Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashoka Sriyani Gamage | -- | 1137 | 2022-10-31 09:32:41 | | | |
| 2 | Conner Chen | Meta information modification | 1137 | 2022-11-01 02:20:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Properties of Starch as Nanocomposites. Encyclopedia. Available online: https://encyclopedia.pub/entry/32042 (accessed on 01 March 2026).
Gamage A, Thiviya P, Mani S, Ponnusamy PG, Manamperi A, Evon P, et al. Properties of Starch as Nanocomposites. Encyclopedia. Available at: https://encyclopedia.pub/entry/32042. Accessed March 01, 2026.
Gamage, Ashoka, Punniamoorthy Thiviya, Sudhagar Mani, Prabaharan Graceraj Ponnusamy, Asanga Manamperi, Philippe Evon, Othmane Merah, Terrence Madhujith. "Properties of Starch as Nanocomposites" Encyclopedia, https://encyclopedia.pub/entry/32042 (accessed March 01, 2026).
Gamage, A., Thiviya, P., Mani, S., Ponnusamy, P.G., Manamperi, A., Evon, P., Merah, O., & Madhujith, T. (2022, October 31). Properties of Starch as Nanocomposites. In Encyclopedia. https://encyclopedia.pub/entry/32042
Gamage, Ashoka, et al. "Properties of Starch as Nanocomposites." Encyclopedia. Web. 31 October, 2022.
Copy Citation
Starch is one of the most abundant natural polymers globally. Starch and its nanocomposites have been extensively studied for their abundance, low cost, ease of processibility, and chemical and physical properties.
biodegradability
carbon nanotubes
graphene
life cycle analysis
nanocomposites
1. Introduction
In recent days, nanocomposites have gained much attention over traditional composite materials and are widely used in food, packaging, biomedical applications, electronics, energy storage, optics, the automotive industry, bio-sorbants for environmental remediation, textiles, and many other applications [1][2]. Polymer nanocomposites consist of polymer matrices embedded with nanofillers [3]. Petroleum-based polymers are produced in huge amounts globally. Petroleum-based polymers are non-biodegradable, non-renewable, and produce hazardous substances which can threaten human health and the environment [4]. Furthermore, the depletion of these non-renewable petroleum-based fuels demands alternative resources [5].
Thus, biopolymer-based nanocomposites can be a sustainable alternative for petroleum-based nanocomposites in many applications due to their biodegradability, eco-friendliness, renewability, relatively inexpensive, low toxicity, abundancy, and improved thermal, mechanical, physical, barrier, and functional properties [3][4]. Various natural biopolymers, including starch, cellulose, pectin, lignin, chitin/chitosan, alginates, hyaluronic acid, gelatin, terpenes, gelatin, gluten, and polyhydroxyalkanoates (PHAs) from plants, animals, algae, microorganisms and synthetic biopolymers, including polycaprolactone (PCL), poly(butylene succinate) (PBS), poly(lactic-co-glycolic acids) (PLGA), and polylactic acids (PLA), have been used in nanocomposite materials for various applications [1][2][3][6][7][8].
Starch is one of the most abundant natural polymers globally. Starch and its nanocomposites have been extensively studied for their abundance, low cost, ease of processibility, and chemical and physical properties [1][4]. Furthermore, starch can be used in natural or modified form. Native starch has drawbacks, such as poor mechanical properties, high hydrophilicity, and high biodegradability. Thus, researchers are exploring starch modification techniques to improve its properties and develop novel composites [1].
Starch can be modified into nanoparticles and can also undergo various physical (milling, blending with other polymers, extrusion, plasticizers, etc.) and chemical (substitution, graft co-polymerization, cross-linking, oxidation, etherification, esterification, dual modification, etc.) modifications to produce materials with novel properties [9][10][11][12].
Starch can be reinforced with starch nanoparticle/starch nanocrystals and nano polymers such as nanoclay (montmorillonites [MMTs], halloysites nanotubes [HNTs]), carbon nanotubes (CNTs), and nanofibers and nanowhiskers (cellulose, chitin) and metal and metal oxides (TiO2 NPs, ZnO NPs, etc.) to achieve desirable properties and produce potential green sustainable nanocomposite materials [4][7][13]. The addition of nanofillers and additives with antioxidant and antimicrobial properties has been shown to improve or minimally affect biodegradation of starch-based nanocomposites [5][14][15]. Lifecycle assessments on starch and starch-based composites ensure their lower environmental impact and sustainable alternative for petrochemical-based polymers [16][17][18].
