
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ali T Alouani | -- | 2036 | 2022-10-31 19:59:15 | | | |
| 2 | Rita Xu | + 236 word(s) | 2272 | 2022-11-01 04:27:24 | | | | |
| 3 | Rita Xu | -250 word(s) | 2022 | 2022-11-01 04:28:41 | | |
Video Upload Options
Traumatic brain injury (TBI) can produce temporary biochemical imbalance due to leaks through cell membranes or disruption of the axoplasmic flow due to the misalignment of intracellular neurofilaments. If untreated, TBI can lead to Alzheimer’s, Parkinson’s, or total disability. Mild TBI (mTBI) accounts for about about 90 percent of all TBI cases. The detection of TBI as soon as it happens is crucial for successful treatment management. Neuroimaging-based tests provide only a structural and functional mapping of the brain with poor temporal resolution. Such tests may not detect mTBI. On the other hand, the electroencephalogram (EEG) provides good spatial resolution and excellent temporal resolution of the brain activities beside its portability and low cost.
1. Introduction
2. Conventional TBI Detection Using EEG
2.1. EEG Signal Preprocessing
2.2. TBI Detection Using EEG
3. TBI Detection Using Artificial Intelligence
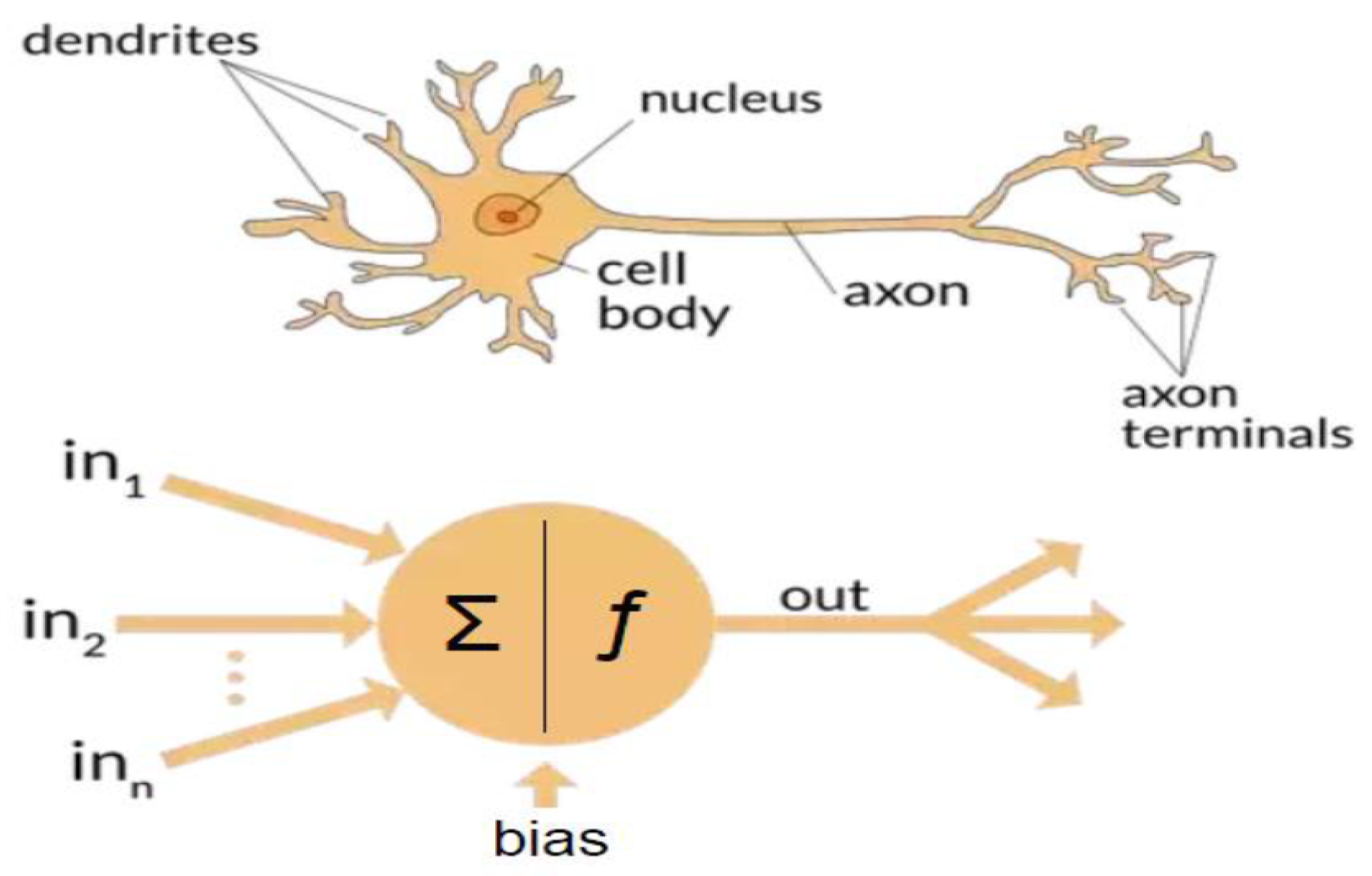
3.1. Artificial Intelligence
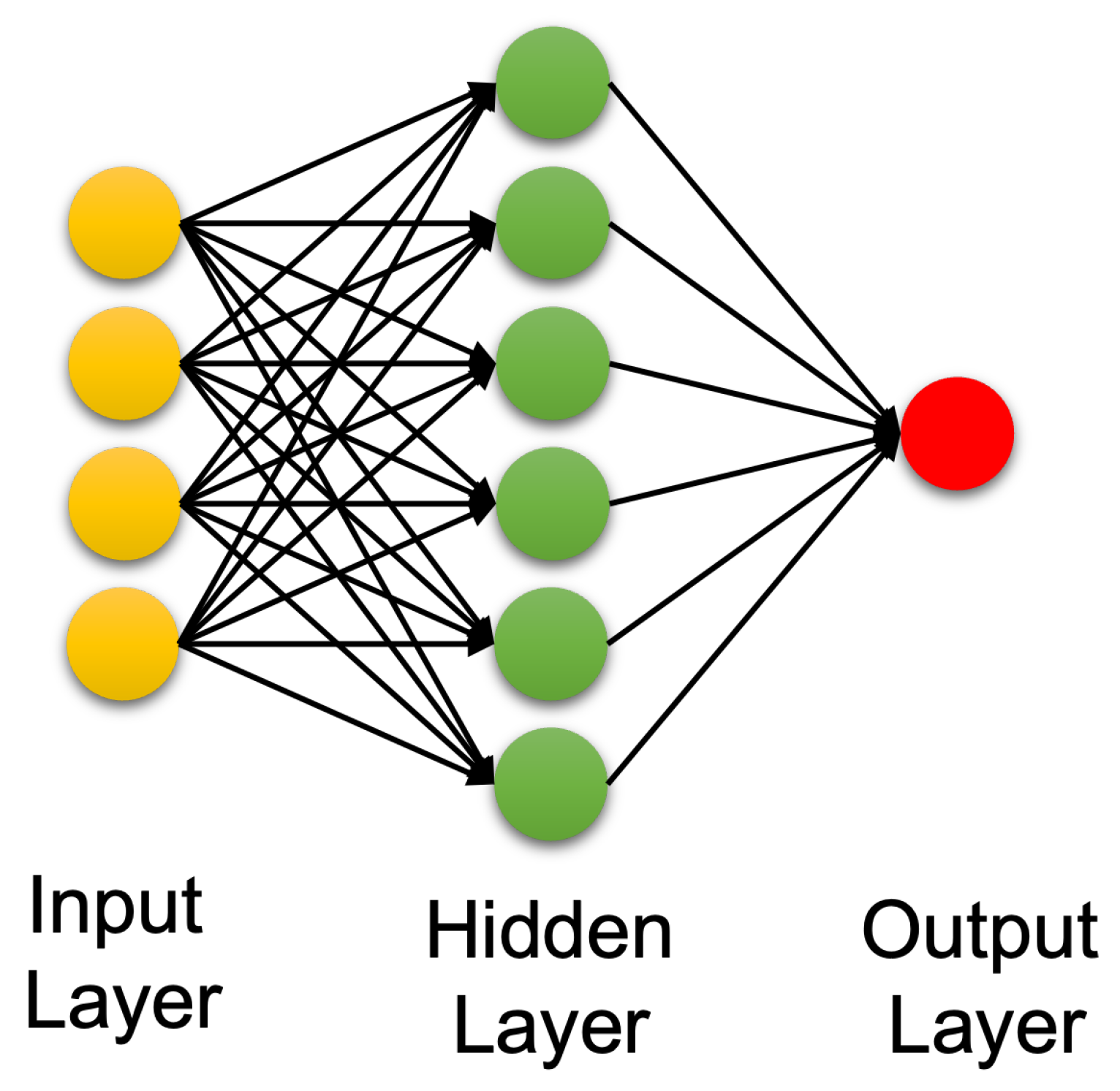
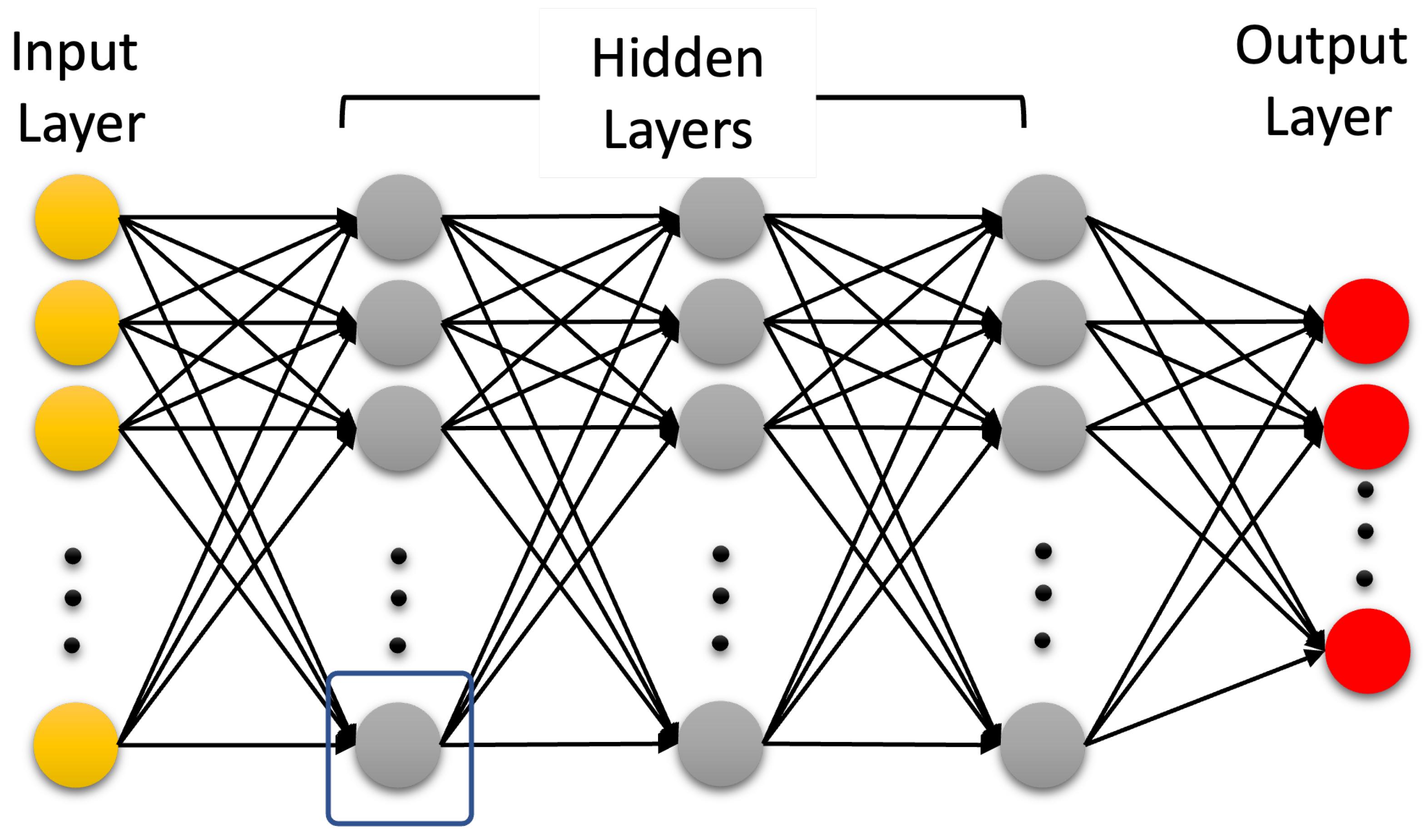
3.2. Machine Learning for TBI Detection
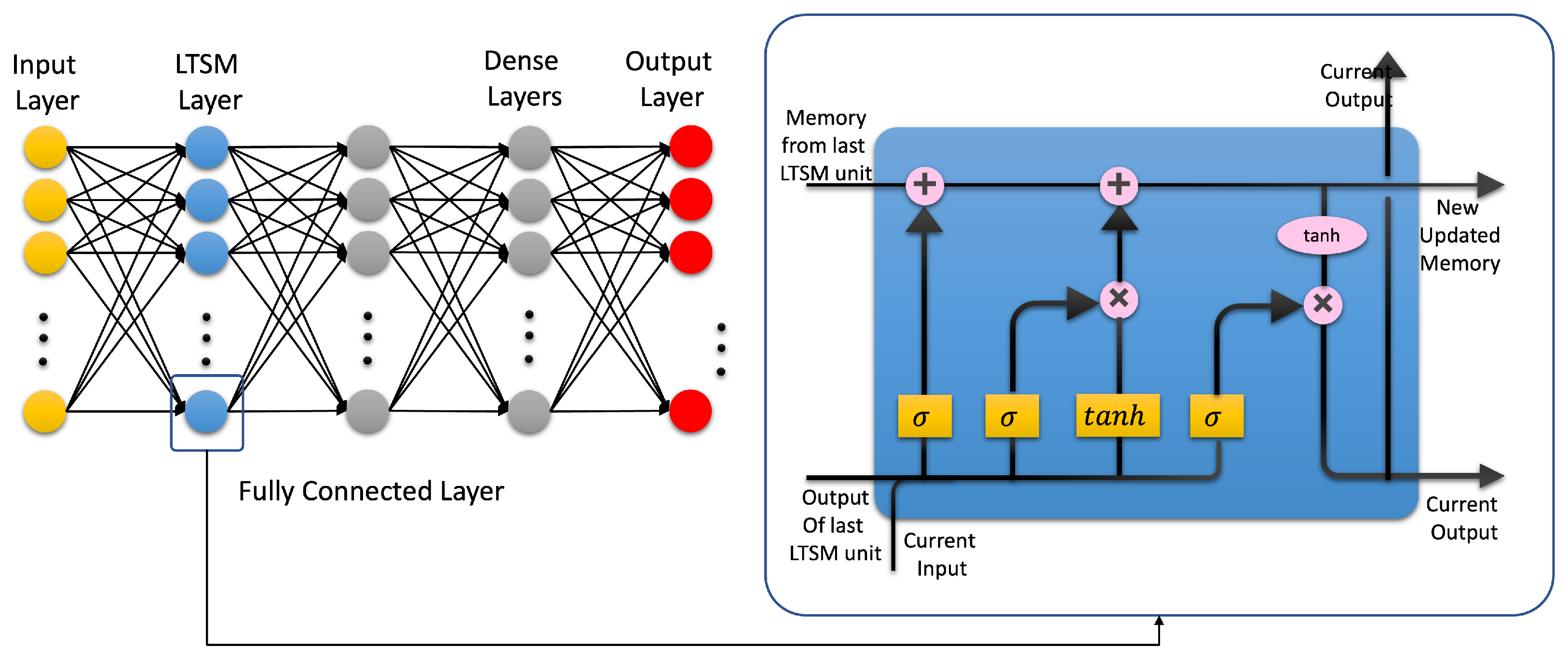
