Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thankhoe Abram Rants’o | -- | 1991 | 2022-10-26 22:50:59 | | | |
| 2 | Peter Tang | Meta information modification | 1991 | 2022-10-31 02:18:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rants’o, T.A.; Koekemoer, L.L.; Panayides, J.; Zyl, R.L.V. Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors. Encyclopedia. Available online: https://encyclopedia.pub/entry/31853 (accessed on 13 January 2026).
Rants’o TA, Koekemoer LL, Panayides J, Zyl RLV. Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors. Encyclopedia. Available at: https://encyclopedia.pub/entry/31853. Accessed January 13, 2026.
Rants’o, Thankhoe A., Lizette L. Koekemoer, Jenny-Lee Panayides, Robyn L. Van Zyl. "Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors" Encyclopedia, https://encyclopedia.pub/entry/31853 (accessed January 13, 2026).
Rants’o, T.A., Koekemoer, L.L., Panayides, J., & Zyl, R.L.V. (2022, October 28). Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors. In Encyclopedia. https://encyclopedia.pub/entry/31853
Rants’o, Thankhoe A., et al. "Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors." Encyclopedia. Web. 28 October, 2022.
Copy Citation
The insect's nervous system is critical for its functionality. The cholinergic system, of which acetylcholinesterase (AChE) is a key enzyme, is essential to the Anopheles (consisting of major malaria vector species) nervous system. Furthermore, the nervous system is also the primary target site for insecticides used in malaria vector control programs. Insecticides, incorporated in insecticide-treated nets and used for indoor residual spraying, are a core intervention employed in malaria vector control.
malaria
insecticides
terpenoids
acetylcholinesterase
1. Introduction
Malaria is a devastating disease caused by a protozoan parasite, namely Plasmodium falciparum which is the major causative agent in the pathogenesis of this infectious disease[1][2]. Anopheles vectors are infected with malaria after they ingest blood from an infected human host. The female Anopheles vectors effectively bite the human hosts between dusk and dawn[3] and it is during this time that she ingests gametocytes. The Plasmodium gametocytes develop into an oocyst in the mosquito midgut, which then matures into sporozoites. The sporozoites are released into the hemolymph and migrate to the salivary glands[1][4]. This parasite developmental process within the vector takes approximately 11–16 days before the female mosquito is able to transmit the parasite to the next human host during a blood feeding. This means that a long lifespan of the Anopheles vector is required for the successful completion of the parasite development and reinfection of the human host. Vertebrate blood is needed every 2–3 days by the female mosquito for nutrition, as well as egg development. The eggs are oviposited into water and fertile eggs hatch into larvae a few days later. Larvae will develop into pupae and finally adults will emerge after a few days[3]. There are more than 400 Anopheles species of which about 30 are major malaria vectors. The African Anopheles vectors have both long lifespans and a higher preference for human feeding and, collectively, these account for the high malaria cases and mortality that is recorded in Africa[3][5]. Other factors, such as climate conditions and political and economic stability, also affect the intensity of transmission and enhance the problem[3].
The malaria vectors have long been controlled by using insecticides. Insecticide classes include organophosphates, carbamates, pyrethroids, organochlorines and neonicotinoids[6]. Larvicides including insect growth inhibitors as well as bacterial larvicides, such as Bacillus thuringiensis subspecies israelensis, Bacillus sphaericus and spinosyns from Saccharopolyspora species, have also gained popularity in mosquito control activities[6][7][8]. The implementation of large-scale larviciding is however challenging in Sub-Saharan Africa and these may be used as a complementary intervention[6][9][10][11]. The Anopheles vectors have developed substantial resistance against almost all current insecticides[12][13][14][15]. To compound the issue, the commercial development of insecticides through various and often complicated synthetic mechanisms is expensive and time-consuming[16][17]. The researchers propose that the identification of potential insecticides from natural product resources, such as essential oils (EOs), is a relatively cost-effective and faster alternative. Target identification and the corresponding mechanism of action are critical components of the drug discovery process[18]. Acetylcholinesterase (AChE) is a validated target in the insect nervous system and inhibitors of this critical enzyme have been useful in the control of malaria vectors for over eighty years[6][19][20].
2. Malaria Vector Control
The early vector control strategies adopted vast activities to reduce larval populations, which included, amongst others, the drainage of breeding sites such as swamps or the application of copper (II) acetoarsenite (Paris green), a highly toxic inorganic compound to the breeding sites[21][22]. In addition, the screening of windows and doors to prevent vectors entering houses and the use of mosquito nets have been at the forefront in protecting people against mosquito bites[6]. The plant-based insecticides were the first preparations used historically. Pyrethrins extracted from the flowers of Chrysanthemum cinerariifolium and Chrysanthemum roseum were used against indoor Anopheles mosquitoes in the 19th century[23][24]. However, the structural modifications of the natural pyrethrins and the generation of first synthetic pyrethroids were first reported in the period 1924 to 1970[25]. The discovery of an organochloride, namely, dichlorodiphenyltrichloroethane (DDT), was reported in 1939[25][26]. DDT has been highly effective against malaria vectors. However, in recent times increasing safety concerns have seen it being replaced in many countries by newer insecticides with reduced toxicity profiles[27][28][29].
