Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dimiter Avtanski | -- | 2256 | 2022-10-31 16:24:10 | | | |
| 2 | Conner Chen | -2 word(s) | 2254 | 2022-11-01 03:39:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Atanasova-Panchevska, N.; Stojchevski, R.; Hadzi-Petrushev, N.; Mitrokhin, V.; Avtanski, D.; Mladenov, M. Antibacterial and Antiviral Properties of Tetrahydrocurcumin-Based Formulations. Encyclopedia. Available online: https://encyclopedia.pub/entry/32131 (accessed on 09 February 2026).
Atanasova-Panchevska N, Stojchevski R, Hadzi-Petrushev N, Mitrokhin V, Avtanski D, Mladenov M. Antibacterial and Antiviral Properties of Tetrahydrocurcumin-Based Formulations. Encyclopedia. Available at: https://encyclopedia.pub/entry/32131. Accessed February 09, 2026.
Atanasova-Panchevska, Natalija, Radoslav Stojchevski, Nikola Hadzi-Petrushev, Vadim Mitrokhin, Dimiter Avtanski, Mitko Mladenov. "Antibacterial and Antiviral Properties of Tetrahydrocurcumin-Based Formulations" Encyclopedia, https://encyclopedia.pub/entry/32131 (accessed February 09, 2026).
Atanasova-Panchevska, N., Stojchevski, R., Hadzi-Petrushev, N., Mitrokhin, V., Avtanski, D., & Mladenov, M. (2022, October 31). Antibacterial and Antiviral Properties of Tetrahydrocurcumin-Based Formulations. In Encyclopedia. https://encyclopedia.pub/entry/32131
Atanasova-Panchevska, Natalija, et al. "Antibacterial and Antiviral Properties of Tetrahydrocurcumin-Based Formulations." Encyclopedia. Web. 31 October, 2022.
Copy Citation
Special attention was given to the bactericidal effects of one of the tetrahydrocurcumin (THC)-phospholipid formulations, which has shown greater bioavailability and activity than pure THC. Similarly, quinoline derivatives and amino acid conjugates of THC have also shown antibacterial effects in the gut. Furthermore, the antiviral characteristics of curcumin (Cur) compared to those of THC are more pronounced in preventing the influenza virus.
tetrahydrocurcumin
intestinal microbiota
antibacterial properties
1. Background
Curcumin (Cur) has occupied scientific interest in the last decade [1] due to its possible bioactivity in humans [2][3]. On the other hand, Cur has very poor oral absorption, insignificant biodistribution, and low systemic bioavailability. To increase Cur bioavailability, several methods such as phytosome, liposome, and micelle formulation, as well as Cur co-formulation with adjuvants such as piperine, have been utilized [4]. Although new formulations improve curcuminoid absorption in the small intestine, a significant proportion of curcuminoids reach the colon and are excreted [5]. This is an essential step since it has been demonstrated that the animal gut microbiota conducts several metabolic/catabolic reactions to curcuminoids, which should be included in the overall assessment of Cur bioactivity and bioavailability [6][7].
Tetrahydrocurcumin (THC), a reduced analog of Cur with phenolic and -diketo moieties [8], has been identified as an active Cur metabolite in the gastrointestinal system. THC has been demonstrated to be superior to Cur in terms of anti-diabetic [9], anti-hyperlipidemic, anti-oxidant, and anti-cancerogenic effects which may be related to the biotransformation of Cur by intestinal microbiota [10][11]. Despite this advantage, there have been several efforts to develop new, even better THC formulations in order to increase the work of the gut flora. Different studies on THCs’ intestinal bioconversion are being conducted in order to reveal the possible pool of catabolites generated from various THC formulations via gut microbiota-driven metabolism [8][9][10]. In this direction, Duan et al. [12] reported antitumor activity of three new THC derivatives, but since the anti-cancerogenic efficacy of THC derivatives is not the objective of this study, regarding their mechanisms of action the reader is directed to the relevant works. More relevantly, the results of Ou et al. [13] have a very important contribution, implying that THC might potentially target specific components of the viral replication machinery or block cellular signaling pathways required for viral reproduction. In light of this, THC was proposed as an alternative to Cur in the prevention of human immunodeficiency virus (HIV) infection. Moreover, the recently employed computer-assisted in silico molecular docking and molecular dynamic simulations, focused on the comparison between the gp120-Cur and gp120-THC inhibitory effects, demonstrate that the THC-gp120 complex has greater chemical stability. This is another example of improved antiviral properties based on increased upregulation as a result of the changed formulation of the THC complex [14].