3.3. Artificial Neural Networks: Deep Learning Neural Networks
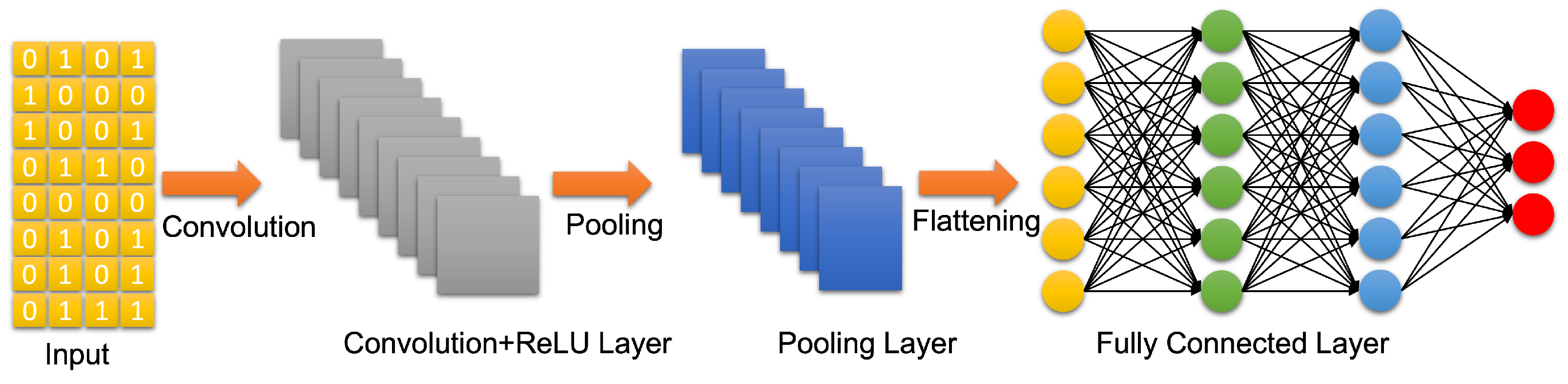
References
- Miller, J.D.; Murray, L.S.; Teasdale, G.M. Development of a traumatic intracranial hematoma after a “minor” head injury. Neurosurgery 1990, 27, 669–673.
- Kristiansson, H.; Nissborg, E.; Bartek, J., Jr.; Andresen, M.; Reinstrup, P.; Romner, B. Measuring elevated intracranial pressure through noninvasive methods: A review of the literature. J. Neurosurg. Anesthesiol. 2013, 25, 372–385.
- Puffer, R.C.; Yue, J.K.; Mesley, M.; Billigen, J.B.; Sharpless, J.; Fetzick, A.L.; Puccio, A.; Diaz-Arrastia, R.; Okonkwo, D.O. Long-term outcome in traumatic brain injury patients with midline shift: A secondary analysis of the Phase 3 COBRIT clinical trial. J. Neurosurg. 2018, 131, 596–603.
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097.
- Miller, G.F.; DePadilla, L.; Xu, L. Costs of nonfatal traumatic brain injury in the United States, 2016. Med. Care 2021, 59, 451–455.
