| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Castro-Barquero | + 2940 word(s) | 2940 | 2020-11-25 07:32:31 | | | |
| 2 | Bruce Ren | Meta information modification | 2940 | 2020-11-25 10:37:28 | | |
Video Upload Options
Obesity in pregnancy has been directly associated with an increased risk of almost all pregnancy complications such as gestational hypertension, preeclampsia, gestational diabetes mellitus (GDM), and premature delivery. Thereby, according to current evidence available, life-style interventions to prevent pre-pregnancy overweight and obesity in women of fertile age are necessary to reduce the negative impact of obesity on mother and child health. Unhealthy dietary patterns, together with the increased consumption of processed foods rich in simple sugar and sweeteners are some of the responsible, among others, for the increase in obesity rates during the last years. Nevertheless, how its consumption can affect pregnancy outcomes and long-term children's health is still uncertain.
1. Introduction
It is well documented that the prevalence of obesity among children and adolescents has doubled around the world in the last 30 years [1]. Currently, one out of three children is overweight or obese [2], leading to possible cardiometabolic disturbances, mental health disorders and obesity during adulthood [3][4]. In addition, obesity has also been linked to some types of cancer and even arthritis [5]. Therefore, obesity comprises a large number of multifactorial problems, including high blood pressure, insulin resistance and type 2 diabetes mellitus (T2DM), high cholesterol concentrations, fatty liver disease, asthma, sleep apnea and joint pain, among others [6].
In most cases, obesity is the result of an excessive intake of calories that the body stores as fat. This excess of energy mainly comes from foods rich in fat and sugar [6]. According to the World Health Organization, free sugars, including added sugars, should be limited to less than 10% of daily calories [7]. The Dietary Guidelines for Americans (2015–2020) also recommends that added sugar should be reduced to less than 10% of total energy [8]. However, the results of 11 European surveys published by Azaïs-Braesco et al. [9] showed that relative intakes of sugar were higher in children (from 16 to 26% of total energy intake for both sexes) than in adults (from 15 and 21% of total energy intake for both sexes). Moreover, the National Health and Nutrition Examination Survey (NHANES) of 2003–2004 to 2011–2012 [10], showed that consumption of added sugars in absolute grams among non-pregnant women was 76.7 g, being higher for pregnant women at 85.1 g. Sugar-sweetened beverages (SSB), cakes, cookies, and pastries, sugars and sweets, juice drinks and smoothies, and milk desserts were the main sugary foods chosen by pregnant and non-pregnant women.
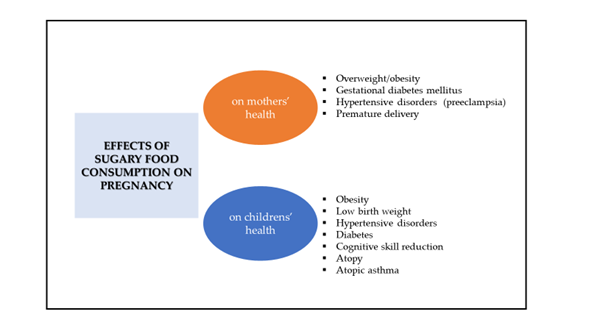
First, several observational studies and meta-analyses, both in adults and children, have reported that consumption of sugary foods, especially SSB, is related to weight gain, obesity, metabolic syndrome, and T2DM [11][12][13]. Several clinical studies have reported that higher consumption of added sugars from SSB is associated with unhealthy lifestyles, poor-quality dietary patterns and greater total energy intake, which might explain weight gain, gestational diabetes mellitus (GDM), hypertensive disorders and premature delivery, among other conditions (Figure 1) [14][15][16][17][18][19][20]. Second, there is a large amount of clinical evidence showing that following a healthy dietary pattern, which is by definition low in sugar-rich foods, such as the Mediterranean diet, can exert a beneficial influence on adverse gestational and birth outcomes [21][22]. Indeed, several studies have confirmed that a healthy dietary pattern during pregnancy reduces the incidence of hypertensive disorders [21], GDM [23], premature birth [24] and low birth weight [25].
Figure 1. Effects of sugary food consumption in pregnancy on mothers’ and childrens’ health.
