida, Y.; Katsuki, N.; Ishikawa, R.; Ibuki, E.; Motoyama, M.; Nii, K.; Yokomise, H.; Bandoh, S.; Haba, R. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am. J. Surg. Pathol. 2017, 41, 1077–1086.
- Yin, Q.; Wang, H.; Cui, H.; Wang, W.; Yang, G.; Qie, P.; Xun, X.; Han, S.; Liu, H. Meta-analysis of association between CT-based features and tumor spread through air spaces in lung adenocarcinoma. J. Cardiothorac. Surg. 2020, 15, 243.
- Ledda, R.E.; Milanese, G.; Gnetti, L.; Borghesi, A.; Sverzellati, N.; Silva, M. Spread through air spaces in lung adenocarcinoma: Is radiology reliable yet? J. Thorac. Dis. 2019, 11, S256–S261.
- Onozato, Y.; Nakajima, T.; Yokota, H.; Morimoto, J.; Nishiyama, A.; Toyoda, T.; Inage, T.; Tanaka, K.; Sakairi, Y.; Suzuki, H.; et al. Radiomics is feasible for prediction of spread through air spaces in patients with nonsmall cell lung cancer. Sci. Rep. 2021, 11, 13526.