Currently, malaria vector control adopts an integrated vector management program through the use of insecticides targeting both the larval and adult stages[6][27]. This is achieved through two main interventions, namely, insecticide-treated mosquito nets and indoor residual spraying, with additional interventions including larviciding[6]. The insecticide-treated nets provide both a physical barrier and insecticidal activity against Anopheles vectors. Indoor residual spraying (IRS) on the other hand, provides host protection through the Anopheles insecticidal effect[3]. Pyrethroids, pyrethroid-PBO combinations and pyrroles are the only insecticide classes used for the insecticide-treated nets, as the latter insecticides pose a low toxicity risk to humans[30]. The pyrethroids used in IRS include deltamethrin, alpha-cypermethrin, etofenprox, lambda-cyhalothrin, bifenthrin and cyfluthrin, while the organochlorines include DDT. On the other hand, the organophosphates approved by WHO for IRS include malathion, fenitrothion, pirimiphos-methyl and the carbamates such as propoxur and bendiocarb[6][31][32][33].
2.1. Insecticide Resistance in Main African Malaria Vectors
Insecticide resistance has been reported in all of the main African malaria vectors and this resistance against WHO approved insecticidal agents is rapidly increasing in intensity and geographical distribution[5][34]. An overview of mosquito resistance has been highlighted below. To keep this brief, only a few examples will be provided to explain the extent of the problem mainly on the African continent. Insecticide resistance in the main vector species has been reported for pyrethroids[34][35][36][37], organochlorides[36][38][39][40], organophosphates[12][41] and carbamates[20][36][40][42].
Common insecticide resistance markers associated with pyrethroid and organochloride resistance include the L1014F and L1014S mutation of the voltage-gated sodium channel gene, known as knockdown resistance (kdr). These mutations shift activation voltage dependence of sodium channels stabilizing them in the closed state. This antagonizes the action of pyrethroids and organochlorines since these compounds bind to open sodium channels[25][38][43]. Apart from the kdr mutations, elevated metabolic enzymes, including P450 monooxygenases, glutathione-S-transferases and non-specific esterases, also convey high resistance to pyrethroids and organochlorides[13][37][44][41]. On the other hand, the resistance mechanism commonly conferring organophosphate and carbamate resistance is a single point polymorphism resulting from glycine conversion to a serine residue at position 119 (G119S; Torpedo californica AChE numbering) or more precisely, position 280 (G280S; Anopheles gambiae AChE numbering) in the AChE target[38][43][45][46]. Resistance mechanisms often prevent the intended biological activity of a specific insecticide.
2.2. Modes of Action of Main Insecticides Used in Malaria Vector Control
Regardless of their small size, insects have a high surface area for the penetration and subsequent systemic distribution of an insecticide from contact exposure. Furthermore, the small size generates short pathways to the insect’s nervous system and as a result, most insecticides act on the insect’s nervous system[47]. Organochlorines act specifically on the peripheral nervous system, where they bind and stabilize the open voltage-gated sodium channels[25]. The stabilized open state of the sodium channels allows for continuous sodium influx and prolonged action potentials leading to spontaneous neuronal firings succeeded by muscle twitches and sustained body tremors[48]. In contrast to the organochlorines, pyrethroids act on both the peripheral and central nervous systems; however, they act in a similar manner to prevent the closing of the voltage-gated sodium channels, resulting in continuous neuronal discharges followed by paralysis[48]. Similarly, the organophosphates and carbamates also exert their effects on the central nervous system. However, these insecticide classes inhibit acetylcholinesterase, a principal enzyme in the insect nervous system, which leads to an increase in the neurotransmitter ACh levels in the synapse. This leads to enhanced ACh effects on the cholinergic receptors resulting in constant neurotransmission and neuronal hyperexcitation[49]. On the other hand, the neonicotinoids enhance cholinergic activity by acting as agonists on nicotinic acetylcholine receptors (nAChRs). Similarly, this disrupts neuronal transmission in the insect nervous system, causing paralysis and subsequent insect death[50].
2.3. Insect Nervous System
The insect nervous system is composed of central, visceral and peripheral nervous systems[51]. The insect central nervous system (CNS) is composed of the ventral nerve cord and brain connected to various ganglia including supra- and sub-esophageal ganglia, thoracic ganglia and abdominal ganglia (Figure 1). The sub-esophageal ganglion transmits impulses to the mouthparts and salivary glands. The insect brain is composed of three cephalic neuromeres, including the protocerebrum, deutocerebrum and tritocerebrum. The deutocerebrum carries out olfactory and sensory functions through the antennae; where the olfactory signal transduction is important in host identification and interaction by the insect. The tritocerebrum nerves innervate the ventral nerve cord and internal organs including the anterior digestive canal[51][52][53]. The insect’s peripheral nervous system, commonly referred to as a stomatogastric nervous system is composed of the peripheral ganglia complex and nerves that innervate visceral organs. This system mainly controls food intake and digestion. Generally, the insect CNS ganglia receive sensory impulses from the appendages and body cuticle after which the efferent signals are sent to the body muscles, internal organs and genitalia[54][55]. The protocerebrum controls the insect’s vision through compound eyes and ocelli. Most importantly, neurosecretory cells are located in the protocerebrum[55][56]. Most insecticides including the organophosphates and carbamates affect neurotransmitter secretion and action[56][57].