2. Effect of the Curcuminoid Formulation on Its Metabolism in the Intestinal Microbiota
The study of Bresciani et al. [15] added a new value to understanding of the metabolism of human intestinal bacteria, especially in terms of the metabolic fate of different formulations of curcuminoids. Concerning qualitative differences, no variance has been found between the lecithin-curcuminoid formulation and the unformulated botanical extract, albeit the phospholipid formulation undergoes faster microbial degradation of the base components compared to the unformulated Cur extract [15]. The curcuminoids in the phospholipid delivery method undergo more efficient microbial biotransformation than the Cur extract alone. The comparison of lecithin to a basic botanical extract formulation also revealed that the primary difference was in the synthesis of metabolites after 24 h of microbial incubation [15]. Curcuminoid catabolites are mostly recovered as tetrahydro-forms after 5 h of incubation due to the activity of microbial reductases. The parent curcuminoids are mainly digested by the colonic microbiota within 24 hours, leading to demethylation and/or bis(demethylation). The main curcuminoid catabolites formed after 24 h of microbial fermentation are bis(dimethyl)-tetrahydrocurcumin (BDM-THC), bis(dimethyl)-hexahydrocurcumin (BDM-HHC), and dimethyl-tetrahydrocurcumin (DM-THC), and the yield of these compounds is substantially greater in case of the phospholipid formulation [15]. Bresciani et al. [15] were the first to report that BDM-HHC is one of the basic curcuminoids extracted from microbial catabolites. These findings demonstrate that the human colonic microbiota is capable of generating demethylated curcuminoids [6]. The curcuminoids administered in a phospholipid formulation are also better absorbed in the upper digestive tract compared to the curcuminoids administered in their parent, unformulated form [16]. However, the formulation’s effect on microbial catabolism of curcuminoids in this part of the gut is unknown. The novel findings in Bresciani et al. [15] study support the importance of the intestinal microbiota in curcuminoid degradation, which was also already shown in the case of other polyphenols [17]. Furthermore, compared to the unformulated extract, the phospholipid formulation leads to a more effective microbial biotransformation of Cur [17] (Figure 1).