Third, it has been well demonstrated that being obese before pregnancy and during gestation increases the risk of obesity in childhood, adolescence, and adulthood. Thus, some authors have pointed out that the consumption of sugary foods during gestation might exert a certain influence on intrauterine programming [26][27] Thereby, it seems clear that obesity has its origin in early life.
The purpose of this review was to investigate the possible relationship between sugary food consumption during gestation and obesity in childhood and mid-childhood, as there is currently scarce documented information, with a simple bibliographic review of the literature published in the last 20 years, evaluating humans, adults (>18 years) and written in Spanish or English. The bibliographic search was performed through PubMed, ScienceDirect, and Google Scholar from June 2020 to September 2020. The keywords used for this search were: pregnancy, gestational diabetes mellitus, premature birth and low birth weight, preeclampsia, obesity and metabolic programming. We also investigated whether the impact of high sugary food intake on maternal health (excessive weight gain) is associated with complications during pregnancy and the impact on the fetus.
2. Cravings during Pregnancy
During pregnancy, women report food cravings and aversions, which may lead to choosing to eat certain unhealthy foods [28][29][30][31]. It is estimated that between 50–90% of pregnant women have one or more food cravings during gestation [32]. These unhealthy foods often provide excess energy intake, which leads to gestational weight gain (GWG) and the development of obesity in pregnancy [32]. On the contrary, aversions are associated with limiting or avoiding the intake of certain foods because of their association with vomiting and nausea [28]. To date, the mechanisms underlying food cravings are unknown, although physical and hormonal changes during pregnancy might play a key role [31]. Additionally, some authors have suggested that energy requirements during pregnancy are increased, which might lead to having a preference for candies and sweet foods [29]. In fact, Belzer et al. [33] reported that US women with mild GDM without dietary restrictions (e.g., weight-loss oriented dietary advice or low-sodium diets) showed a higher preference for this type of food. The most commonly craved foods during pregnancy seem to be dairy products (ice cream) and sweet foods (chocolate, fruit and fruit juice) and, in a lower proportion, salty foods (chips) [34].
A prenatal healthy dietary pattern is essential to avoid adverse gestational and birth outcomes [34]. Therefore, cravings for sugary foods are far from being considered part of a healthy diet.
3. Sugar Consumption and Pregnancy Complications
There is a large amount of evidence showing that sugar intake during pregnancy is directly associated with GWG and the development of several pregnancy complications such as GDM, preeclampsia and preterm birth (Figure 2). Below, we discuss how added sugars, sugary products, intrinsic sugar and SSB intake can impact maternal health during pregnancy.
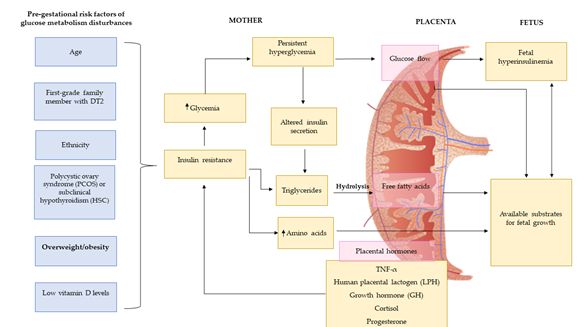
Figure 2. Pre-gestational risk factors of glucose metabolism disturbances. Figure adapted from Agha-Jaffar et al. [26].
3.1. Weight Gain during Pregnancy
On one hand, it is clear that during pregnancy there is a steady stream of glucose from the mother’s placenta to the fetus, being the main energy substrate for intrauterine growth [35]. On the other hand, adequate GWG is also important to ensure the healthy growth and development of the fetus [36]. According to the Institute of Medicine, obese women should limit their GWG to 5–9 Kg [37]. Nevertheless, among 1,309,136 pregnant women of US analyzed, it was estimated that close to 50% of pregnant women showed a higher than recommended GWG [36]. Currently, the prevalence of weight gain and obesity is increasing in the obstetric population by more than 40 and 50%, respectively [38][39].
It is necessary to highlight that excessive gestational weight is associated with adverse effects during gestation, such as small or large fetus for gestational age, macrosomia, cesarean delivery, preeclampsia, gestational hypertension, preterm birth, small or large size for gestational age at birth or offspring obesity [40]. Nevertheless, up to now, few studies have investigated the possible direct association between sugary food intake and GWG.