Figure 1. The nervous system ganglia of Anopheles[51].
2.4. Molecular Characterization of Acetylcholinesterase
There are apparent AChE structural differences between insects and mammals. These span from their distinct genomics, amino acid sequences to their active and peripheral anionic site conformations[58]. Recent biochemical studies have revealed critical differences between the Anopheles AChE and human AChE that could serve as potential drug targets for directed insecticide design.
The amino acid sequence of Anopheles AChE is reported to be 48–49% identical to that of the human AChE[59][60]. Unlike humans where there is a single ace gene coding for AChE, mosquitoes have two ace genes, ace-1 and ace-2, coding for AChE1 and AChE2 enzymes, respectively[61][62]. These genes are crucial in all life stages of the mosquito, ranging from egg through to adult stages[63]. AChE1 is the main catalytic enzyme, while AChE2 is involved in non-catalytic activities such as reproduction. As a result, target site insensitivity on insect AChEs, such as G280S genotype, is linked to mutations in ace-1 but not ace-2[46][61][57]. AChE is characterized by a deep and narrow active-site gorge (Figure 2). There are differences in these gorge structures between Anopheles and human AChEs and this may affect ligand binding and specificity[46][59][64]. Notably, a free cysteine residue (Cys447) is available at the entrance to the active site gorge of Anopheles AChE (Figure 2A,B), but not in human AChE. Instead, a human AChE has a bulky phenylalanine (Phe295) at the active site entrance (Figure 2C). Additionally, in Anopheles AChE, a smaller aspartic acid residue (Asp602; Figure 2A,B) replaces a larger tyrosine residue (Tyr449) at the base of the active site gorge[46]. Moreover, a conserved arginine residue (Arg339; not shown in order to maintain the catalytic side resolution) has also been identified in Anopheles AChE[65]. In addition, the displayed An. gambiae AChE catalytic site in Figure 2B shows a G280S mutated site (pointed).
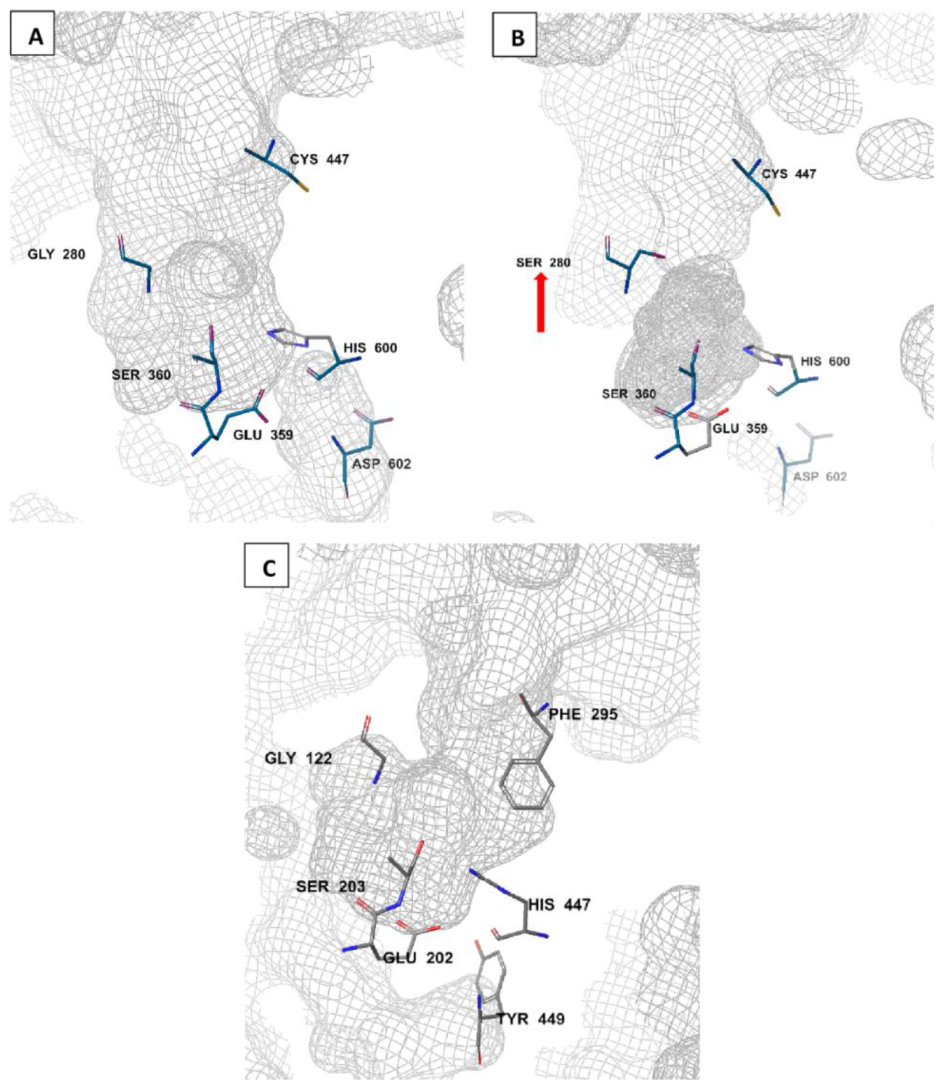
Figure 2. Molecular comparison of An. gambiae wild-type (A) and resistant (B) AChE catalytic sites (PDB IDs: 5YDI and 6ARY, respectively) to the human AChE (PDB ID: 7E3H) (C). generated by Schrodinger’s Maestro 2018-2 software (New York, NY, USA). The G280S mutation is shown (red arrow) in the resistant Anopheles AChE phenotype (B)[46].