- Agimi, Y.; Regasa, L.E.; Stout, K.C. Incidence of traumatic brain injury in the US Military, 2010–2014. Mil. Med. 2019, 184, e233–e241.
- Dinh, M.M.; Bein, K.; Roncal, S.; Byrne, C.M.; Petchell, J.; Brennan, J. Redefining the golden hour for severe head injury in an urban setting: The effect of prehospital arrival times on patient outcomes. Injury 2013, 44, 606–610.
- Hu, W.; Freudenberg, V.; Gong, H.; Huang, B. The “Golden Hour” and field triage pattern for road trauma patients. J. Saf. Res. 2020, 75, 57–66.
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113.
- Reith, F.; Van den Brande, R.; Synnot, A.; Gruen, R.; Maas, A.I. The reliability of the Glasgow Coma Scale: A systematic review. Intensive Care Med. 2016, 42, 3–15.
- Shan, R.; Szmydynger-Chodobska, J.; Warren, O.U.; Mohammad, F.; Zink, B.J.; Chodobski, A. A new panel of blood biomarkers for the diagnosis of mild traumatic brain injury/concussion in adults. J. Neurotrauma 2016, 33, 49–57.
- Congedo, M.; Gouy-Pailler, C.; Jutten, C. On the blind source separation of human electroencephalogram by approximate joint diagonalization of second order statistics. Clin. Neurophysiol. 2008, 119, 2677–2686.
- Mammone, N.; La Foresta, F.; Morabito, F.C. Automatic Artifact Rejection From Multichannel Scalp EEG by Wavelet ICA. IEEE Sens. J. 2012, 12, 533–542.
- Zou, Y.; Nathan, V.; Jafari, R. Automatic Identification of Artifact-Related Independent Components for Artifact Removal in EEG Recordings. IEEE J. Biomed. Health Inform. 2016, 2, 73–81.
- Mannan, M.M.N.; Jeong, M.Y.; Kamran, M.A. Hybrid ICA—Regression: Automatic identification and removal of ocular artifacts from electroencephalographic signals. Front. Hum. Neurosci. 2016, 10, 193.
- Mannan, M.M.N.; Kamran, M.A.; Jeong, M.Y. Identification and Removal of Physiological Artifacts From Electroencephalogram Signals: A Review. IEEE Access 2018, 6, 30630–30652.
- Dai, C.; Wang, J.; Xie, J.; Li, W.; Gong, Y.; Li, Y. Removal of ECG Artifacts From EEG Using an Effective Recursive Least Square Notch Filter. IEEE Access 2019, 7, 158872–158880.
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans. Biomed. Eng. 2020, 67, 1114–1121.
- Robbins, K.A.; Touryan, J.; Mullen, T.; Kothe, C.; Bigdely-Shamlo, N. How Sensitive Are EEG Results to Preprocessing Methods: A Benchmarking Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1081–1090.
- Blanco, S.; Garcia, H.; Quiroga, R.Q.; Romanelli, L.; Rosso, O.A. Stationarity of the EEG series. IEEE Eng. Med. Biol. Mag. 1995, 14, 395–399.
- Penttonen, M.; Buzsáki, G. Natural logarithmic relationship between brain oscillators. Thalamus Relat. Syst. 2003, 2, 145–152.
- Nuwer, M.R.; Hovda, D.A.; Schrader, L.M.; Vespa, P.M. Routine and quantitative EEG in mild traumatic brain injury. Clin. Neurophysiol. 2005, 116, 2001–2025.
- Islam, M.K.; Rastegarnia, A.; Yang, Z. Methods for artifact detection and removal from scalp EEG: A review. Neurophysiol. Clin. Neurophysiol. 2016, 46, 287–305.
- Albert, B.; Zhang, J.; Noyvirt, A.; Setchi, R.; Sjaaheim, H.; Velikova, S.; Strisland, F. Automatic EEG processing for the early diagnosis of traumatic brain injury. Procedia Comput. Sci. 2016, 96, 703–712.