According to the last Cochrane systematic review including 27 studies and 3964 women, there is no evidence that a behavioral intervention based on the promotion of healthy dietary habits and regular physical activity can prevent GWG during gestation. However, the results obtained in several studies have reported that exercise and caloric restriction in obese pregnant women might prevent GWG. A meta-analysis that included 36 randomized trials with 12,526 women showed significant reductions of GWG in obese women that followed-up behavioral interventions (diet and physical activity).
A few interventional studies have also described this association . In a randomized controlled trial (RCT) carried out by Poston et al. in 1555 obese pregnant women (mean body mass index, BMI = 36.3 kg/m2, 15–18 weeks plus 6 days of gestation, and ≥16 years old), the women were randomized into two study arms: a behavioral intervention and standard antenatal care (control group). After 8 health trainer-led sessions, the authors reported that the women in the intervention group had less GWG over the total pregnancy than the control group, although the primary outcomes did not differ between groups (25% vs. 26%, respectively; p = 0.68). In another RCT, 61 pregnant women (BMI > 25) in the first trimester were included and randomized into two study arms: a Therapeutic Lifestyle Changes (TLC) Program and a control group. The TLC Program included changes in diet (overweight: 1700 kcal/day, obese: 1800 kcal/day) and mild physical activity (30 min/day, 3 times/week). After the intervention, the authors reported that women assigned to the TLC group showed less GWG (6.7 ± 4.3 kg) compared to controls (10.1 ± 5.6 kg, p = 0.047). Similar results were reported by Renault et al., who showed that only the consumption of added sugar was associated with GWG. Furthermore, the sugary foods most strongly associated with weight gain were sweets, snacks, cakes, and soft drinks. Women who consumed ≥ 2 units/day of sweets showed a 5.4 kg greater weight gain than those with a low (<1 week) sweet intake. Finally, Wattar et al. carried out an RCT including 1252 pregnant women with metabolic risk factors. Of these, 593 women were randomly allocated to Mediterranean-style diet intervention. The authors reported a lower GWG in the intervention group (mean 6.8 versus 8.3 kg; adjusted difference −1.2 kg, 95% confidence interval [CI] −2.2 to −0.2, p = 0.03) compared to the control group.
Several observational studies have also reported associations between sugar intake during gestation and excess maternal weight gain [41][42][43]. A large, prospective, Danish, cohort study, which included 46,262 pregnant women, found a strong association between added sugar intake and GWG (Q5 vs. Q1: 34, 95% CI 28 to 40 g/week, p for trend <0.0001), with an average extra weight gain of 1.4 kg during pregnancy. On the other hand, a higher protein/carbohydrate ratio was related to lower GWG, possibly because of decreased added sugar intake. Additionally, Diemert et al. found that 60% of pregnant women (N = 200) gained more weight than recommended. Saturated fat and sugar were among the nutrients that most contributed to total energy consumption. There was a positive correlation (p = 0.006) between weight gain and monosaccharides and saccharose. Similar results were reported by Olafsdottir et al. after analyzing 495 pregnant women in Iceland. The authors reported that women who ate more sweets in early pregnancy increased the risk of gaining excessive weight (OR = 2.52, CI =1.10–5.77, p = 0.029). After analyzing 3360 pregnant Finnish women, Uusatilo et al. [43] also reported, that adherence to a higher fast-food dietary pattern, characterized by high consumption of hamburgers, pizza, sweets, soft drinks and added sugars, was strongly associated with GWG. Despite the fact that it is an observational study, causation cannot be proved, while the results supported that frequent consumption of fast foods and snacks might influence excessive GWG. Thus, recent evidence has shown that unhealthy dietary patterns are correlated with excessive GWG [41][42][43].
3.2. Sugar Consumption and Gestational Diabetes
Up to 16% of pregnant women are diagnosed with GDM during pregnancy [44]. For women with GDM, progression to T2DM is estimated to be between 15 to 50% at 5 years [45]. At present, the prevalence of GDM is rising because of the high incidence of both overweight and obesity around the world [46], highlighting that weight gain is a significant predictor of T2DM at 15 years of follow-up [47]. In the short- and long-term, GDM is associated with serious obstetric and neonatal complications during gestation and childbirth, including an increased risk for both the mother and child. Some examples are macrosomia, birth injury, cesarean delivery, offspring obesity, epigenetic changes in children with a higher predisposition to both obesity and T2DM in adulthood, etc. [48].