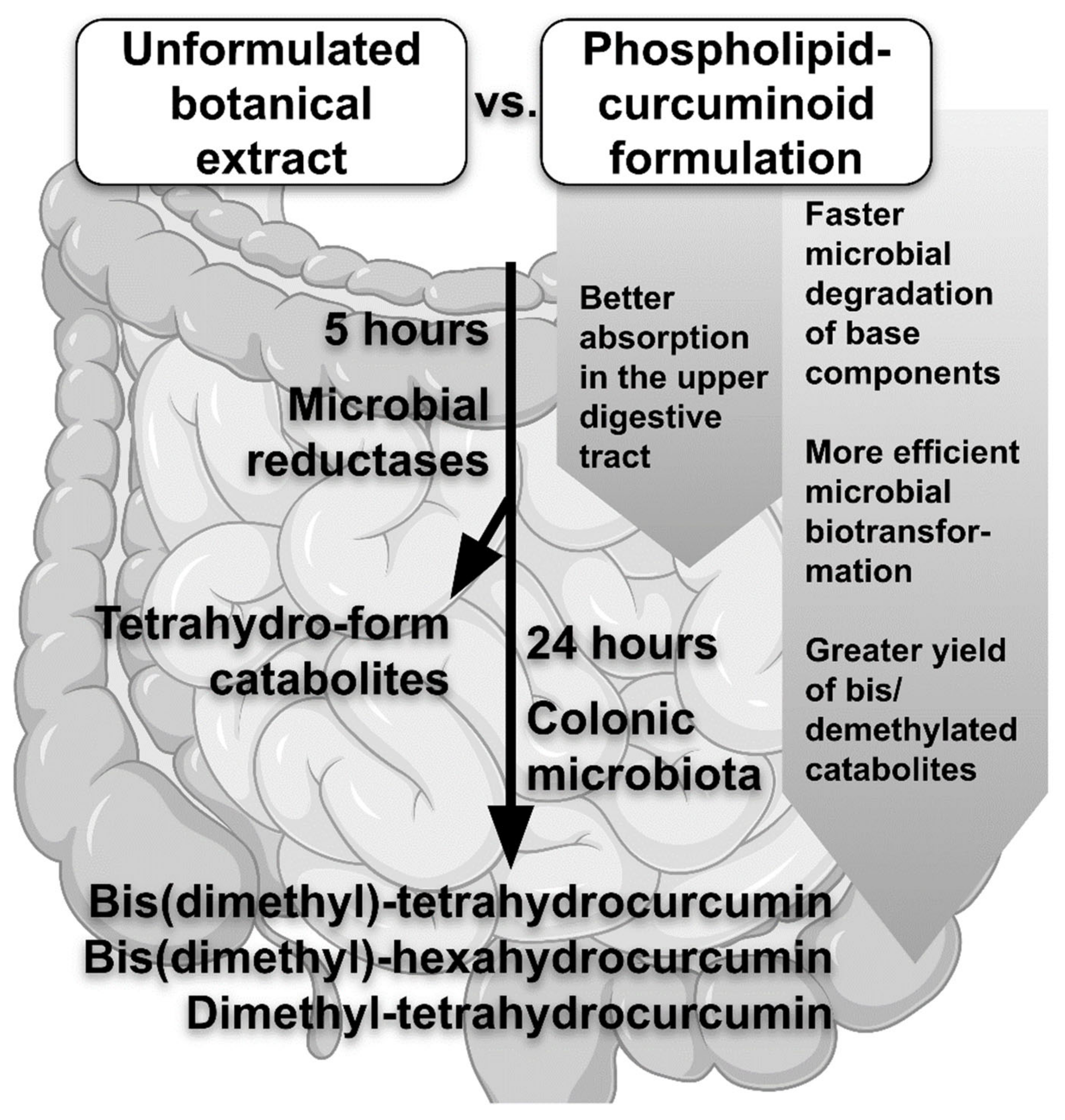
Figure 1. Effects of the curcuminoid formulation on its metabolism in the intestinal microbiota.
From all the above, the in vivo bioavailability and the potential bioactivity attributed to curcuminoids should be reassessed. The phospholipid formulation by combining different curcuminoids might create a composition with considerably increased bioavailability, activity, and microbial stability.
3. Quinoline Derivatives and AA Conjugates of THC and Their Antibacterial Properties
Manjunatha et al. [18] investigated the antibacterial activity of quinoline derivatives of THC (Figure 1) against two Gram-positive (B. cereus and S. aureus) and two Gram-negative bacteria (E. coli and Y. enterocolitica).
The same group also tested amino acid (AA) conjugates of THC (presented in Figure 2a–g) and reported antibacterial activity against two Gram-positive (B. cereus and S. aureus) and two Gram-negative (E. coli and Y. enterocolitica) species [19].