2. Starch
Starch is a polysaccharide and is renewable, inexpensive, biodegradable, and readily available. Starch contains two polymers (glucans) known as amylose (10–30%) and amylopectin (70–90%). Amylose is a linear chain of D-glucose units linked by the α-(1,4) glycosylic bonds, while amylopectin is a highly branched and high molecular weight chain composed of D-glucose repeating units linked by α-(1,4) glycosylic bonds and α-(1,6) glycosidic bonds. The amylopectin chain contains 10–60 glucose units, and the side chains consist of 15–45 glucose units with about 5% of α-(1,6) branching points [6][7]. Amylose and amylopectin are radially arranged in an alternating concentric (amorphous and semi-crystalline) ring in starch granules. Amylopectin is radially arranged in granules and contributes to its crystalline nature (double helices region), and single helices amylose is randomly distributed among amylopectin clusters. Amylose and the branching point of amylopectin form the amorphous region [19][20][21]. Figure 1 illustrates the structure of the starch granule and the chemical structure of amylopectin and amylose.
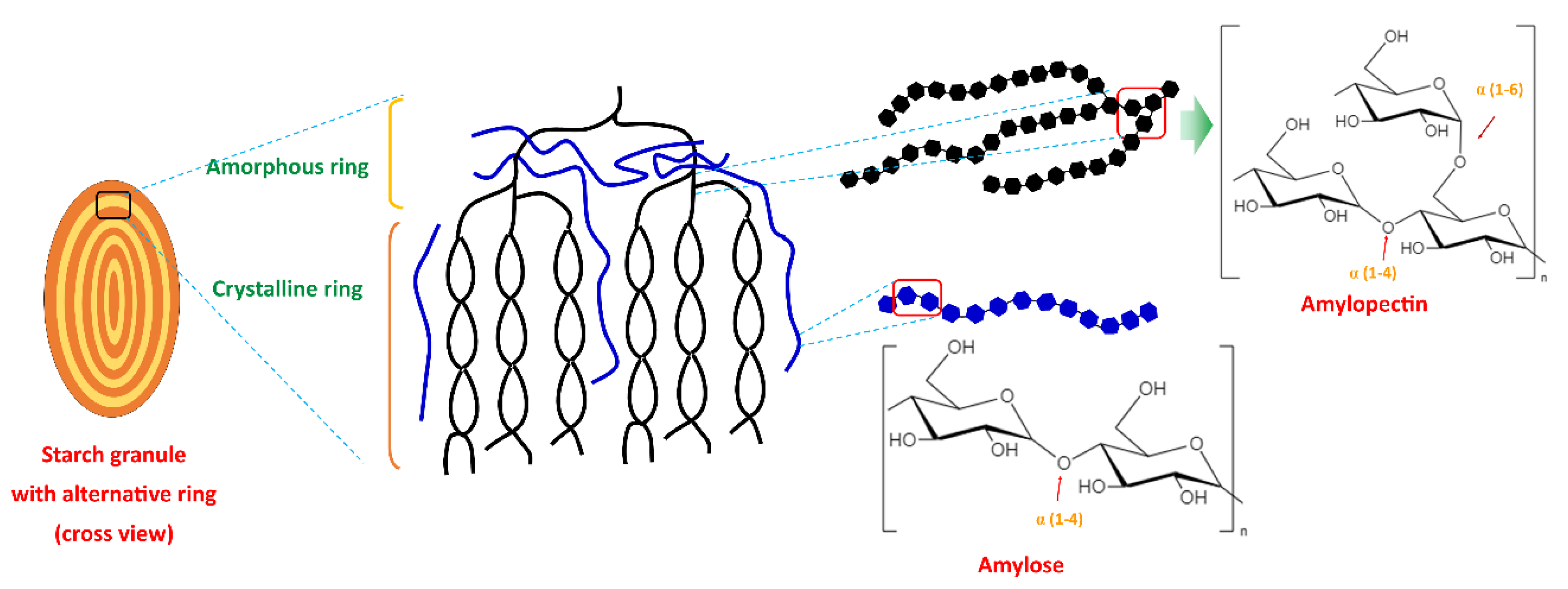
Figure 1. Starch granule structure and the chemical structure of amylopectin and amylose.