There is robust evidence that reinforces how a healthy dietary pattern such as the Mediterranean diet can reduce the incidence of GDM during pregnancy [49][50][51][52][53]. One of the main variables directly related to the intake of sugary foods is GWG which might also be a predictor for GDM [54][55]. Scientific evidence supports that a diet rich in simple sugars might decrease insulin sensitivity and insulin secretion [56][57].
In this sense, Mijatovic-Vukas et al. carried out a systematic review including the data of 30,871 pregnant women. The authors reported significant associations between SSB and the risk of GDM (RR for pregnant women ≥5 weeks = 1.23, 95% CI: 1.05–1.45, p-value = 0.005). After considering different sub-types of SSB, the highest association with GMD was observed for sugar-sweetened cola (RR high vs. low intake = 1.29, 95% CI: 1.07–1.55). No significant associations were observed for non-cola SSB (RR high vs. low = 0.99, 95% CI: 0.78–1.25). Wattar et al. [18] reported a reduction in the odds of GDM by 35%. The same authors designed a meta-analysis of RCTs, which included 2 trials and 2397 pregnant women who followed a Mediterranean diet supplemented with nuts and extra virgin olive oil, where authors reported a significant reduction in GDM (OR = 0.67, 95% CI 0.53–0.84, I2 = 0%).
Nevertheless, RCTs based on behavioral interventions (changes in eating behavior and promotion of physical activity) have shown contradictory results in GDM prevention. On one hand, some authors showed that energy restriction plus the promotion of regular physical activity was associated with improved pregnancy complications, such as GDM, gestational hypertension and preterm delivery in obese women . In contrast, Poston et al. observed that the incidence of GMD in obese women was similar between the participants assigned to the intervention group and the control group (26 and 25%, respectively). In addition, Wattar et al. reported a lower risk of GDM (35%) in pregnant women who present metabolic risk factors (obesity, chronic hypertension, or hypertriglyceridemia) but followed a Mediterranean-style diet.
Finally, the results obtained after analyzing 253 pregnant women (aged between 16 to 41 years) from the NHANES survey showed that women who followed a diet rich in added sugar and viscera; low fruits, vegetables and seafood had a higher risk of developing GDM than those with a diet based on a high intake of nuts, seeds, fat and soybean and low milk and cheese intake. Additionally, in a prospective study that included 13,475 US women pre-pregnancy, 860 incident GDM cases were identified after 10 years of follow-up. Furthermore, the authors reported that pre-pregnancy women with a sugar-sweetened cola consumption ≥ 5 servings per week had a higher risk of GDM (22%) compared to those with a consumption of less than 1 serving/month. In this study, the authors did not include juice intake in the analysis. Another study, the “Seguimiento Universidad de Navarra” (SUN) cohort, also evaluated SSB consumption and the risk of developing GDM. In this case, the authors followed 3396 pre-pregnancy women over 10 years. During this period, 172 new cases of GDM were diagnosed, and the authors reported that SSB consumption was strongly associated with a higher risk of GDM when they became pregnant (OR = 2.03, 95% CI: 1.25–3.31). Nevertheless, they found no association between sugar-free soft drink intake and the risk of GDM. A prospective Canadian study including 205 women with singleton pregnancies without type 1 or type 2 diabetes found that adding sugar to coffee and tea was directly associated with a higher risk of hyperglycemia.
3.3. Sugar Consumption and Preeclampsia
Preeclampsia can be defined as a disorder during pregnancy characterized by hypertension and often proteinuria in healthy women [58]. It is a common pregnancy complication and affects between 2 to 8% of pregnancies worldwide [59]. In addition, it is one of the most common causes of morbidity and mortality in both pregnant women and their offspring [58][60]. Both type 1 and 2 diabetes can further increase the risk of preeclampsia [61].
Although the known risk is associated with preeclampsia, the number of studies that correlate the consumption of sugary foods with preeclampsia risk during pregnancy is limited .
