| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liuchen Liu | + 1785 word(s) | 1785 | 2020-11-18 06:56:41 | | | |
| 2 | Bruce Ren | Meta information modification | 1785 | 2020-11-25 02:54:44 | | |
Video Upload Options
Supercritical CO2 power cycles have been deeply investigated in recent years. However, their potential in waste heat recovery is still largely unexplored. This paper presents a critical review of engineering background, technical challenges, and current advances of the s-CO2 cycle for waste heat recovery. Firstly, common barriers for the further promotion of waste heat recovery technology are discussed. Afterwards, the technical advantages of the s-CO2 cycle in solving the abovementioned problems are outlined by comparing several state-of-the-art thermodynamic cycles. On this basis, current research results in this field are reviewed for three main applications, namely the fuel cell, internal combustion engine, and gas turbine. For low temperature applications, the transcritical CO2 cycles can compete with other existing technologies, while supercritical CO2 cycles are more attractive for medium- and high temperature sources to replace steam Rankine cycles. Moreover, simple and regenerative configurations are more suitable for transcritical cycles, whereas various complex configurations have advantages for medium- and high temperature heat sources to form cogeneration system. Finally, from the viewpoints of in-depth research and engineering applications, several future development directions are put forward. This review hopes to promote the development of s-CO2 cycles for waste heat recovery.
1. Introduction
Efficient conversion and utilization of energy is an effective pathway to address energy shortage and environmental problems faced by mankind. However, several losses occur during the energy conversion process, starting from the primary energy carrier to the end-user, mainly in the form of waste heat. The waste heat discharged to the environment has high exergy value and also contains large quantities of pollutants,thus, contributing to global warming.The recovery of this waste heat for targeted use can significantly raise the process efficiency and reduce the primary energy consumption [1][2]. Such an energy-saving potential is particularly significant for industrial processes, thermal engines, and mechanical devices [3][4][5]. Take China as an example, the annual energy consumption of the industrial sector accounts for more than 70% of the total national energy consumption, of which at least 50% is converted to waste heat at different temperatures and with different carriers. The estimated waste heat in the cement, iron/steel, and glass industries in China, in 2016, were, 41.0 GWth, 2.9 GWth, and 1.8 GWth, respectively [3].
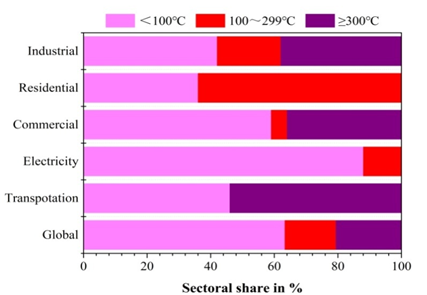
Generally speaking, there are many kinds and forms of waste heat resources. According to the investigation by Galanis et al. [6], waste energy is released from industrial processes to the environment via four main states of matter; namely, liquid streams at temperatures between 50 °C and 300 °C, exhaust at temperatures ranging between 150 °C to 800 °C, steam at temperatures ranging from 100 °C to 250 °C, as well as the process gases and vapors within a temperature range of 80 °C to 500 °C. Furthermore, low-temperature(<350 °C) waste heat accounts for the majority in most end-use energy sectors. As shown in Figure 1, evaluations reveal that 63% of the waste heat streams arise at temperatures below 100 °C, and the largest proportion of which is produced by the electricity sector, followed by the commercial and transportation sector [1].
Figure 1. Sectoral shares of worldwide waste heat distribution [1].
From a thermodynamic point of view, energy from waste heat can be recovered in various ways. Direct heat exchange can take place between waste heat and other fluids, namely the heat transfer fluids for the heating and cooling process [7][8]. Conversion of waste heat into useful power is done using a thermodynamic cycle. The waste heat can be used as a heat source, i.e., the high-temperature side, which is used to heat the working fluid to obtain a gas phase of certain temperature and pressure. This working fluid, or the waste heat fluid itself, can be used to perform expansion work [9][10]. Another method is to raise the temperature of the waste heat to a required value using a heat pump for special applications such as distributed energy systems [11][12]. These diverse pathways face challenges, such as the low-grade and fluctuation and intermittency of heat sources, inefficiencies in the energy conversion process, and cascade utilization of different energy sources.