2.5 Acetylcholinesterase Inhibition in Anopheles
The catalytic site in Anopheles is characterized with a catalytic triad made of His-Ser-Glu (His600-Ser360-Glu359; Figure 3A) amino acid combination. The catalytic serine (Ser360;
Figure 2A) is the target for covalent insecticides, including organophosphates and carbamates[56][57]. These insecticides establish a covalent bond with AChE through phosphorylation and carbamoylation, respectively[66]. The Anopheles resistance to the anticholinesterase insecticide classes is usually caused by ace-1 G280S mutation (Figure 3B) and metabolic resistance resulting from the elevated levels of monooxygenases, glutathione S-transferases and general esterases[57][67][68][69]. Given the widespread resistance that has largely rendered organophosphates and carbamates noneffective, there is an urgent need to identify novel anticholinesterase insecticides. AChE has proven to be a valid target in Anopheles vectors[59] and EOs have also shown to be the promising sources of novel insecticides[70][71][72]
Figure 2A) is the target for covalent insecticides, including organophosphates and carbamates[56][57]. These insecticides establish a covalent bond with AChE through phosphorylation and carbamoylation, respectively[66]. The Anopheles resistance to the anticholinesterase insecticide classes is usually caused by ace-1 G280S mutation (Figure 3B) and metabolic resistance resulting from the elevated levels of monooxygenases, glutathione S-transferases and general esterases[57][67][68][69]. Given the widespread resistance that has largely rendered organophosphates and carbamates noneffective, there is an urgent need to identify novel anticholinesterase insecticides. AChE has proven to be a valid target in Anopheles vectors[59] and EOs have also shown to be the promising sources of novel insecticides[70][71][72]
References
- Shigeharu Sato; Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology. Journal of Physiological Anthropology 2021, 40, 1-13, 10.1186/s40101-020-00251-9.
- Malaria . World Health Organization. Retrieved 2022-10-28
- James M. Crutcher, Stephen L. Hoffman. Malaria; Samuel Baron, Ed., Eds.; University of Texas Medical Branch: Galveston, TX, USA, 1996; pp. 1-17.
- Ryan C. Smith; Marcelo Jacobs-Lorena; Plasmodium-Mosquito Interactions. Advances in Insect Physiology 2010, 39, 119-149, 10.1016/b978-0-12-381387-9.00004-x.
- World Malaria Report 2020: 20 Years of Global Progress and Challenges . World Health Organization. Retrieved 2022-10-28
- Guidelines for Malaria Vector Control. . World Health Organization. Retrieved 2022-10-28
- Herbert A Kirst; The spinosyn family of insecticides: realizing the potential of natural products research. The Journal of Antibiotics 2010, 63, 101-111, 10.1038/ja.2010.5.
- Christophe Antonio-Nkondjio; Nino Ndjondo Sandjo; Parfait Awono-Ambene; Charles S. Wondji; Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: key parameters for success. Parasites & Vectors 2018, 11, 57, 10.1186/s13071-018-2627-9.
- Peter Dambach; Issouf Traoré; Achim Kaiser; Ali Sié; Rainer Sauerborn; Norbert Becker; Challenges of implementing a large scale larviciding campaign against malaria in rural Burkina Faso – lessons learned and recommendations derived from the EMIRA project. BMC Public Health 2016, 16, 1-7, 10.1186/s12889-016-3587-7.
- Nina Berlin Rubin; Leonard E.G. Mboera; Adriane Lesser; Marie Lynn Miranda; Randall Kramer; Process Evaluation of a Community-Based Microbial Larviciding Intervention for Malaria Control in Rural Tanzania. International Journal of Environmental Research and Public Health 2020, 17, 7309, 10.3390/ijerph17197309.
- Leslie Choi; Silas Majambere; Anne L Wilson; Larviciding to prevent malaria transmission. Cochrane Database of Systematic Reviews 2019, 8, CD012736, 10.1002/14651858.cd012736.pub2.
- Daniel N. Munywoki; Elizabeth D. Kokwaro; Joseph M. Mwangangi; Ephantus J. Muturi; Charles M. Mbogo; Insecticide resistance status in Anopheles gambiae (s.l.) in coastal Kenya. Parasites & Vectors 2021, 14, 1-10, 10.1186/s13071-021-04706-5.