- Lewine, J.D.; Plis, S.; Ulloa, A.; Williams, C.; Spitz, M.; Foley, J.; Paulson, K.; Davis, J.; Bangera, N.; Snyder, T.; et al. Quantitative EEG biomarkers for mild traumatic brain injury. J. Clin. Neurophysiol. 2019, 36, 298–305.
- Kostarelos, F.; MacNamee, C.; Mullane, B. A hardware implementation of a qEEG-based discriminant function for brain injury detection. In Proceedings of the 2021 IEEE Biomedical Circuits and Systems Conference (BioCAS), Berlin, Germany, 7–9 October 2021; pp. 1–6.
- Sjaaheim, H.; Albert, B.; Setchi, R.; Noyvirt, A.; Strisland, F. A portable medical system for the early diagnosis and treatment of traumatic brain injury. In Proceedings of the 2014 IEEE International Conference on Systems, Man, and Cybernetics (SMC), San Diego, CA, USA, 5–8 October 2014; pp. 2529–2534.
- Rapp, P.E.; Keyser, D.O.; Albano, A.; Hernandez, R.; Gibson, D.B.; Zambon, R.A.; Hairston, W.D.; Hughes, J.D.; Krystal, A.; Nichols, A.S. Traumatic Brain Injury Detection Using Electrophysiological Methods. Front. Hum. Neurosci. 2015, 9, 11.
- Dingle, A.A.; Jones, R.D.; Carroll, G.J.; Fright, W.R. A multistage system to detect epileptiform activity in the EEG. IEEE Trans. Biomed. Eng. 1993, 40, 1260–1268.
- Chamanzar, A.; George, S.; Venkatesh, P.; Chamanzar, M.; Shutter, L.; Elmer, J.; Grover, P. An algorithm for automated, noninvasive detection of cortical spreading depolarizations based on EEG simulations. IEEE Trans. Biomed. Eng. 2018, 66, 1115–1126.
- Fisher, J.A.; Huang, S.; Ye, M.; Nabili, M.; Wilent, W.B.; Krauthamer, V.; Myers, M.R.; Welle, C.G. Real-time detection and monitoring of acute brain injury utilizing evoked electroencephalographic potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1003–1012.
- Jo, T. Machine Learning Foundations; Springer: Berlin/Heidelberg, Germany, 2021.
- Alzubi, J.; Nayyar, A.; Kumar, A. Machine learning from theory to algorithms: An overview. Proc. J. Phys. Conf. Ser. 2018, 142, 012012.
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2.
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction; MIT Press: Cambridge, MA, USA, 2018.
- Hosseini, M.P.; Hosseini, A.; Ahi, K. A review on machine learning for EEG signal processing in bioengineering. IEEE Rev. Biomed. Eng. 2020, 14, 204–218.
- Rothmann, M.; Porrmann, M. A Survey of Domain-Specific Architectures for Reinforcement Learning. IEEE Access 2022, 10, 13753–13767.
- Roh, Y.; Heo, G.; Whang, S.E. A survey on data collection for machine learning: A big data-ai integration perspective. IEEE Trans. Knowl. Data Eng. 2019, 33, 1328–1347.
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260.
- Bonaccorso, G. Machine Learning Algorithms; Packt Publishing Ltd.: Birmingham, UK, 2017.
- Zhou, L.; Pan, S.; Wang, J.; Vasilakos, A.V. Machine learning on big data: Opportunities and challenges. Neurocomputing 2017, 237, 350–361.
- L’heureux, A.; Grolinger, K.; Elyamany, H.F.; Capretz, M.A. Machine learning with big data: Challenges and approaches. IEEE Access 2017, 5, 7776–7797.
- Cao, C.; Tutwiler, R.L.; Slobounov, S. Automatic classification of athletes with residual functional deficits following concussion by means of EEG signal using support vector machine. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 327–335.