Starch is a primary energy source in plants, which is stored in various parts, including the roots, tubers, seeds, and stems [6]. Various plant sources, such as corn, potato, wheat, cassava, rice, corn, barley, rye, millet, peas, mung beans, lentils, arrowroot, sago, sorghum, banana, yam, and many others, are utilized to obtain starch [22][23][24].
Starches from different sources show variation in their chemical composition (α-glucans, moisture, lipids, proteins, and phosphorylated residues), the structure of glucan components (amylose and amylose), and starch granule size and shape due to genetic and environmental factors [25][26].
Starch granules’ size and shape can vary with the content, structure, and arrangement of amylose and amylopectin [25]. Starch granules are found in various sizes ranging from 2–150 µm and packed with amylose and amylopectin content. Regular starch granules contain amylose in the range of 15–30% but can be varied in the range of 0–78%. Waxy starch contains lower or no amylose, whereas high-amylose starch consists of more than 50% amylose [7][23]. Table 1 shows the amylose contents of various starch sources.
Table 1. Amylose and amylopectin contents of starch from various sources.
| Starch Source | Amylose (%) | Reference |
|---|---|---|
| Arrowroot | 35.52 | [27] |
| Banana (pulp) | 16.36–26.2 | [28][29][30] |
| Banana (peel) | 25.7 | [29] |
| Barley (regular) | 24.7 | [31] |
| Cassava | 2.5–32.12 | [28][32][33] |
| Corn | 0–79.05 | [28][32] |
| Maize (normal) | 22.7–28.9 | [31][34] |
| Maize (waxy) | 0.18 | [34] |
| Maize (high amylose content) | 35.5–64.8 | [34] |
| Potato | 18.6–31.9 | [28][31][32][33] |
| Rice | 0.1–28.7 | [20][35] |
| Sweet potato (normal) | 30.4 | [36] |
| Wheat | 6.2–22.8 | [31][32] |
Starch-based hydrogel is formed via gelatinization of starch during heating with excess water and followed by three-dimensional network formation by retrogradation [37]. Gelatinization of starch is an irreversible process that occurs through the absorption of water and disruption of the crystalline structure of starch granules by hydrogen bond breakage, swelling, the disintegration of starch granules, leaching of amylose that increases viscosity and solubilization of starch molecules [32][35][37].
Amylose and amylopectin content, amylopectin structure (molar mass or chain length), and starch granule size influence the chemical, physical, optical/transparency, and functional properties (water uptake, swelling, gelatinization, pasting [pasting viscosity and temperature], retrogradation, and susceptibility to enzymatic hydrolysis of starch [7][20][23][36][38].
Amylopectin contributes to water absorption, swelling, and pasting of starch granules, whereas amylose hinders the swelling property in the presence of lipids, thus preventing gelatinization power [32][38]. Furthermore, short-chain amylopectin showed better swelling power than that of long-chain amylopectin, indicating that starch with higher crystallinity reduces the swelling power [38]. Smaller granule size increases hydration, thus increasing the swelling, viscosity, and gelatinization properties [26].
Amylose content is negatively correlated with swelling power, gelatinization temperature, and the enthalpy of gelatinization required to disrupt the crystalline structure [35]. Waxy starch has a higher degree of crystallinity and higher gelatinization temperature than starch with high amylose content [31][35]. Amylose in starch has a high tendency for retrogradation due to its linear structure. However, the retrogradation properties of starch are mainly determined by the degree of crystallinity and gelatinization temperature than the amylose content [35].
Amylose–amylopectin ratio also influences thermal, mechanical, and barrier properties. Basiak et al. [23] reported that potato starch, containing lower amylose (20%) than that of wheat (25%) and corn (27%) starch, exhibited greater mechanical properties and lower water solubility, water vapor, and oxygen permeability. Other than that, optical properties were influenced by the amylose/amylopectin ratio: the potato (lower amylose) film was transparent, whereas corn and wheat films were opalescent.