- Abdullahi Muhammad; Sulaiman S. Ibrahim; Muhammad M. Mukhtar; Helen Irving; Maduamaka C. Abajue; Noutcha M. A. Edith; Sabitu S. Da’U; Mark J. I. Paine; Charles S. Wondji; High pyrethroid/DDT resistance in major malaria vector Anopheles coluzzii from Niger-Delta of Nigeria is probably driven by metabolic resistance mechanisms. PLOS ONE 2021, 16, e0247944, 10.1371/journal.pone.0247944.
- Roland Bamou; Edmond Kopya; Leslie Diane Nkahe; Benjamin D. Menze; Parfait Awono-Ambene; Timoléon Tchuinkam; Flobert Njiokou; Charles S. Wondji; Christophe Antonio-Nkondjio; Increased prevalence of insecticide resistance in Anopheles coluzzii populations in the city of Yaoundé, Cameroon and influence on pyrethroid-only treated bed net efficacy. Parasite 2021, 28, 8, 10.1051/parasite/2021003.
- Moussa Keïta; Nafomon Sogoba; Fousseyni Kané; Boissé Traoré; Francis Zeukeng; Boubacar Coulibaly; Ambiélè Bernard Sodio; Sekou Fantamady Traoré; Rousseau Djouaka; Seydou Doumbia; et al. Multiple Resistance Mechanisms to Pyrethroids Insecticides in Anopheles gambiae sensu lato Population From Mali, West Africa. The Journal of Infectious Diseases 2021, 223, S81-S90, 10.1093/infdis/jiaa190.
- Rosemary Lees; Giorgio Praulins; Rachel Davies; Faye Brown; George Parsons; Anthony White; Hilary Ranson; Graham Small; David Malone; A testing cascade to identify repurposed insecticides for next-generation vector control tools: screening a panel of chemistries with novel modes of action against a malaria vector. Gates Open Research 2019, 3, 1464, 10.12688/gatesopenres.12957.2.
- Erika L. Flannery; Arnab K. Chatterjee; Elizabeth Winzeler; Antimalarial drug discovery — approaches and progress towards new medicines. Nature Reviews Genetics 2013, 11, 849-862, 10.1038/nrmicro3138.
- Monica Schenone; Vlado Dančík; Bridget K Wagner; Paul A Clemons; Target identification and mechanism of action in chemical biology and drug discovery. Nature Chemical Biology 2013, 9, 232-240, 10.1038/nchembio.1199.
- Hafez Ayad; George P. Georghiou; Resistance to Organophosphates and Carbamates in Anopheles albimanus Based on Reduced Sensitivity of Acetylcholinesterase12. Journal of Economic Entomology 1975, 68, 295-297, 10.1093/jee/68.3.295.
- Nazaire Aïzoun; Rock Aïkpon; Virgile Gnanguenon; Olivier Oussou; Fiacre Agossa; Gil Germain Padonou; Martin Akogbéto; Status of organophosphate and carbamate resistance in Anopheles gambiae sensu lato from the south and north Benin, West Africa. Parasites & Vectors 2013, 6, 274-274, 10.1186/1756-3305-6-274.
- Maggio, Francesco; Sollod, L. Brianna; Tedford, H. William; King, F. Glenn. Comprehensive Molecular Insect Science - Spider Toxins and their Potential for Insect Control; Gilbert, L.I., Ed., Eds.; Elsevier: Amsterdam: The Netherlands, 2005; pp. 221–238.
- Sur, S.N.; Sarkar, H.; Paris Green as an Anopheline Larvicide.. The Indian medical gazette 1929, 64, 376-378.
- Park G. Ross; Insecticide as a major measure in the control of malaria, being an account of the methods and organizations put into force in Natal and Zululand during the past six years. Quarterly Bulletin of the Health Organization 1936, 5, 114–133.
- Volodymyr Volodymyrovych Oberemok; Kateryna Volodymyrivna Laikova; Yuri Ivanovich Gninenko; Aleksei Sergeevich Zaitsev; Palmah Mutah Nyadar; Tajudeen Adesoji Adeyemi; A short history of insecticides. Journal of Plant Protection Research 2015, 55, 221-226, 10.1515/jppr-2015-0033.
- Davies, T.G.E.; Field L.; Usherwood, P.N.R.; Williamson, M.S.; DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151-162, 10.1080/15216540701352042.
- John E. Casida; Gary B. Quistad; Golden Age of Insecticide Research: Past, Present, or Future?. Annual Review of Entomology 1998, 43, 1-16, 10.1146/annurev.ento.43.1.1.
- Mahbubar Rahman; Insecticide substitutes for DDT to control mosquitoes may be causes of several diseases. Environmental Science and Pollution Research 2012, 20, 2064-2069, 10.1007/s11356-012-1145-0.
- Walter J Rogan; Aimin Chen; Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). The Lancet 2005, 366, 763-773, 10.1016/s0140-6736(05)67182-6.
- Amir Attaran; Rajendra Maharaj; Amir Attaran Richard Liroff; Ethical debate: Doctoring malaria, badly: the global campaign to ban DDT DDT for malaria control should not be banned Commentary: Reduction and elimination of DDT should proceed slowly. BMJ 2000, 321, 1403-1405, 10.1136/bmj.321.7273.1403.