In a prospective Norwegian study that included 32,933 normal and overweight pregnant women, a high intake of SSB (≥125 mL/day) was associated with a higher risk of preeclampsia (OR = 1.27, 95% CI: 1.05, 1.54) [61], while a high intake of intrinsic sugars (such as dried and fresh fruit) was associated with a lower risk (OR = 0.79, 95% CI: 0.67, 0.93 and OR = 0.79, 95% CI: 0.68, 0.92, respectively). The authors also reported that high intake of sugar-sweetened beverages in women with a BMI < 25 showed a stronger association with the risk of preeclampsia than those with a BMI ≥ 25 (OR 1.32 v. 1.28, respectively). Moreover, Clausen et al. reported that the risk of preeclampsia was directly associated with high sucrose intake (>25% of total energy) after analyzing 3133 pregnant Norwegian women. The NHANES survey showed that every 12 oz. (~354 mL) of SBBs was associated with a reduction of 2.3 points of the Alternate Healthy Eating Index modified for Pregnancy (AHEI-P) score, which measured the quality of the diet. SSB intake was also associated with an intake of 124 more calories. The authors estimated that SSB consumption, which was set at 0, should be an average AHEI-P of 6.4 and with an average total calorie intake less than 203.5. Brantsaeter et al. investigated the association between different dietary patterns during pregnancy and the risk of preeclampsia in 23,423 pregnant Norwegian women. They showed that an unhealthy pattern, characterized by high consumption of processed meat, salty snacks, and sweet drinks, was strongly associated with an increased risk of preeclampsia [OR for tertile 3 vs. tertile 1: 1.21; 95% CI: 1.03, 1.42]. However, the authors considered their results could be influenced by non-included confounding factors in the analysis, thereby a causal inference between dietary habits and the risk of preeclampsia should be evaluated. A prospective longitudinal cohort study, including 55,139 Danish women, found a harmful association between following a Western diet and pregnancy-associated hypertension (PAH) which included gestational hypertension (GH) and preeclampsia. Concretely, a higher adherence to a Western diet was associated with a higher risk for GH (OR = 1.18; 95% CI 1.05–1.33) and preeclampsia (OR = 1.40; 95% CI 1.11–1.76). The authors did not find any significant associations between a diet high in sugary products and GH (OR = 1.05; 95% CI 0.94–1.16) or preeclampsia (OR = 1.10; 95% CI 0.90–1.35). Similar results were reported by Schoenaker et al., who analyzed 3582 women participating in the 9-year Australian Longitudinal Study on Women’s Health and found that a Mediterranean-style dietary pattern was inversely related to the risk of developing PAH compared to three other dietary patterns (1. based on meat, high-fat, and sugary foods; 2. based on fruit and low-fat dairy; and 3. based on cooked vegetables). However, the authors found no significant association between SSB and PAH.
References
- Zylke, J.W.; Bauchner, H. JAMA—Journal of the American Medical Association; American Medical Association: Chicago, IL, USA, 2018; pp. 443–444.
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA J. Am. Med. Assoc. 2014, 311, 806–814.
- Daniels, S.R.; Arnett, D.K.; Eckel, R.H.; Gidding, S.S.; Hayman, L.L.; Kumanyika, S.; Robinson, T.N.; Scott, B.J.; St. Jeor, S.; Williams, C.L. Overweight in Children and Adolescents. Circulation 2005, 111, 1999–2012.
- Crume, T.L.; Harrod, C.S. Childhood obesity is there effective treatment? JAMA Pediatr. 2013, 167, 697–699.
- Scimeca, G.; Alborghetti, A.; Bruno, A.; Troili, G.M.; Pandolfo, G.; Muscatello, M.R.A.; Zoccali, R.A. Self-worth and psychological adjustment of obese children: An analysis through the Draw-A-Person. World J. Psychiatry 2016, 6, 329.
- Thompson, A.E. Childhood obesity. JAMA J. Am. Med. Assoc. 2015, 314, 850.
- Guideline: Sugars Intake for Adults and Children. Available online: https://www.who.int/publications-detail/9789241549028 (accessed on 8 April 2020).
- 2015–2020 Dietary Guidelines|Health.gov. Available online: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/ (accessed on 8 April 2020).
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 2017, 16, 6.