However, applications of starch have been limited due to their poor performance, such as through their brittleness, high water sensitivity, poor gas and moisture barrier, susceptibility to retrogradation, high viscosity, and limited solubility [13][39]. Therefore, plasticizers, chemical modifiers, and incorporating nanofillers, such as starch nanoparticles, nanoparticles, nanoclay, nanofibers, and others, have been used to improve the properties of starch [39].
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362.
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva Lactuca Algae Based Chitosan Bio-Composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242.
- Puiggalí, J.; Katsarava, R. Chapter 7—Bionanocomposites. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–272. ISBN 978-0-323-46153-5.
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Thakur, V.K. Recent Advances in Starch–Clay Nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345.
- Balakrishnan, P.; Geethamma, V.G.; Gopi, S.; Thomas, M.G.; Kunaver, M.; Huskić, M.; Kalarikkal, N.; Volova, T.; Rouxel, D.; Thomas, S. Thermal, Biodegradation and Theoretical Perspectives on Nanoscale Confinement in Starch/Cellulose Nanocomposite Modified via Green Crosslinker. Int. J. Biol. Macromol. 2019, 134, 781–790.
- Arora, B.; Bhatia, R.; Attri, P. 28—Bionanocomposites: Green Materials for a Sustainable Future. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Wilmington, NC, USA, 2018; pp. 699–712. ISBN 978-0-12-811033-1.
- García, N.L.; Famá, L.; D’Accorso, N.B.; Goyanes, S. Biodegradable Starch Nanocomposites. In Eco-friendly Polymer Nanocomposites: Processing and Properties; Thakur, V.K., Thakur, M.K., Eds.; Advanced Structured Materials; Springer: New Delhi, India, 2015; pp. 17–77. ISBN 978-81-322-2470-9.
- Mohammad, F.; Arfin, T.; Bwatanglang, I.B.; Al-lohedan, H.A. Starch-Based Nanocomposites: Types and Industrial Applications. In Bio-Based Polymers and Nanocomposites: Preparation, Processing, Properties & Performance; Sanyang, M.L., Jawaid, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 157–181. ISBN 978-3-030-05825-8.
- BeMiller, J.N. Chapter 5—Physical Modification of Starch. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; pp. 223–253. ISBN 978-0-08-100868-3.
- Gunawardene, O.H.P.; Gunathilake, C.A.; Amaraweera, A.P.S.M.; Fernando, N.M.L.; Manipura, A.; Manamperi, W.A.; Kulatunga, K.M.A.K.; Rajapaksha, S.M.; Gamage, A.; Dassanayake, R.S.; et al. Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch. J. Compos. Sci. 2021, 5, 46.
- Okoli, C.P.; Ofomaja, A.E. Degree of Time Dependency of Kinetic Coefficient as a Function of Adsorbate Concentration; New Insights from Adsorption of Tetracycline onto Monodispersed Starch-Stabilized Magnetic Nanocomposite. J. Environ. Manag. 2018, 218, 139–147.
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832.
- Le Corre, D.; Angellier-Coussy, H. Preparation and Application of Starch Nanoparticles for Nanocomposites: A Review. React. Funct. Polym. 2014, 85, 97–120.
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation Behavior of Starch-PVA Films as Affected by the Incorporation of Different Antimicrobials. Polym. Degrad. Stab. 2016, 132, 11–20.
- Mohan, T.; Devchand, K.; Kanny, K. Barrier and Biodegradable Properties of Corn Starch-Derived Biopolymer Film Filled with Nanoclay Fillers. J. Plast. Film Sheeting 2017, 33, 309–336.
- Venkatesh, G.; Nyflött, Å.; Bonnerup, C.; Lestelius, M. An Economic-Environmental Analysis of Selected Barrier-Coating Materials Used in Packaging Food Products: A Swedish Case Study. Env. Dev. Sustain. 2018, 20, 1483–1497.
- Wani, A.A.; Singh, P. Application of Life Cycle Assessment for Starch and Starch Blends. In Starch-Based Polymeric Materials and Nanocomposites; Ahmed, J., Tiwari, B.K., Imam, S.H., Rao, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-0-429-10818-1.