- Katherine Gleave; Natalie Lissenden; Marty Richardson; Leslie Choi; Hilary Ranson; Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database of Systematic Reviews 2018, 11, CD012776, 10.1002/14651858.cd012776.pub2.
- Julie-Anne Tangena; Chantal M.J. Hendriks; Maria Devine; Meghan Tammaro; Anna E. Trett; Ignatius Williams; Adilson José DePina; Achamylesh Sisay; Ramandimbiarijaona Herizo; Hmooda Toto Kafy; et al.Elizabeth ChizemaAllan WereJennifer RozierMichael ColemanCatherine L. Moyes Indoor residual spraying for malaria control in Sub-Saharan Africa 1997 to 2017; an adjusted retrospective analysis. Malaria Journal 2020, 19, 150, 10.21203/rs.2.17606/v2.
- Thomas Syme; Augustin Fongnikin; Damien Todjinou; Renaud Govoetchan; Martial Gbegbo; Mark Rowland; Martin Akogbeto; Corine Ngufor; Which indoor residual spraying insecticide best complements standard pyrethroid long-lasting insecticidal nets for improved control of pyrethroid resistant malaria vectors?. PLOS ONE 2021, 16, e0245804, 10.1371/journal.pone.0245804.
- Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination, 2nd ed. . World Health Organization. Retrieved 2022-10-28
- Hilary Ranson; Natalie Lissenden; Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends in Parasitology 2016, 32, 187-196, 10.1016/j.pt.2015.11.010.
- B.D. Brooke; G. Kloke; R.H. Hunt; L.L. Koekemoer; E.A. Tem; M.E. Taylor; G. Small; J. Hemingway; M. Coetzee; Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bulletin of Entomological Research 2001, 91, 265-272, 10.1079/ber2001108.
- Richard H Hunt; Godwin Fuseini; Steve Knowles; Joseph Stiles-Ocran; Rolf Verster; Maria L Kaiser; Kwang Shik Choi; Lizette L Koekemoer; Maureen Coetzee; Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasites & Vectors 2011, 4, 107-107, 10.1186/1756-3305-4-107.
- Hunt, R.H.; Brooke, B.D.; Pillay C.,; Koekemoer, L.L.; Coetzee, M.; Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Medical and Veterinary Entomology 2005, 19, 271-275, 10.1111/j.1365-2915.2005.00574.x.
- Oumou. K. Gueye; Magellan Tchouakui; Abdoulaye K. Dia; Mouhamed B. Faye; Amblat A. Ahmed; Murielle J. Wondji; Daniel N. Nguiffo; Leon. M. J. Mugenzi; Frederic Tripet; Lassana Konaté; et al.Abdoulaye DiabateIbrahima DiaOumar GayeOusmane FayeEl Hadji A. NiangCharles S. Wondji Insecticide Resistance Profiling of Anopheles coluzzii and Anopheles gambiae Populations in the Southern Senegal: Role of Target Sites and Metabolic Resistance Mechanisms. Genes 2020, 11, 1403, 10.3390/genes11121403.
- Lizette L. Koekemoer; Belinda L. Spillings; Riann N. Christian; Te-Chang M. Lo; Maria L. Kaiser; Ryan A.I. Norton; Shune V. Oliver; Kwang S. Choi; Basil D. Brooke; Richard H. Hunt; et al.Maureen Coetzee Multiple Insecticide Resistance inAnopheles gambiae(Diptera: Culicidae) from Pointe Noire, Republic of the Congo. Vector-Borne and Zoonotic Diseases 2011, 11, 1193-1200, 10.1089/vbz.2010.0192.
- Themba Mzilahowa; Martin Chiumia; Rex B. Mbewe; Veronica T. Uzalili; Madalitso Luka-Banda; Anna Kutengule; Don P. Mathanga; Doreen Ali; John Chiphwanya; John Zoya; et al.Shadreck MulengaWilfred DodoliJennifer Bergeson-LockwoodPeter TroellJessica OyugiKim LindbladeJohn E. Gimnig Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malaria Journal 2016, 15, 1-15, 10.1186/s12936-016-1610-1.
- Majidah Hamid-Adiamoh; Alfred Amambua-Ngwa; Davis Nwakanma; Umberto D’Alessandro; Gordon A. Awandare; Yaw A. Afrane; Insecticide resistance in indoor and outdoor-resting Anopheles gambiae in Northern Ghana. Malaria Journal 2020, 19, 1-12, 10.1186/s12936-020-03388-1.
- Javan Chanda; Kochelani Saili; Foustina Phiri; Jennifer C. Stevenson; Mulenga Mwenda; Sandra Chishimba; Conceptor Mulube; Brenda Mambwe; Christopher Lungu; Duncan Earle; et al.Adam BennettThomas P. EiseleMulakwa KamuliwoRichard W. SteketeeJoseph KeatingJohn M. MillerChadwick H. Sikaala Pyrethroid and Carbamate Resistance in Anopheles funestus Giles along Lake Kariba in Southern Zambia. The American Journal of Tropical Medicine and Hygiene 2020, 103, 90-97, 10.4269/ajtmh.19-0664.