- Cioffi, C.E.; Figueroa, J.; Welsh, J.A. Added Sugar Intake among Pregnant Women in the United States: National Health and Nutrition Examination Survey 2003–2012. J. Acad. Nutr. Diet. 2018, 118, 886–895.e1.
- Malik, V.S.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and BMI in children and adolescents: Reanalyses of a meta-analysis. Am. J. Clin. Nutr. 2009, 89, 438–439.
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483.
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102.
- Collison, K.S.; Zaidi, M.Z.; Subhani, S.N.; Al-Rubeaan, K.; Shoukri, M.; Al-Mohanna, F.A. Sugar-sweetened carbonated beverage consumption correlates with BMI, waist circumference, and poor dietary choices in school children. BMC Public Health 2010, 10, 234.
- Chen, L.; Hu, F.B.; Yeung, E.; Willett, W.; Zhang, C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care 2009, 32, 2236–2241.
- Gamba, R.J.; Leung, C.W.; Petito, L.; Abrams, B.; Laraia, B.A. Sugar sweetened beverage consumption during pregnancy is associated with lower diet quality and greater total energy intake. PLoS ONE 2019, 14, e0215686.
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Wah Cheung, N.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: A Systematic review and meta-analysis. Nutrients 2018, 10, 698.
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857.
- Ikem, E.; Halldorsson, T.; Birgisdóttir, B.; Rasmussen, M.; Olsen, S.; Maslova, E. Dietary patterns and the risk of pregnancy-associated hypertension in the Danish National Birth Cohort: A prospective longitudinal study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 663–673.
- Halldorsson, T.I.; Strøm, M.; Petersen, S.B.; Olsen, S.F. Intake of artificially sweetened soft drinks and risk of preterm delivery: A prospective cohort study in 59,334 Danish pregnant women. Am. J. Clin. Nutr. 2010, 92, 626–633.
- Brantsæter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A Dietary Pattern Characterized by High Intake of Vegetables, Fruits, and Vegetable Oils Is Associated with Reduced Risk of Preeclampsia in Nulliparous Pregnant Norwegian Women. J. Nutr. 2009, 139, 1162–1168.
- Englund-Ögge, L.; Brantsæter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446.
- de Seymour, J.; Chia, A.; Colega, M.; Jones, B.; McKenzie, E.; Shirong, C.; Godfrey, K.; Kwek, K.; Saw, S.M.; Conlon, C.; et al. Maternal dietary patterns and gestational diabetes mellitus in a multi-ethnic Asian cohort: The GUSTO study. Nutrients 2016, 8, 574.
- Rasmussen, M.A.; Maslova, E.; Halldorsson, T.I.; Olsen, S.F. Characterization of dietary patterns in the Danish National Birth Cohort in relation to preterm birth. PLoS ONE 2014, 9, e93644.
- Agha-Jaffar, R.; Oliver, N.; Johnston, D.; Robinson, S. Gestational diabetes mellitus: Does an effective prevention strategy exist? Nat. Rev. Endocrinol. 2016, 12, 533–546.
- Kjøllesdal, M.K.R.; Holmboe-Ottesen, G. Dietary Patterns and Birth Weight—A Review. AIMS Public Health 2014, 1, 211–225.
- Horan, M.K.; Donnelly, J.M.; McGowan, C.A.; Gibney, E.R.; McAuliffe, F.M. The association between maternal nutrition and lifestyle during pregnancy and 2-year-old offspring adiposity: Analysis from the ROLO study. J. Public Health 2016, 24, 427–436.
- Bayley, T.M.; Dye, L.; Jones, S.; DeBono, M.; Hill, A.J. Food cravings and aversions during pregnancy: Relationships with nausea and vomiting. Appetite 2002, 38, 45–51.
- Pope, J.F.; Skinner, J.D.; Carruth, B.R. Cravings and aversions of pregnant adolescents. J. Am. Diet. Assoc. 1992, 92, 1479–1482.
- Hook, E.B. Influence of Cravings and Aversions on Diet In Pregnancy. Ecol. Food Nutr. 1985, 17, 117–129.
- Hook, E.B. Dietary cravings and aversions during pregnancy. Am. J. Clin. Nutr. 1978, 31, 1355–1362.