- Kakadellis, S.; Harris, Z.M. Don’t Scrap the Waste: The Need for Broader System Boundaries in Bioplastic Food Packaging Life-Cycle Assessment—A Critical Review. J. Clean. Prod. 2020, 274, 122831.
- Bertolini, A. (Ed.) Starches: Characterization, Properties, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-14172-0.
- Govindaraju, I.; Zhuo, G.-Y.; Chakraborty, I.; Melanthota, S.K.; Mal, S.S.; Sarmah, B.; Baruah, V.J.; Mahato, K.K.; Mazumder, N. Investigation of Structural and Physico-Chemical Properties of Rice Starch with Varied Amylose Content: A Combined Microscopy, Spectroscopy, and Thermal Study. Food Hydrocoll. 2022, 122, 107093.
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Chapter 5—Structural Features of Starch Granules I. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. 149–192. ISBN 978-0-12-746275-2.
- Alves, Z.; Abreu, B.; Ferreira, N.M.; Marques, E.F.; Nunes, C.; Ferreira, P. Enhancing the Dispersibility of Multiwalled Carbon Nanotubes within Starch-Based Films by the Use of Ionic Surfactants. Carbohydr. Polym. 2021, 273, 118531.
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of Starch Type on the Physico-Chemical Properties of Edible Films. Int. J. Biol. Macromol. 2017, 98, 348–356.
- Chaudhary, A.K.; Vijayakumar, R.P. Synthesis of Polystyrene/Starch/CNT Composite and Study on Its Biodegradability. J. Polym. Res. 2020, 27, 187.
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534.
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The Structural Characteristics of Starches and Their Functional Properties. CyTA—J. Food 2018, 16, 1003–1017.
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta Arundinaceae L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72.
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. The Important Role of Crystallinity and Amylose Ratio in Thermal Stability of Starches. J. Anal. Calorim. 2018, 131, 2555–2567.
- Li, Z.; Guo, K.; Lin, L.; He, W.; Zhang, L.; Wei, C. Comparison of Physicochemical Properties of Starches from Flesh and Peel of Green Banana Fruit. Molecules 2018, 23, 2312.
- Thanyapanich, N.; Jimtaisong, A.; Rawdkuen, S. Functional Properties of Banana Starch (Musa Spp.) and Its Utilization in Cosmetics. Molecules 2021, 26, 3637.
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and Morphological Characterization of Different Starches with Variable Amylose/Amylopectin Ratio. Food Hydrocoll. 2013, 32, 52–63.
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in Research and Applications of Cassava Flour and Starch: A Review. J. Food Sci. Technol. 2019, 56, 2799–2813.
- Han, H.; Hou, J.; Yang, N.; Zhang, Y.; Chen, H.; Zhang, Z.; Shen, Y.; Huang, S.; Guo, S. Insight on the Changes of Cassava and Potato Starch Granules during Gelatinization. Int. J. Biol. Macromol. 2019, 126, 37–43.
- Zhong, Y.; Liu, L.; Qu, J.; Blennow, A.; Hansen, A.R.; Wu, Y.; Guo, D.; Liu, X. Amylose Content and Specific Fine Structures Affect Lamellar Structure and Digestibility of Maize Starches. Food Hydrocoll. 2020, 108, 105994.
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical Properties of Starches from Diverse Rice Cultivars Varying in Apparent Amylose Content and Gelatinisation Temperature Combinations. Food Chem. 2015, 172, 433–440.
- Zhou, W.; Yang, J.; Hong, Y.; Liu, G.; Zheng, J.; Gu, Z.; Zhang, P. Impact of Amylose Content on Starch Physicochemical Properties in Transgenic Sweet Potato. Carbohydr. Polym. 2015, 122, 417–427.
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; Halal, S.L.; De, M.E.; Lim, L.-T.; Dias, Á.R.G.; da Zavareze, E.R. Starch Hydrogels: The Influence of the Amylose Content and Gelatinization Method. Int. J. Biol. Macromol. 2018, 113, 443–449.
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of Granule Size Distribution and Amylopectin Structure with Pasting, Thermal, and Retrogradation Properties in Wheat Starch. J. Agric. Food Chem. 2010, 58, 1180–1188.
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor Indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch—Stärke 2021, 73, 2000218.
More
Information
Subjects:
Agricultural Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
01 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