- Dieudonné Diloma Soma; Barnabas Mahugnon Zogo; Anthony Somé; Bertin N’Cho Tchiekoi; Domonbabele François De Sales Hien; Hermann Sié Pooda; Sanata Coulibaly; Jacques Edounou Gnambani; Ali Ouari; Karine Mouline; et al.Amal DahountoGeorges Anicet OuédraogoFlorence FournetAlphonsine Amanan KoffiCédric PennetierNicolas MoirouxRoch Kounbobr Dabiré Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: A pre-intervention study. PLOS ONE 2020, 15, e0236920, 10.1371/journal.pone.0236920.
- Hunt, R.H.; Edwardes, M.; Coetzee, M.; Pyrethroid resistance in southern African Anopheles funestus extends to Likoma Island in Lake Malawi. Parasites & Vectors 2010, 3, 122-122, 10.1186/1756-3305-3-122.
- Emmanuel Elanga-Ndille; Lynda Nouage; Cyrille Ndo; Achille Binyang; Tatiane Assatse; Daniel Nguiffo-Nguete; Doumani Djonabaye; Helen Irwing; Billy Tene-Fossog; Charles S. Wondji; et al. The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation. Genes 2019, 10, 790, 10.3390/genes10100790.
- Jonah Cheung; Arshad Mahmood; Ravi Kalathur; Lixuan Liu; Paul R. Carlier; Structure of the G119S Mutant Acetylcholinesterase of the Malaria Vector Anopheles gambiae Reveals Basis of Insecticide Resistance. Structure 2017, 26, 130-136.e2, 10.1016/j.str.2017.11.021.
- Lewis, C.T.. Cuticle Techniques in Arthropods - The penetration of cuticle by insecticides; Miller, T.A., Eds.; Springer: New York, NY, USA, 1980; pp. 367–400.
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Advances in Insect Physiology - Voltage-Gated Sodium Channels as Insecticide Targets; Elsevier: Amsterdam: The Netherlands, 2014; pp. 389–433.
- English, B.A.; Webster, A.A.. Primer on the Autonomic Nervous System, 3rd ed - Acetylcholinesterase and its Inhibitors; Robertson, D., Biaggioni, I., Burnstock, G., Low, P.A., Paton, J.F.R., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 631–633.
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al.Goulson, DKreutzweiser, D.P.Krupke, C.H.Liess, MLong, E.McField, M.Mineau, P.Mitchell, E.Morrissey, CNoome, D.A.Pisa, L.Settele, J.Stark, J.D.Tapparo, A.Van Dyck, H.Van Praagh, J.van der Sluijs, J.P.Whitehorn, P.R.Wiemers, M. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environmental Science and Pollution Research 2014, 22, 5-34, 10.1007/s11356-014-3470-y.
- Richard H. Osborne; Insect neurotransmission: Neurotransmitters and their receptors. Pharmacology & Therapeutics 1996, 69, 117-142, 10.1016/0163-7258(95)02054-3.
- Stefan M. Kanzok; Liangbiao Zheng; The mosquito genome – a turning point?. Trends in Parasitology 2003, 19, 329-331, 10.1016/s1471-4922(03)00145-4.
- Clare C. Rittschof; Stefanie Schirmeier; Insect models of central nervous system energy metabolism and its links to behavior. Glia 2017, 66, 1160-1175, 10.1002/glia.23235.
- Nino Mancini; Martin Giurfa; Jean-Christophe Sandoz; Aurore Avarguès-Weber; Aminergic neuromodulation of associative visual learning in harnessed honey bees. Neurobiology of Learning and Memory 2018, 155, 556-567, 10.1016/j.nlm.2018.05.014.
- Verlinden, H.; Vleugels, R.; Zels, S.; Dillen, S.; Lenaerts, C.; Crabbé, K.; Spit, J.; Vanden Broeck, J.. Advances in Insect Physiology - Receptors for Neuronal or Endocrine Signalling Molecules as Potential Targets for the Control of Insect Pests.; Cohen, E., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 167–303.
- Sofie Knutsson; Cecilia Engdahl; Rashmi Kumari; Nina Forsgren; Cecilia Lindgren; Tomas Kindahl; Stanley Kitur; Lucy Wachira; Luna Kamau; Fredrik J. Ekström; et al.Anna Linusson Noncovalent Inhibitors of Mosquito Acetylcholinesterase 1 with Resistance-Breaking Potency. Journal of Medicinal Chemistry 2018, 61, 10545-10557, 10.1021/acs.jmedchem.8b01060.
- Haoues Alout; Arnaud Berthomieu; Andreas Hadjivassilis; Mylène Weill; A new amino-acid substitution in acetylcholinesterase 1 confers insecticide resistance to Culex pipiens mosquitoes from Cyprus. Insect Biochemistry and Molecular Biology 2007, 37, 41-47, 10.1016/j.ibmb.2006.10.001.
- John E. Casida; Kathleen A. Durkin; Anticholinesterase insecticide retrospective. Chemico-Biological Interactions 2012, 203, 221-225, 10.1016/j.cbi.2012.08.002.
