
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberto Arrigoni | -- | 1384 | 2022-10-31 15:31:48 | | | |
| 2 | Catherine Yang | Meta information modification | 1384 | 2022-11-01 02:07:21 | | |
Video Upload Options
Tuberculosis (TB) is an infectious disease caused by the bacillus Mycobacterium tuberculosis (Mtb). TB treatment is based on the administration of three major antibiotics: isoniazid, rifampicin, and pyrazinamide. However, multi-drug resistant (MDR) Mtb strains are increasing around the world, thus, allowing TB to spread around the world. The stringent response is demonstrated by Mtb strains in order to survive under hostile circumstances, even including exposure to antibiotics. The stringent response is mediated by alarmones, which regulate bacterial replication, transcription and translation. Moreover, the Mtb cell wall contributes to the mechanism of antibiotic resistance along with efflux pump activation and biofilm formation.
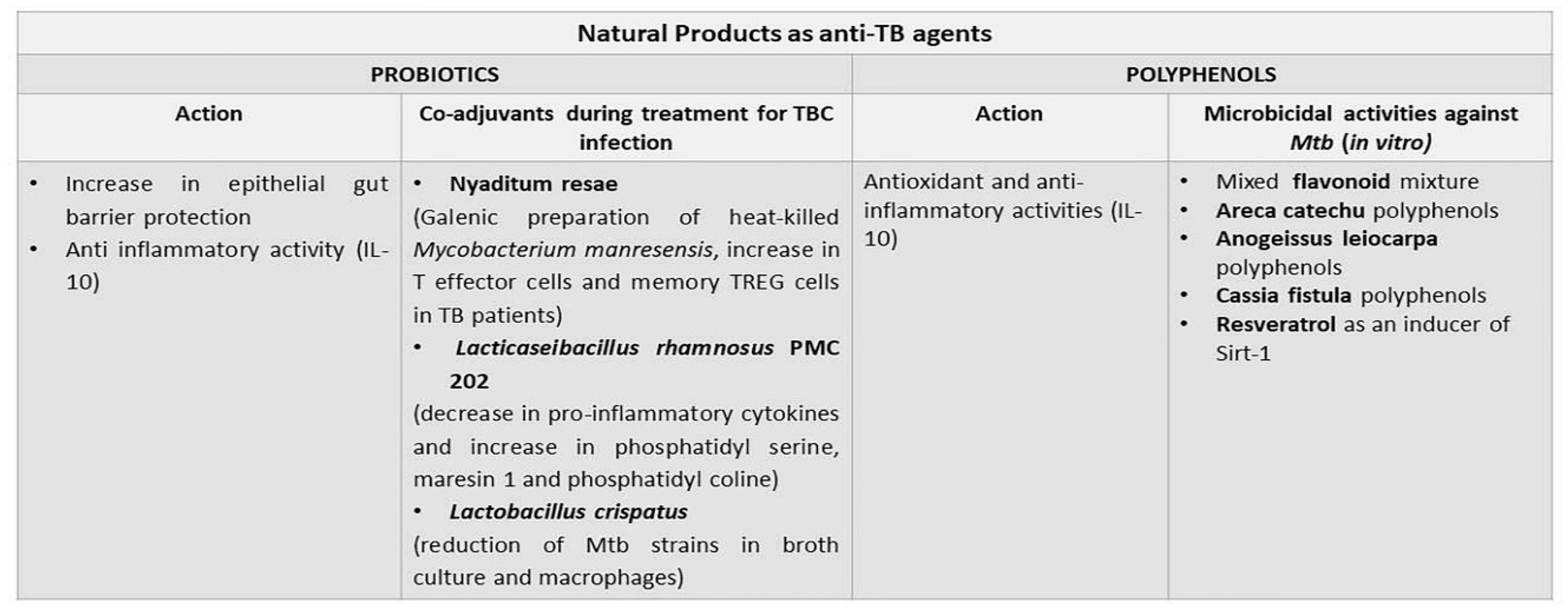
1. Probiotics
2. Polyphenols
3. Antimicrobial Peptides
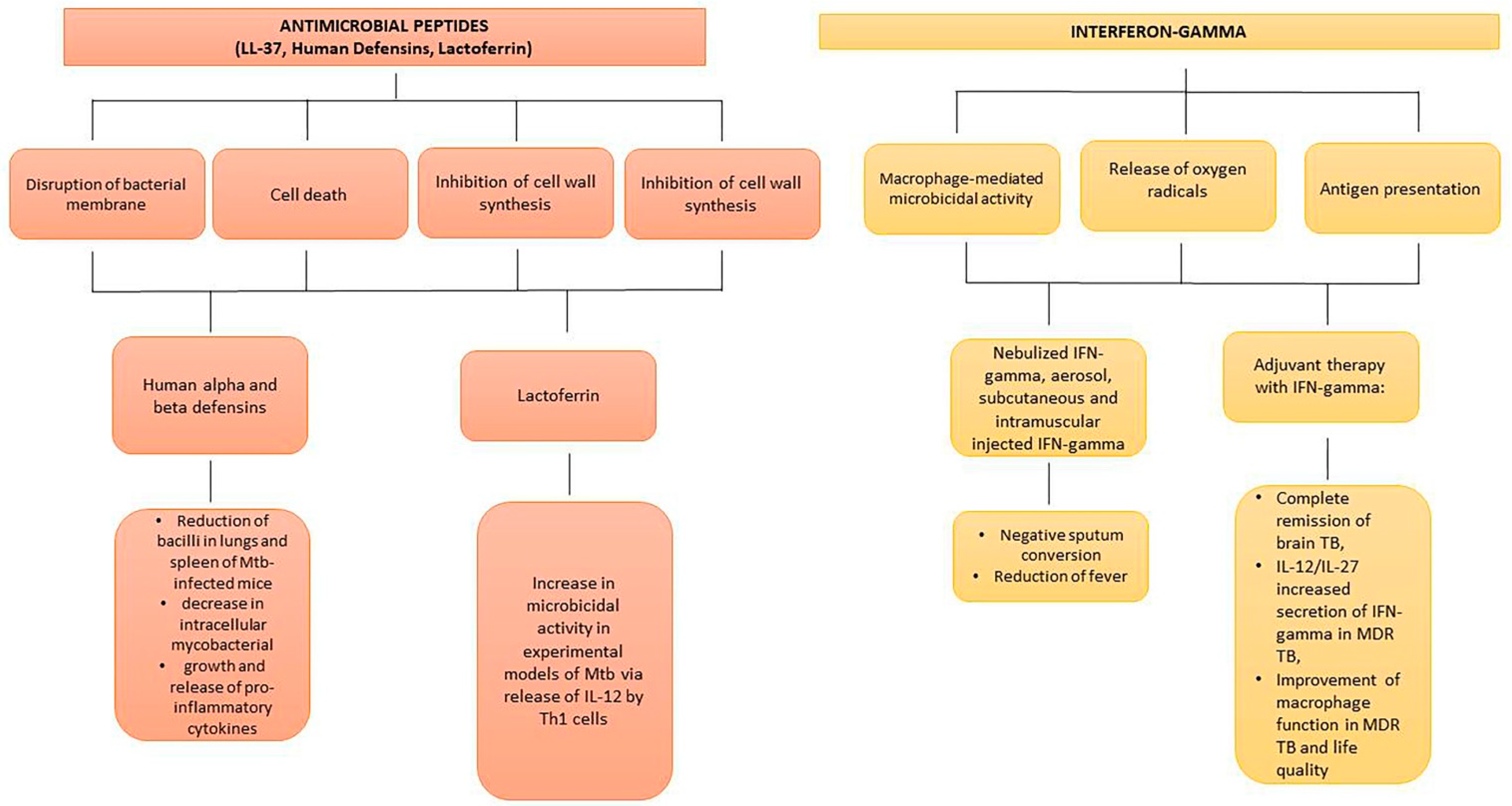
4. Interferon-Gamma and Mesenchymal Stem Cells
References
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. (Landmark Ed.) 2021, 26, 135–148.
- Negatu, D.A.; Liu, J.J.J.; Zimmerman, M.; Kaya, F.; Dartois, V.; Aldrich, C.C.; Gengenbacher, M.; Dick, T. Whole-Cell Screen of Fragment Library Identifies Gut Microbiota Metabolite Indole Propionic Acid as Antitubercular. Antimicrob. Agents Chemother. 2018, 62, e01571-17.