- Lai, C.Q.; Abdullah, M.Z.; Abdullah, J.M.; Azman, A.; Ibrahim, H. Screening of moderate traumatic brain injury from power feature of resting state electroencephalography using support vector machine. In Proceedings of the 2019 2nd International Conference on Electronics and Electrical Engineering Technology, Penang, Malaysia, 25–27 September 2019; pp. 99–103.
- Schmid, E.; Fan, Y.; Chi, T.; Golanov, E.; Regnier-Golanov, A.S.; Austerman, R.J.; Podell, K.; Cherukuri, P.; Bentley, T.; Steele, C.T.; et al. Review of wearable technologies and machine learning methodologies for systematic detection of mild traumatic brain injuries. J. Neural Eng. IOP Publ. 2021, 18, 041006.
- Vivaldi, N.; Caiola, M.; Solarana, K.; Ye, M. Evaluating performance of eeg data-driven machine learning for traumatic brain injury classification. IEEE Trans. Biomed. Eng. 2021, 68, 3205–3216.
- Noor, N.S.E.M.; Ibrahim, H. Machine learning algorithms and quantitative electroencephalography predictors for outcome prediction in traumatic brain injury: A systematic review. IEEE Access 2020, 8, 102075–102092.
- Gravesteijn, B.Y.; Nieboer, D.; Ercole, A.; Lingsma, H.F.; Nelson, D.; Van Calster, B.; Steyerberg, E.W.; Åkerlund, C.; Amrein, K.; Andelic, N.; et al. Machine learning algorithms performed no better than regression models for prognostication in traumatic brain injury. J. Clin. Epidemiol. 2020, 122, 95–107.
- Nti, I.K.; Quarcoo, J.A.; Aning, J.; Fosu, G.K. A mini-review of machine learning in big data analytics: Applications, challenges, and prospects. Big Data Min. Anal. 2022, 5, 81–97.
- Hussein, S.; Kandel, P.; Bolan, C.W.; Wallace, M.B.; Bagci, U. Lung and Pancreatic Tumor Characterization in the Deep Learning Era: Novel Supervised and Unsupervised Learning Approaches. IEEE Trans. Med. Imaging 2019, 38, 1777–1787.
- Roy, S.; Menapace, W.; Oei, S.; Luijten, B.; Fini, E.; Saltori, C.; Huijben, I.; Chennakeshava, N.; Mento, F.; Sentelli, A.; et al. Deep Learning for Classification and Localization of COVID-19 Markers in Point-of-Care Lung Ultrasound. IEEE Trans. Med. Imaging 2020, 39, 2676–2687.
- White, J.; Kameneva, T.; McCarthy, C. Vision Processing for Assistive Vision: A Deep Reinforcement Learning Approach. IEEE Trans. Hum. Mach. Syst. 2022, 52, 123–133.
- Wei, X.S.; Song, Y.Z.; Mac Aodha, O.; Wu, J.; Peng, Y.; Tang, J.; Yang, J.; Belongie, S. Fine-Grained Image Analysis with Deep Learning: A Survey. IEEE Trans. Pattern Anal. Mach. Intell. 2021.
- Khodayar, M.; Liu, G.; Wang, J.; Khodayar, M.E. Deep learning in power systems research: A review. CSEE J. Power Energy Syst. 2021, 7, 209–220.
- Tang, C.; Yu, C.; Gao, Y.; Chen, J.; Yang, J.; Lang, J.; Liu, C.; Zhong, L.; He, Z.; Lv, J. Deep learning in nuclear industry: A survey. Big Data Min. Anal. 2022, 5, 140–160.
- Hale, A.T.; Stonko, D.P.; Lim, J.; Guillamondegui, O.D.; Shannon, C.N.; Patel, M.B. Using an artificial neural network to predict traumatic brain injury. J. Neurosurgery Pediatr. PED 2019, 23, 219–226.