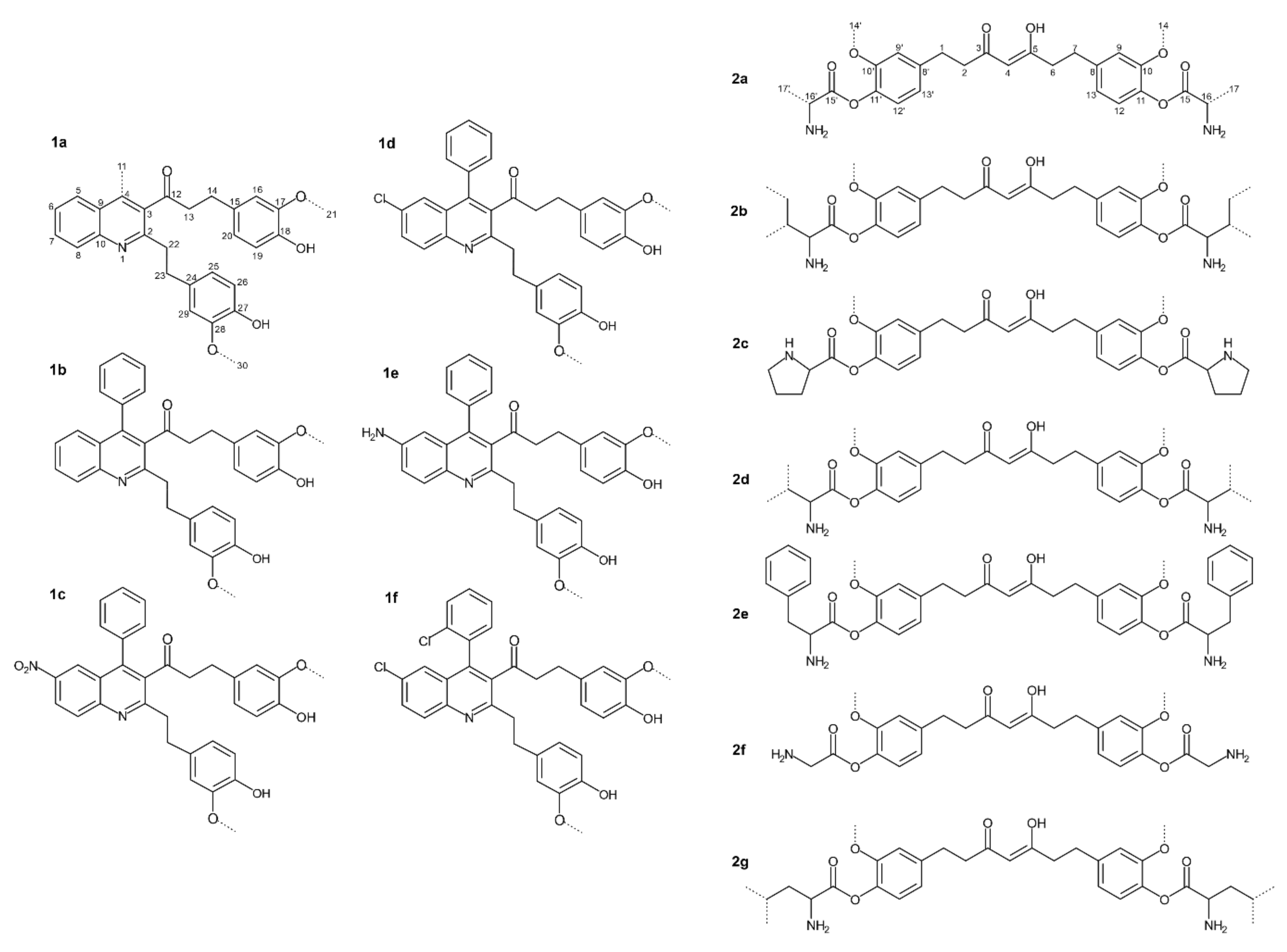
Figure 2. Quinoline derivatives and amino acid conjugates of THC: 1a.—1-(2-(4-hydroxy-3-methoxyphenethyl)-4-methylquinolin-3-yl)-3-(4-hydroxy-3-methoxyphenyl)propan-1-one; 1b.—1-(2-(4-Hydroxy-3-methoxyphenethyl)-4-phenylquinolin-3-yl)-3-(4-hydroxy-3-methoxyphenyl) propan-1-one; 1c.—1-(2-(4-Hydroxy-3-methoxyphenethyl)-6-nitro-4-phenylquinolin-3-yl)-3-(4-hydroxy-3-methoxyphenyl)propan-1-one; 1d.—1-(6-Chloro-2-(4-hydroxy-3-methoxyphenethyl)-4-phenylquinolin-3-yl)-3-(4-hydroxy-3-methoxyphenyl)propan-1-one; 1e.—1-(6-Amino-2-(4-hydroxy-3-methoxyphenethyl)-4-phenylquinolin-3-yl)-3-(4-hydroxy-3-methoxyphenyl)propan-1-one; 1f.—1-(6-Chloro-4-(2-chlorophenyl)-2-(4-hydroxy-3-methoxypheneth-yl) quinolin-3-yl)-3-(4-hydroxy-3-methoxyphenyl) propan-1-one; 2a. Alanine. 2b. Isoleucine. 2c. Proline. 2d. Valine. 2e. Phenylalanine. 2f. Glycine. 2g. Leucine. (Modified from Manjunatha et al. [18][19]).
At the same time, THC-glycine and THC-valine conjugates (2f and 2d, respectively) are the most effective against B. cereus. According to Manjunatha et al. [19], THC and its AA conjugates suppress bacterial growth in the following order of strength: 2f > 2d > 2a > 2c > 2b > 2e > 2g > THC. Actually, the authors have shown that compound 2f inhibited the growth of S. aureus the most, whereas compound 2g led to minimal inhibition, following the growth inhibition trend: 2f > 2c > 2d > 2a = 2b = 2e > THC > 2g. The same research has confirmed that 2f mostly inhibited the growth of E. coli and Y. enterocolitica, and their MIC values were associated with the trend of inhibition.
Kapoor et al. [20] have also found that some Cur bio-conjugates comprising esters and peptides have stronger antifungal and antibacterial properties, which can be related to improved cell uptake, increased cellular concentration, and improved receptor binding capacity. The AA part of the derivatives appears to make the THC conjugates hydrophilic, which aids in the increased absorption of the covalently bound THC into bacterial cells.