- Orloff, N.C.; Hormes, J.M. Pickles and ice cream! Food cravings in pregnancy: Hypotheses, preliminary evidence, and directions for future research. Front. Psychol. 2014, 5, 1076.
- Belzer, L.M.; Smulian, J.C.; Lu, S.E.; Tepper, B.J. Food cravings and intake of sweet foods in healthy pregnancy and mild gestational diabetes mellitus. A prospective study. Appetite 2010, 55, 609–615.
- Kaiser, L.L.; Allen, L. Position of the American Dietetic Association: Nutrition and lifestyle for a healthy pregnancy outcome. J. Am. Diet. Assoc. 2002, 102, 1479–1490.
- Desoye, G.; Nolan, C.J. The fetal glucose steal: An underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016, 59, 1089–1094.
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2017, 317, 2207–2225.
- Institute of Medicine and National Research Council. Composition and Components of Gestational Weight Gain: Physiology and Metabolism. In Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Press (US): Washington, DC, USA, 2009; pp. 71–110, ISBN 13: 978-0-309-13113-1.
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA J. Am. Med. Assoc. 2016, 315, 2284–2291.
- Gavard, J.A.; Artal, R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: A population-based historical cohort study. Matern. Child Health J. 2014, 18, 1038–1047.
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715.
- Wrottesley, S.V.; Pisa, P.T.; Norris, S.A. The influence of maternal dietary patterns on body mass index and gestational weight gain in urban black South African women. Nutrients 2017, 9, 732.
- Tielemans, M.J.; Erler, N.S.; Leermakers, E.T.M.; van den Broek, M.; Jaddoe, V.W.V.; Steegers, E.A.P.; Kiefte-de Jong, J.C.; Franco, O.H. A Priori and a Posteriori dietary patterns during pregnancy and gestational weight gain: The generation R study. Nutrients 2015, 7, 9383–9399.
- Starling, A.P.; Sauder, K.A.; Kaar, J.L.; Shapiro, A.L.; Siega-Riz, A.M.; Dabelea, D. Maternal Dietary Patterns during Pregnancy Are Associated with Newborn Body Composition. J. Nutr. 2017, 147, 1334–1339.
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E.; Dyer, A.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: Paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010, 202, 654.e1–654.e6.
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868.
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735.
- Linne, Y.; Barkeling, B.; Rossner, S. Natural course of gestational diabetes mellitus: Long term follow up of women in the SPAWN study. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 1227–1231.
- Lehnen, H.; Zechner, U.; Haaf, T. Epigenetics of Gestational Diabetes Mellitus and Offspring Health: The Time for Action Is in Early Stages of Life. Mol. Hum. Reprod. 2013, 19, 415–422.
- Assaf-Balut, C.; De La Torre, N.G.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873.
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13.
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; Van der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational diabetes mellitus and diet: A systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care 2018, 41, 1346–1361.
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 2019, 22, 506–520.
- Donazar-Ezcurra, M.; López-del Burgo, C.; Bes-Rastrollo, M. Primary prevention of gestational diabetes mellitus through nutritional factors: A systematic review. BMC Pregnancy Childbirth 2017, 17, 30.
- Brunner, S.; Stecher, L.; Ziebarth, S.; Nehring, I.; Rifas-Shiman, S.L.; Sommer, C.; Hauner, H.; von Kries, R. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: A meta-analysis. Diabetologia 2015, 58, 2229–2237.
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2019, 78, 262–271.
- Zhang, C.; Ning, Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am. J. Clin. Nutr. 2011, 94, 1975S–1979S.
- Davis, J.N.; Alexander, K.E.; Ventura, E.E.; Kelly, L.A.; Lane, C.J.; Byrd-Williams, C.E.; Toledo-Corral, C.M.; Roberts, C.K.; Spruijt-Metz, D.; Weigensberg, M.J.; et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth 1–3. Am. J. Clin. Nutr. 2007, 86, 1331–1338.
- Lain, K.Y.; Roberts, J.M. Contemporary concepts of the pathogenesis and management of preeclampsia. J. Am. Med. Assoc. 2002, 287, 3183–3186.
- WHO|WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Available online: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548335/en/ (accessed on 8 April 2020).
- Arulkumaran, N.; Lightstone, L. Severe pre-eclampsia and hypertensive crises. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 877–884.
- American College of Obstetricians; Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131.