- Genestet, C.; Bernard-Barret, F.; Hodille, E.; Ginevra, C.; Ader, F.; Goutelle, S.; Lina, G.; Dumitrescu, O.; Lyon, T.B.; Lyon TB study group. Antituberculous Drugs Modulate Bacterial Phagolysosome Avoidance and Autophagy in Mycobacterium tuberculosis-Infected Macrophages. Tuberculosis 2018, 111, 67–70.
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58, S164–S179.
- Pamer, E.G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016, 352, 535–538.
- Hörmannsperger, G.; Haller, D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: Clinical relevance in the context of inflammatory bowel disease. Int. J. Med. Microbiol. 2010, 300, 63–73.
- Ghadimi, D.; Fölster-Holst, R.; de Vrese, M.; Winkler, P.; Heller, K.J.; Schrezenmeir, J. Effects of Probiotic Bacteria and Their Genomic DNA on TH1/TH2-Cytokine Production by Peripheral Blood Mononuclear Cells (PBMCs) of Healthy and Allergic Subjects. Immunobiology 2008, 213, 677–692.
- Cardona, P.J. The Progress of Therapeutic Vaccination with Regard to Tuberculosis. Front. Microbiol. 2016, 7, 1536.
- Montané, E.; Barriocanal, A.M.; Arellano, A.L.; Valderrama, A.; Sanz, Y.; Perez-Alvarez, N.; Cardona, P.; Vilaplana, C.; Cardona, P.-J. Pilot, Double-Blind, Randomized, Placebo-Controlled Clinical Trial of the Supplement Food Nyaditum Resae® in Adults with or without Latent TB Infection: Safety and Immunogenicity. PLoS ONE 2017, 12, e0171294.
- Rahim, M.A.; Seo, H.; Kim, S.; Tajdozian, H.; Barman, I.; Lee, Y.; Lee, S.; Song, H.-Y. In Vitro Anti-Tuberculosis Effect of Probiotic Lacticaseibacillus rhamnosus PMC203 Isolated from Vaginal Microbiota. Sci. Rep. 2022, 12, 8290.
- Jiang, L.; Wang, J.; Xu, L.; Cai, J.; Zhao, S.; Ma, A. Lactobacillus Casei Modulates Inflammatory Cytokines and Metabolites during Tuberculosis Treatment: A Post Hoc Randomized Controlled Trial. Asia. Pac. J. Clin. Nutr. 2022, 31, 66–77.
- Lee, Y.; Seo, H.; Kim, S.; Rahim, M.D.A.; Yoon, Y.; Jung, J.; Lee, S.; Beom Ryu, C.; Song, H.-Y. Activity of Lactobacillus Crispatus Isolated from Vaginal Microbiota against Mycobacterium tuberculosis. J. Microbiol. 2021, 59, 1019–1030.
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35.
- Watson, R.R. Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Elsevier: San Diego, CA, USA, 2018.
- Cao, R.; Teskey, G.; Islamoglu, H.; Gutierrez, M.; Salaiz, O.; Munjal, S.; Fraix, M.P.; Sathananthan, A.; Nieman, D.C.; Venketaraman, V. Flavonoid Mixture Inhibits Mycobacterium tuberculosis Survival and Infectivity. Molecules 2019, 24, 851.
- Raju, A.; Degani, M.S.; Khambete, M.P.; Ray, M.K.; Rajan, M.G. Antifolate Activity of Plant Polyphenols against Mycobacterium tuberculosis. Phytother. Res. 2015, 29, 1646–1651.
- Salih, E.Y.A.; Julkunen-Tiitto, R.; Luukkanen, O.; Sipi, M.; Fahmi, M.K.M.; Fyhrquist, P.J. Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols. Antibiotics 2020, 9, 364.
- Chakraborty, A.K.; Saha, S.; Poria, K.; Samanta, T.; Gautam, S.; Mukhopadhyay, J. A Saponin-Polybromophenol Antibiotic (CU1) from Cassia Fistula Bark Against Multi-Drug Resistant Bacteria Targeting RNA Polymerase. Curr. Res. Pharm. Drug Discov. 2022, 3, 100090.
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory Stimuli Induce Inhibitory S-Nitrosylation of the Deacetylase SIRT1 to Increase Acetylation and Activation of P53 and P65. Sci. Signal 2014, 7, ra106.