A similar pattern has been discovered for various natural and synthetic compounds, which exhibit varying activity based on the derivative structure and the studied bacterial strain [21][22]. Comparing the activity of various THC derivatives against bacteria demonstrated that virtually all of the investigated THC derivatives (Figure 2) have higher MIC values against Gram-negative than Gram-positive bacteria, as this impact is primarily due to differences in the compound structure [23][24]. Gram-positive bacteria have an exterior peptidoglycan layer that acts as a permeability barrier [25]. The outer phospholipid membrane of Gram-negative bacteria is impervious to lipophilic solutes [26]. Furthermore, porins in the cell membrane serve as a selective barrier to hydrophilic solutes, rendering cells resistant to antibacterial compounds [26].
Finally, in vitro experiments demonstrated that specific THC quinoline or AA conjugates have a substantial antimicrobial impact. The findings demonstrated that altering THC’s side chain carbonyl activity improved its antibacterial properties [18][19]. Manjunatha et al. [18] discovered a quinoline derivative with an electron-donating amino group with the highest free radical scavenging activity. These derivatives might have relevant pharmaceutical use and might significantly impact the use of THC in fostering or inhibiting some specific intestinal microbial species in relation to different physiological conditions. In this direction, both THC quinolone and THC amino acidic conjugates might have significant pharmaceutical relevance in the treatment of diseases associated with the change of intestinal microbiota such as diabetes or any other immunologically associated conditions.