- Paul R. Carlier; Troy D. Anderson; Dawn M. Wong; Danny C. Hsu; Joshua Hartsel; Ming Ma; Eric A. Wong; Ranginee Choudhury; Polo C.-H. Lam; Maxim M. Totrov; et al.Jeffrey R. Bloomquist Towards a species-selective acetylcholinesterase inhibitor to control the mosquito vector of malaria, Anopheles gambiae. Chemico-Biological Interactions 2008, 175, 368-375, 10.1016/j.cbi.2008.04.037.
- Cecilia Engdahl; Sofie Knutsson; Sten-Åke Fredriksson; Anna Linusson; Göran Bucht; Fredrik Ekström; Acetylcholinesterases from the Disease Vectors Aedes aegypti and Anopheles gambiae: Functional Characterization and Comparisons with Vertebrate Orthologues. PLOS ONE 2015, 10, e0138598, 10.1371/journal.pone.0138598.
- Mylène Weill; Philippe Fort; Arnaud Berthomieu; Marie Pierre Dubois; Nicole Pasteur; Michel Raymond; A novel acetylcholinesterase gene in mosquitoes codes for the insecticide target and is non–homologous to theacegeneDrosophila. Proceedings of the Royal Society B: Biological Sciences 2002, 269, 2007-2016, 10.1098/rspb.2002.2122.
- Frédéric Hoffmann; Didier Fournier; Pierre Spierer; Minigene rescues acetylcholinesterase lethal mutations in Drosophila melanogaster. Journal of Molecular Biology 1992, 223, 17-22, 10.1016/0022-2836(92)90710-2.
- Yanhui Lu; Yoonseong Park; Xiang Gao; Xiaoping Zhang; Jianxiu Yao; Yuan-Ping Pang; Hualiang Jiang; Kun Yan Zhu; Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Scientific Reports 2012, 2, 288, 10.1038/srep00288.
- Jonah Cheung; Michael J. Rudolph; Fiana Burshteyn; Michael S. Cassidy; Ebony N. Gary; James Love; Matthew C. Franklin; Jude J. Height; Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. Journal of Medicinal Chemistry 2012, 55, 10282-10286, 10.1021/jm300871x.
- Yuan-Ping Pang; Novel Acetylcholinesterase Target Site for Malaria Mosquito Control. PLOS ONE 2006, 1, e58-e58, 10.1371/journal.pone.0000058.
- Qudsia Yousafi; Ayesha Sarfaraz; Muhammad Saad Khan; Shahzad Saleem; Umbreen Shahzad; Azhar Abbas Khan; Mazhar Sadiq; Allah Ditta Abid; Muhammad Sohail Shahzad; Najam Ul Hassan; et al. In silico annotation of unreviewed acetylcholinesterase (AChE) in some lepidopteran insect pest species reveals the causes of insecticide resistance. Saudi Journal of Biological Sciences 2021, 28, 2197-2209, 10.1016/j.sjbs.2021.01.007.
- Takeshi Nabeshima; Akio Mori; Toshinori Kozaki; Yoichi Iwata; Osamu Hidoh; Shizuko Harada; Shinji Kasai; David W Severson; Yoshiaki Kono; Takashi Tomita; et al. An amino acid substitution attributable to insecticide-insensitivity of acetylcholinesterase in a Japanese encephalitis vector mosquito, Culex tritaeniorhynchus. Biochemical and Biophysical Research Communications 2004, 313, 794-801, 10.1016/j.bbrc.2003.11.141.
- Mylène Weill; Georges Lutfalla; Knud Mogensen; Fabrice Chandre; Arnaud Berthomieu; Claire Berticat; Nicole Pasteur; Alexandre Philips; Philippe Fort; Michel Raymond; et al. Insecticide resistance in mosquito vectors. Nature 2003, 423, 136-137, 10.1038/423136b.
- Rock Aïkpon; Michel Sezonlin; Razaki Ossè; Martin Akogbeto; Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin. Parasites & Vectors 2014, 7, 568, 10.1186/preaccept-1676019280144188.
- Edmund J. Norris; Aaron D. Gross; Brendan M. Dunphy; Steven Bessette; Lyric Bartholomay; Joel R. Coats; Comparison of the Insecticidal Characteristics of Commercially Available Plant Essential Oils AgainstAedes aegyptiandAnopheles gambiae(Diptera: Culicidae). Journal of Medical Entomology 2015, 52, 993-1002, 10.1093/jme/tjv090.
- Prabhakaran Vasantha-Srinivasan; Sengottayan Senthil-Nathan; Athirstam Ponsankar; Annamalai Thanigaivel; Edward-Sam Edwin; Selvaraj Selin-Rani; Muthiah Chellappandian; Venkatraman Pradeepa; Jalasteen Lija-Escaline; Kandaswamy Kalaivani; et al.Wayne B. HunterVeeramuthu DuraipandiyanNaif Abdullah Al-Dhabi Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide Temephos and plant derived essential oil derived from Piper betle L.. Ecotoxicology and Environmental Safety 2017, 139, 439-446, 10.1016/j.ecoenv.2017.01.026.
- Veena Prajapati; A K Tripathi; K K Aggarwal; S P S Khanuja; Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresource Technology 2005, 96, 1749-1757, 10.1016/j.biortech.2005.01.007.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
31 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