2. Barriers to Waste Heat Recovery
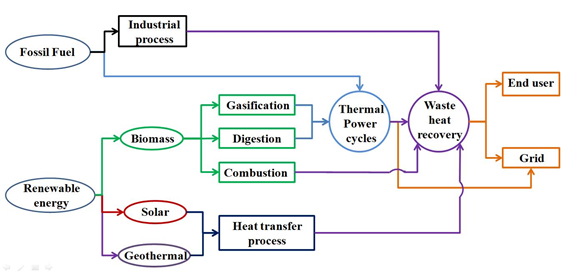
Using waste heat as an energy source is based on many aspects, which are elaborated as follows: Many factors make waste heat recovery very difficult, such as operating principle of heat recovery facilities, user demand, and characteristics of the source of the waste heat. Each method of waste heat recovery is posed with different problems, thus, the technologies face a number of barriers. A graphical representation of the various energy conversion pathways for waste heat is shown in Figure 2. Waste heat is known to mainly originate from two types of sources, i.e., fossil fuel and renewable energy. Most of the waste heat from fossil fuel involves industrial processes while renewable energy can be used directly through an air pre-heater, waste heat boiler, and economizer, and a small part of them need to go through a thermal power cycle before being used [13]. This is one of the main reasons for the temperature grade diversification of waste heat resources. Meanwhile, as mentioned above, the diverse forms of waste heat recovery technologies depend on the specific energy form needed by the end-user. At this point, the limitation of space for equipment as well as the economic and environmental boundaries should be taken into account. These abovementioned issues make an efficient waste heat recovery challenging.
Figure 2. Waste heat recovery from various heat sources [13].
From a technical perspective, the following aspects need to be highlighted. First of all, waste heat sources are prone to more fluctuation and intermittence than traditional heat sources, severely affecting the operation stability of the recovery system. This is especially challenging in context to an evaporation process where the energy transfer takes place directly between the heat source and the working fluid. The abrupt fluctuation of the temperature of the heat source during a transient scan cause the variation in physical and chemical properties of the working fluid [14]. The indirect evaporation with an intermediate heat transfer fluid between the fluctuating waste heat stream and the working fluid can solve such problem effectively. Moreover, the development of a proper control strategy can also avoid large fluctuations in the performance of the system [15][16].
A second technical aspect involves systems that have more than one heat source, such as the waste heat from internal combustion engines(ICE) and from other complex industrial processes. For such a case, a waste heat recovery system must be designed and optimized for the multiple heat sources. In ICEs, the engine coolant is low-grade waste heat with temperature below 100 °C which is used as a preheat source, and the exhaust gas is high-grade waste heat with temperatures ranging from 500 °C to 700 °C [17]. Technical solutions involve matching the characteristic temperature drop of each source of waste heat.
Thirdly, due to the variety and complexity of waste heat resources, where the waste heat is present, the chemical composition of the waste heat carrier is important when considering recovery from steam generation [18][19]. Furthermore, the waste heat sources are usually dispersed geographically, which makes it challenging to integrate the recovery system with the original industrial process. In these cases, additional problems like the pressure drop of the flue gases in the heat absorber [20] should be taken into account.
To realize an economical and efficient waste heat recovery process, the barriers to the wide application of recovery technology for low-temperature waste heat have been described as follows: (1) The mismatch of energy grade of the waste heat resource and user demand in terms of time and space, (2) the intermittent of waste heat sources, and (3) the lack of a comprehensive methodology for global energy transfer and transformation optimization at the system level [21][22][23], especially for larger zones.
3. State-of-the-Art Thermodynamic Cycles for Waste Heat Recovery
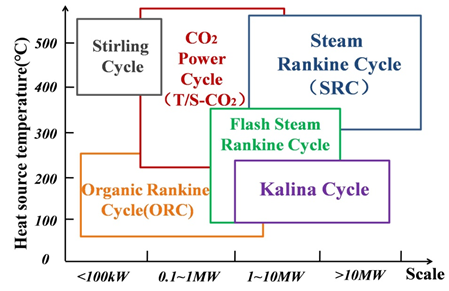
Various implementation pathways can be adopted with respect to the quality of waste heat sources [2]. The main approach is to utilize the available waste heat as heat source to drive the thermodynamic cycle and convert the thermal energy into useful power. One of the most important indicators for choosing a proper cycle is the operation temperature. Figure 3 depicts the possible thermodynamic cycles and the range of their relevant operation temperatures. The most suitable waste heat temperature range for the Steam Rankine Cycle(SRC) is medium-high temperature, at about more than 300 °C. Systems for lower temperature heat sources are much less cost-effective and may lead to surface corrosion problem. Furthermore, the Organic Rankine Cycle(ORC) which uses lower boiling point organic fluids, has been also extensive investigated in last few decades. A suitable waste heat temperature to obtain competitive efficiency of the ORC ranges from 90 °C to 250 °C. Additionally, the Kalina Cycle uses a mixture of ammonia and water as working fluid to closely meet the temperature profile of waste heat sources during the phase change heat transfer process, between 100 °C and 450 °C.