4. Anti-Influenza Virus Activity of THC
In their study, Ou et al. [13] examined the anti-type A influenza virus (anti-IAV) bioactivity of numerous curcuminoids in order to elucidate the antiviral mechanism of Cur and to develop stable derivatives with higher biological activity. In this direction, they examined the following questions: (i) Does the stable metabolite THC possess an anti-IAV function? (ii) What is the essential structure responsible for the Cur-mediated anti-IAV activity? It should be noted that Cur can be degraded or bio-transformed very fast in a neutral pH environment and is unstable in physiological conditions [20]. Hence, it is critical to determine whether Cur may exhibit antiviral actions in physiological conditions via its metabolites. THC, as one such metabolite, can cause a decrease in viral production, despite its lesser potency than Cur, indicating that THC may serve as a viable antiviral drug against IAV [13].
The modern pharmaceutical approach declared that it is critical to design antiviral medications that target key components and cellular factors or pathways essential for successful viral replication. The curcuminoids assessed in Ou’s study [13] have been proven to significantly suppress the propagation of the IAV, probably through multiple mechanisms [13]. First, after virus entry, THC dramatically decreased IAV yields, demonstrating that THC likely targets particular phases of the viral replication machinery or dampens cellular signaling involved in the viral replication [27]. Furthermore, the Ras/Mitogen-activated protein kinase (MAPK)/ERK kinase (MEK)/extracellular-signal-regulated kinase (ERK) cascade [28][29][30], the nuclear factor kappa B (NF-κB) pathway [31][32], and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling pathways [33][34], are activated and necessary for viral replication. Interestingly, it was proven that THC strongly inhibited the activation of PI3K/PKB and MAPK signaling pathways in human HL-60 leukemia cells [35]. Because THC only mildly decreased viral particle infectivity and did not entirely block viral hemagglutinating activity (HA), it could be expected that modulation of these signaling pathways can contribute significantly to THC-mediated anti-IAV action. In addition, desmethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC), which have one or no methoxy groups, have the same inhibitory effect as Cur, demonstrating that the methoxy groups are not responsible for the HA [13].
From a chemical point of view, Cur has a symmetrical structure composed of two aromatic rings connected by two unsaturated carbonyl (enone) groups. The enone group serves as the acceptor in the Michael reaction of addition, which is important in intermolecular conjugation with certain proteins [36]. Cur may interact with viral surface proteins and interfere with their function, therefore inactivating virus infectivity via the Michael addition reaction. Hence, in terms of HA inhibition, THC, unlike Cur, failed to reduce viral HA activity [13]. Because each double bond conjugated to the carbonyl moiety of Cur is saturated, the resultant THC cannot function as a Michael reaction acceptor [37]. Furthermore, the presence of glutathione (GSH), one of the most abundant endogenous antioxidants, reduces Cur’s inhibitory effects. This means that incubating Cur with a protein or peptide (e.g., GSH) that has exposed electron-donating functional groups (e.g., the SH group of cysteine in GSH) prevents the Michael acceptor electrophile (MAE) region of Cur from being accessible, thus limiting Cur’s ability to change viral surface proteins [13]. It is crucial to note that GSH does not influence the hemagglutination inhibition (HI) effect. It can be believed that either the Michael addition reaction is insufficient (other Cur-dependent actions contribute to its anti-IAV effectiveness) or that GSH is not an adequate molecule to compete with the IAV surface protein (i.e., hemagglutinin) for the conjugation to Cur. A variety of phyto-compounds with MAE characteristics failed to suppress IAV’s HA activity, demonstrating that Michael’s addition is not the main contributor to anti-IAV activity [38]. The docking simulation indicated that Cur might create one and two hydrogen bonds with the Asn133 and Gln226 residues on the receptor binding site of the viral HA protein. This explains why GSH competition is ineffective because cysteine is unlikely to be the major residue on the HA protein’s ribosomal binding site (RBS) region that interacts with Cur [38]. Soundararajan et al. [38], employing docking studies, imply that conjugation of Cur with RBS residues on HA reduces the chance of IAV to connect with its cellular receptor, hence preventing viral entrance. This is consistent with the experimental results from Ou et al. [13], showing that incubating viral particles with Cur before cell attachment limits IAV-induced red blood cells agglutination (HI effect), decreases plaque development on Madin–Darby canine kidney (MDCK) cells, and reduces virus production.