- Yang, H.; Chen, J.; Chen, Y.; Jiang, Y.; Ge, B.; Hong, L. Sirtuin Inhibits, M. Tuberculosis -Induced Apoptosis in Macrophage through Glycogen Synthase Kinase-3β. Arch. Biochem. Biophys. 2020, 694, 108612.
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230.
- Gaglione, R.; Pizzo, E.; Notomista, E.; de la Fuente-Nunez, C.; Arciello, A. Host Defence Cryptides from Human Apolipoproteins: Applications in Medicinal Chemistry. Curr. Top. Med. Chem. 2020, 20, 1324–1337.
- Mehta, K.; Sharma, P.; Mujawar, S.; Vyas, A. Role of Antimicrobial Peptides in Treatment and Prevention of Mycobacterium tuberculosis: A Review. Int. J. Pept. Res. Ther. 2022, 28, 132.
- Jadhav, K.; Singh, R.; Ray, E.; Singh, A.K.; Verma, R.K. Taming the devil: Antimicrobial peptides for safer TB therapeutics. Curr. Protein Pept. Sci. 2022.
- Travis, S.M.; Anderson, N.N.; Forsyth, W.R.; Espiritu, C.; Conway, B.D.; Greenberg, E.P.; McCray, P.B., Jr.; Lehrer, R.I.; Welsh, M.J.; Tack, B.F. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 2000, 68, 2748–2755.
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557.
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4.
- Silva, T.; Gomes, M.S. Immuno-Stimulatory Peptides as a Potential Adjunct Therapy against Intra-Macrophagic Pathogens. Molecules 2017, 22, 1297.
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425.
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R.; et al. Significance of LL-37 on Immunomodulation and Disease Outcome. Biomed Res. Int. 2020, 2020, 8349712.
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773.
- Mily, A.; Rekha, R.S.; Kamal, S.M.M.; Arifuzzaman, A.S.M.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138340.
- Torres-Juarez, F.; Cardenas-Vargas, A.; Montoya-Rosales, A.; González-Curiel, I.; Garcia-Hernandez, M.H.; Enciso-Moreno, J.A.; Hancock, R.E.W.; Rivas-Santiago, B. LL-37 Immunomodulatory Activity during Mycobacterium tuberculosis Infection in Macrophages. Infect. Immun. 2015, 83, 4495–4503.
- Cobongela, S.; Makatini, M.M.; Mdluli, P.S.; Sibuyi, N. Acyldepsipeptide Analogues: A Future Generation Antibiotics for Tuberculosis Treatment. Pharmaceutics 2022, 14, 1956.
- Desvignes, L.; Wolf, A.J.; Ernst, J.D. Dynamic Roles of Type I and Type II IFNs in Early Infection with Mycobacterium tuberculosis. J. Immunol. 2012, 188, 6205–6215.
- Petruccioli, E.; Scriba, T.J.; Petrone, L.; Hatherill, M.; Cirillo, D.M.; Joosten, S.A.; Ottenhoff, T.H.; Denkinger, C.M.; Goletti, D. Correlates of Tuberculosis Risk: Predictive Biomarkers for Progression to Active Tuberculosis. Eur. Respir. J. 2016, 48, 1751–1763.
- Berns, S.A.; Isakova, J.A.; Pekhtereva, P.I. Therapeutic Potential of Interferon-Gamma in Tuberculosis. Admet Dmpk 2022, 10, 63–73.
- Ernst, J.D. The Immunological Life Cycle of Tuberculosis. Nat. Rev. Immunol. 2012, 12, 581–591.
- Hickman-Davis, J.M.; Fang, F.C.; Nathan, C.; Shepherd, V.L.; Voelker, D.R.; Wright, J.R. Lung surfactant and reactive oxygen-nitrogen species: Antimicrobial activity and host-pathogen interactions. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L517–L523.
- Fortes, A.; Pereira, K.; Antas, P.R.Z.; Franken, C.L.M.C.; Dalcolmo, M.; Ribeiro-Carvalho, M.M.; Cunha, K.S.; Geluk, A.; Kritski, A.; Kolk, A.; et al. Detection of in Vitro Interferon-Gamma and Serum Tumour Necrosis Factor-Alpha in Multidrug-Resistant Tuberculosis Patients. Clin. Exp. Immunol. 2005, 141, 541–548.