Recent years, the use of CO2 power cycles for waste heat recovery are gained more and more attentions. Such cycles are usually operated under trans-critical or supercritical conditions,due to the relatively low critical point of carbon dioxide. Its main advantages can be summarized as follows. First of all, the CO2 cycles are suitable for the recovery of waste heat sources with a wide range of temperatures, while the steam based Rankine cycle is only efficientto recovery waste heat at high-temperature. Therefore, the main propose to investigate the CO2 power cycles was not only to replace the SRC [24] but also as a more wide range to waste heat recovery temperatures [25]. The special physical properties of CO2 can reduce the heat transfer loss between working fluid and heat source [26]. Furthermore, the CO2 is a non-combustible and nontoxic refrigerant which frees up space for the cycle operation temperature to rise [27][28]. In addition, the CO2 power cycles occupy a smaller space as compared with SRC, which has a large working fluid steam volume and therefore shows great potential for system downsizing and lightweight. Accordingly, the chemistry and condensingprocesses are also simpler [28][29].
Figure 3. Thermodynamic cycles for waste heat recovery at various temperature-levels and scales.
Besides to thermodynamic cycles, other choices is to use thermoelectric (TE) materialsfor power conversion of waste heat. Relevant investigations focus on the thermoelectric generator [30], electrochemical systems [31], thermogalvanic cells [32] and pyroelectric energy conversion [33], which are still under study and has no large scale market application.
In summary, with respect to conventional thermodynamic cycles, supercritical carbon dioxide(s-CO2) cycles offer a wide range of operating temperatures and potentially higher efficiencies, and a much smaller environmental footprint [34][35][36]. Power cycles running on s-CO2 have received wide attention, and the number of publications has risen exponentially in recent years. However, the great potential for this technology in waste heat recovery remains to be further explored, which makes this comprehensive review of recent advances in s-CO2 cycles for waste heat recovery highly relevant.
References
- Formann, C.; Muritala, I.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sust. Energy Rev. 2016, 1, 1568–1579.
- Xu, Z.; Wang, R.; Yang, C. Perspectives for low-temperature waste heat recovery. Energy2019, 1761, 1037–1043.
- Lu, H.; Price, L.; Zhang, Q. Capturing the invisible resource: Analysis of waste heat potential in Chinese industry. Appl. Energy 2016, 161, 497–511.
- Hong, G.; Pan, T.; Chan, D.; Liu, I. Bottom-up analysis of industrial waste heat potential in Taiwa. Energy 2020, 198, 117393.
- Yong, Z.; Bashir, M.; Ng, C.; Sethupathi, S.; Lim, J.; Show, P. Sustainable waste-to-energy development in malaysia: Appraisal of environmental, financial, and public issues related with energy recovery from municipal solid waste. Processes 2019, 7, 676.
- Galanis, N.; Cayer, E.; Roy, P.; Denis, E.; Désilets, M. Electricity generation from low temperature sources. J.Appl. Fluid. Mech. 2009, 2, 55–67.
- Sun, F.; Li, J.; Fu, L.; Li, Y.; Wang, R.; Zhang, S. New configurations of district heating and cooling system based on absorption and compression chillers driven by waste heat of flue gas from coke ovens. Energy 2020, 193, 116707.
- Hsua, C.; Lin, T.; Liang, D.; Lai, C.; Chen, S. Optimization analysis of waste heat recovery district cooling system on a remote island: Case study Green Island. Energy Convers. Manag. 2019, 183, 660–670.
- Liao, G.; Jiaqiang, E.; Zhang, F.; Chen, J.; Leng, E. Advanced exergy analysis for Organic Rankine Cycle-based layout to recover waste heat of flue gas. Appl. Energy 2020, 266, 114891.
- Lin, S.; Zhao, L.; Deng, S.; Ni, J.; Ma, M. Dynamic performance investigation for two types of ORC system driven by waste heat of automotive internal combustion engine. Energy 2019, 169, 958–971.
- Wang, M.; Deng, C.; Wang, Y.; Feng, X. Exergoeconomic performance comparison, selection and integration of industrial heat pumps for low grade waste heat recovery. Energy Convers. Manag. 2020, 2071, 112532.
- Han, X.; Zou, H.; Jiang, W.; Tian, C.; Huang, G. Investigation on the heating performance of the heat pump with waste heat recovery for the electric bus. Renew. Energ. 2020, 152, 835–848.
- Johnson, I.; Choate, W.; Davidson, A. Waste Heat Recovery. Technology and Opportunities in US Industry; BCS, Inc.: Laurel, MD, USA, 2008.
- Zhai, H.; An, Q.; Shi, L.; Lemort, V.; Quoilin, S. Categorization and analysis of heat sources for organic Rankine cycle systems. Renew. Sust. Energy Rev. 2016, 64, 790–805.