Although Cur successfully prevents viral entrance by interacting with the viral HA protein, resistant virus variants would seem to emerge in response to Cur. Nonetheless, Chen et al. [39] reported no IAV variants against Cur, even after five rounds of the blind passage under Cur exposition. This is explained by the fact that Cur-dependent antiviral ability also occurs through acting on cellular factors [39][40][41], and accumulating evidence suggests that antiviral compounds targeting cellular factors appear to be a high barrier to the development of resistant virus variants [32][42].
References
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011, 50, 151–161.
- Shen, L.; Ji, H.-F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902.
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112.
- Tsai, Y.-M.; Jan, W.-C.; Chien, C.-F.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925.
- Tan, S.; Calani, L.; Bresciani, L.; Dall’asta, M.; Faccini, A.; Augustin, M.A.; Gras, S.L.; Del Rio, D. The degradation of curcuminoids in a human faecal fermentation model. Int. J. Food Sci. Nutr. 2015, 66, 790–796.
- Burapan, S.; Kim, M.; Han, J. Curcuminoid Demethylation as an Alternative Metabolism by Human Intestinal Microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310.
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892.
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853.
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39.
- Lai, C.-S.; Ho, C.-T.; Pan, M.-H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules 2020, 10, 831.
- Duan, M.; Mahal, A.; Mohammed, B.; Zhu, Y.; Tao, H.; Mai, S.; Al-Haideri, M.; Zhu, Q. Synthesis and antitumor activity of new tetrahydrocurcumin derivatives via click reaction. Nat. Prod. Res. 2021, 36, 5268–5276.
- Ou, J.-L.; Mizushina, Y.; Wang, S.-Y.; Chuang, D.-Y.; Nadar, M.; Hsu, W.-L. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013, 280, 5829–5840.
- Mirani, A.; Kundaikar, H.; Velhal, S.; Patel, V.; Bandivdekar, A.; Degani, M.; Patravale, V. Tetrahydrocurcumin-loaded vaginal nanomicrobicide for prophylaxis of HIV/AIDS: In silico study, formulation development, and in vitro evaluation. Drug Deliv. Transl. Res. 2019, 9, 828–847.
- Bresciani, L.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; Del Rio, D. The Effect of Formulation of Curcuminoids on Their Metabolism by Human Colonic Microbiota. Molecules 2020, 25, 940.
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177.
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669.
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of quinoline derivatives of tetrahydrocurcumin and zingerone and evaluation of their antioxidant and antibacterial attributes. Food Chem. 2013, 136, 650–658.
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of amino acid conjugates of tetrahydrocurcumin and evaluation of their antibacterial and anti-mutagenic properties. Food Chem. 2013, 139, 332–338.
- Kapoor, N.; Narain, U.; Misra, K. Bio-active conjugates of curcumin having ester, peptide, thiol and disulfide links. J. Sci. Ind. Res. 2007, 66, 647–650.
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Evaluation of Antioxidant and Antimutagenic Activities of the Extracts from the Fruit Rinds of Garcinia cowa. Int. J. Food Prop. 2010, 13, 1256–1265.
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009, 115, 265–271.
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria. LWT-Food Sci. Technol. 2008, 41, 1857–1861.
- Nostro, A.; Germanò, M.P.; D’angelo, V.; Marino, A.; Cannatelli, M.A. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 2000, 30, 379–384.
- Scherrer, R.; Gerhardt, P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 1971, 107, 718–735.
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32.