- Brooks, B.M.; Hart, C.A.; Coleman, J.W. Differential Effects of Beta-Lactams on Human IFN-Gamma Activity. J. Antimicrob. Chemother. 2005, 56, 1122–1125.
- Gao, X.-F.; Yang, Z.-W.; Li, J. Adjunctive Therapy with Interferon-Gamma for the Treatment of Pulmonary Tuberculosis: A Systematic Review. Int. J. Infect. Dis. 2011, 15, 594–600.
- Condos, R.; Rom, W.N.; Schluger, N.W. Treatment of Multidrug-Resistant Pulmonary Tuberculosis with Interferon-Gamma via Aerosol. Lancet 1997, 349, 1513–1515.
- Park, S.-K.; Cho, S.; Lee, I.-H.; Jeon, D.-S.; Hong, S.-H.; Smego, R.A.; Cho, S.-N. Subcutaneously Administered Interferon-Gamma for the Treatment of Multidrug-Resistant Pulmonary Tuberculosis. Int. J. Infect. Dis. 2007, 11, 434–440.
- Suárez-Méndez, R.; García-García, I.; Fernández-Olivera, N.; Valdés-Quintana, M.; Milanés-Virelles, M.T.; Carbonell, D.; Machado-Molina, D.; Valenzuela-Silva, C.M.; López-Saura, P.A. Adjuvant interferon gamma in patients with drug-resistant pulmonary tuberculosis: A pilot study. BMC Infect. Dis. 2004, 4, 44.
- Raad, I.; Hachem, R.; Leeds, N.; Sawaya, R.; Salem, Z.; Atweh, S. Use of Adjunctive Treatment with Interferon-Gamma in an Immunocompromised Patient Who Had Refractory Multidrug-Resistant Tuberculosis of the Brain. Clin. Infect. Dis. 1996, 22, 572–574.
- Pisarenko, M.S. Peculiarities of IFN-g secretion in drug-resistant pulmonary tuberculosis. Fundam. Res. 2013, 9, 444–447.
- Khan, T.A.; Mazhar, H.; Saleha, S.; Tipu, H.N.; Muhammad, N.; Abbas, M.N. Interferon-Gamma Improves Macrophages Function against M. Tuberculosis in Multidrug-Resistant Tuberculosis Patients. Chemother. Res. Pr. 2016, 2016, 7295390.
- Ballini, A.; De Frenza, G.; Cantore, S.; Papa, F.; Grano, M.; Mastrangelo, F.; Tetè, S.; Grassi, F.R. In vitro stem cell cultures from human dental pulp and periodontal ligament: New prospects in dentistry. Int. J. Immunopathol. Pharmacol. 2007, 20, 9–16.
- Charitos, I.A.; Ballini, A.; Cantore, S.; Boccellino, M.; Di Domenico, M.; Borsani, E.; Nocini, R.; Di Cosola, M.; Santacroce, L.; Bottalico, L. Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues. Stem. Cells Int. 2021, 2021, 9978837.
- Raghuvanshi, S.; Sharma, P.; Singh, S.; Van Kaer, L.; Das, G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21653–21658.
- Das, B.; Kashino, S.S.; Pulu, I.; Kalita, D.; Swami, V.; Yeger, H.; Felsher, D.W.; Campos-Neto, A. CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci. Transl. Med. 2013, 5, 170ra13.
- Zhang, X.; Huang, F.; Li, W.; Dang, J.L.; Yuan, J.; Wang, J.; Zeng, D.L.; Sun, C.X.; Liu, Y.Y.; Ao, Q.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front. Immunol. 2018, 9, 878.
- Zhang, X.; Xie, Q.; Ye, Z.; Li, Y.; Che, Z.; Huang, M.; Zeng, J. Mesenchymal Stem Cells and Tuberculosis: Clinical Challenges and Opportunities. Front. Immunol. 2021, 12, 695278.
- Maslennikov, A.A.; Obolonkova, N.I.; Belgorod State National Research University. Efficiency of ingaron in the treatment of patients with destructive pulmonary bacteriologicaly proven tuberculosis. Res. Result 2016, 2, 10–16.