- Manente, G.; Toffolo, A.; Lazzaretto, A.; Paci, M. An Organic Rankine Cycle off-design model for the search of the optimal control strategy. Energy 2013, 58, 97–106.
- Quoilin, S.; Aumann, R.; Grill, A.; Schuster, A.; Lemort, V.; Spliethoff, H. Dynamic modeling and optimal control strategy of waste heat recovery organic Rankine cycles. Appl. Energy 2011, 88, 2183–2190.
- Wang, T.; Zhang, Y.; Zhang, J.; Peng, Z.; Shu, G. Comparisons of system benefits and thermo-economics for exhaust energy recovery applied on a heavy-duty diesel engine and a light-duty vehicle gasoline engine. Energy Convers. Manag. 2014, 84, 97–107.
- Pandis, P.; Papaioannou, S.; Siaperas, V.; Terzopoulos, A.; Stathopoulos, V. Evaluation of Zn- and Fe-rich organic coatings for corrosion protection and condensation performance on waste heat recovery surfaces. Int. J. Therm. Fluid. 2020, 3, 100025.
- Li, M.; Tang, S.; Wang, F.; Zhao, Q.; Tao, W. Gas-side fouling, erosion and corrosion of heat exchangers for middle/low temperature waste heat utilization: A review on simulation and experiment. Appl. Therm. Eng. 2017, 126, 737–761.
- Lei, B.; Wu, Y.; Ma, C.; Wang, W.; Zhi, R. Theoretical analyses of pressure losses in organic Rankine cycles. Energy Convers. Manag. 2017, 153, 157–162.
- Hipólito-Valencia, B.; Rubio-Castro, E.; Ponce-Ortega, J.; Serna-González, M.; Nápoles-Rivera, F.; El-Halwagi, M. Optimal integration of organic Rankine cycles with industrial processes. Energy Convers. Manag. 2013, 73, 285–302.
- Stijepovic, M.; Linke, P. Optimal waste heat recovery and reuse in industrial zones. Energy 2011, 36, 4019–4031.
- Dou, Y.; Togawa, T.; Dong, L.; Fujii, M.; Ohnishi, S.; Tanikawa, H.; Fujita, T. Innovative planning and evaluation system for district heating using waste heat considering spatial configuration: A case in Fukushima, Japan. Resour. Conserv. Recycl. 2018, 128, 406–416.
- Li, C.; Wang, H. Power cycles for waste heat recovery from medium to high temperature flue gas sources—From a view of thermodynamic optimization. Appl. Energy 2016, 180, 707–721.
- Poullikkas, A. An overview of current and future sustainable gas turbine technologies. Renew. Sustain. Energy Rev. 2005, 9, 409–443.
- Chen, Y.; Lundqvist, P. The CO2transcritical power cycle for low grade heat recovery-discussion on temperature profiles in system heat exchangers. In Proceedings of the ASME 2011 Power Conference (POWER2011), Denver, CO, USA, 12–14 June 2011.
- Boewe, D.; Bullard, C.; Yin, J.; Hrnjak, P. Contribution of internal heat exchanger to transcritical R-744 cycle performance. HVAC&R Res. 2001, 7, 155–168.
- Marcus, Y. Some Advances in Supercritical Fluid Extraction for Fuels, Bio-Materials and Purification. Processes 2019, 7, 156.
- Bae, S.; Lee, J.; Ahn, Y.; Lee, J. Preliminary studies of compact Brayton cycle performance for small modular high temperature gas-cooled reactor system. Ann. Nucl. Energy 2015, 75, 11–19.
- Bell, L. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461.
- Lee, S.; Yang, Y.; Lee, H.; Ghasemi, H.; Kraemer, D.; Chen, G.; Cui, Y. An electrochemical system for efficiently harvesting low-grade heat energy. Nat. Commun. 2014, 5, 3942.
- Duan, J.; Feng, G.; Yu, B.; Li, J.; Chen, M.; Yang, P.; Feng, J.; Liu, K.; Zhou, J. Aqueous thermogalvanic cells with a high Seebeck coefficient for low-grade heat harvest. Nat. Commun. 2018, 9, 5146.
- Pandya, S.; Wilbur, J.; Kim, J.; Gao, R.; Dasgupta, A.; Dames, C.; Martin, L. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nat. Mater. 2018, 17, 432.
- Feher, E. The supercritical thermodynamic power cycle. Energy Convers. Manag. 1968, 8, 85–90.
- Siddiqui, M.; Taimoor, A.; Almitani, K. Energy and Exergy Analysis of the S-CO2Brayton Cycle Coupled with Bottoming Cycles. Processes 2018, 6, 153.
- Siddiqui, M.; Almitani, K.; Energy Analysis of the S-CO2Brayton Cycle with Improved Heat Regeneration. Processes 2019, 7, 3.