- Lappi, J.; Mykkänen, H.; Bach Knudsen, K.E.; Kirjavainen, P.; Katina, K.; Pihlajamäki, J.; Poutanen, K.; Kolehmainen, M. Postprandial glucose metabolism and SCFA after consuming wholegrain rye bread and wheat bread enriched with bioprocessed rye bran in individuals with mild gastrointestinal symptoms. Nutr. J. 2014, 13, 104.
- Luig, C.; Köther, K.; Dudek, S.E.; Gaestel, M.; Hiscott, J.; Wixler, V.; Ludwig, S. MAP kinase-activated protein kinases 2 and 3 are required for influenza A virus propagation and act via inhibition of PKR. FASEB J. 2010, 24, 4068–4077.
- Ludwig, S.; Planz, O.; Pleschka, S.; Wolff, T. Influenza-virus-induced signaling cascades: Targets for antiviral therapy? Trends Mol. Med. 2003, 9, 46–52.
- Pleschka, S.; Wolff, T.; Ehrhardt, C.; Hobom, G.; Planz, O.; Rapp, U.R.; Ludwig, S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 2001, 3, 301–305.
- Ludwig, S.; Planz, O. Influenza viruses and the NF-kappaB signaling pathway—Towards a novel concept of antiviral therapy. Biol. Chem. 2008, 389, 1307–1312.
- Mazur, I.; Wurzer, W.J.; Ehrhardt, C.; Pleschka, S.; Puthavathana, P.; Silberzahn, T.; Wolff, T.; Planz, O.; Ludwig, S. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell. Microbiol. 2007, 9, 1683–1694.
- Ehrhardt, C.; Wolff, T.; Pleschka, S.; Planz, O.; Beermann, W.; Bode, J.G.; Schmolke, M.; Ludwig, S. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 2007, 81, 3058–3067.
- Ehrhardt, C.; Ludwig, S. A new player in a deadly game: Influenza viruses and the PI3K/Akt signalling pathway. Cell. Microbiol. 2009, 11, 863–871.
- Wu, J.-C.; Lai, C.-S.; Badmaev, V.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol. Nutr. Food Res. 2011, 55, 1646–1654.
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791.
- Hadzi-Petrushev, N.; Bogdanov, J.; Krajoska, J.; Ilievska, J.; Bogdanova-Popov, B.; Gjorgievska, E.; Mitrokhin, V.; Sopi, R.; Gagov, H.; Kamkin, A.; et al. Comparative study of the antioxidant properties of monocarbonyl curcumin analogues C66 and B2BrBC in isoproteranol induced cardiac damage. Life Sci. 2018, 197, 10–18.
- Soundararajan, V.; Zheng, S.; Patel, N.; Warnock, K.; Raman, R.; Wilson, I.A.; Raguram, S.; Sasisekharan, V.; Sasisekharan, R. Networks link antigenic and receptor-binding sites of influenza hemagglutinin: Mechanistic insight into fitter strain propagation. Sci. Rep. 2011, 1, 200.
- Chen, D.-Y.; Shien, J.-H.; Tiley, L.; Chiou, S.-S.; Wang, S.-Y.; Chang, T.-J.; Lee, Y.-J.; Chan, K.-W.; Hsu, W.-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351.
- Si, X.; Wang, Y.; Wong, J.; Zhang, J.; McManus, B.M.; Luo, H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J. Virol. 2007, 81, 3142–3150.
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247.
- Ehrhardt, C.; Rückle, A.; Hrincius, E.R.; Haasbach, E.; Anhlan, D.; Ahmann, K.; Banning, C.; Reiling, S.J.; Kühn, J.; Strobl, S.; et al. The NF-κB inhibitor SC75741 efficiently blocks influenza virus propagation and confers a high barrier for development of viral resistance. Cell. Microbiol. 2013, 15, 1198–1211.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
972
Revisions:
2 times
(View History)
Update Date:
01 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





