Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anatoly Antipov | -- | 2690 | 2022-10-28 18:11:57 | | | |
| 2 | Rita Xu | Meta information modification | 2690 | 2022-10-31 03:34:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Antipov, A.; Pichugov, R.; Abunaeva, L.; Tong, S.; Petrov, M.; Pustovalova, A.; Speshilov, I.; Kartashova, N.; Loktionov, P.; Modestov, A.; et al. Halogen Hybrid Flow Batteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/31845 (accessed on 09 March 2026).
Antipov A, Pichugov R, Abunaeva L, Tong S, Petrov M, Pustovalova A, et al. Halogen Hybrid Flow Batteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/31845. Accessed March 09, 2026.
Antipov, Anatoly, Roman Pichugov, Lilia Abunaeva, Shengfu Tong, Mikhail Petrov, Alla Pustovalova, Ivan Speshilov, Natalia Kartashova, Pavel Loktionov, Alexander Modestov, et al. "Halogen Hybrid Flow Batteries" Encyclopedia, https://encyclopedia.pub/entry/31845 (accessed March 09, 2026).
Antipov, A., Pichugov, R., Abunaeva, L., Tong, S., Petrov, M., Pustovalova, A., Speshilov, I., Kartashova, N., Loktionov, P., Modestov, A., & Glazkov, A. (2022, October 28). Halogen Hybrid Flow Batteries. In Encyclopedia. https://encyclopedia.pub/entry/31845
Antipov, Anatoly, et al. "Halogen Hybrid Flow Batteries." Encyclopedia. Web. 28 October, 2022.
Copy Citation
Among the most effective energy systems for stationary applications, a special place is occupied by redox flow battery (RFB) technology, encompassing easy scalability with independent scaling of power density and energy capacity, no detrimental effects of a deep discharge, very low self-discharge, low cost for a large system compared to other types of batteries, and long cycle life.
hybrid flow batteries
zinc-bromine flow batteries
hydrogen-bromate flow batteries
1. Introduction
The well-known 17 Sustainable Development Goals (SDGs) are at the hearing today to form the 2030 ecological agenda majors [1]. Among them, the reliable technologies to provide energy in a sustainable way are limited by insufficient usage of renewable energy sources. Due to the intermittent availability of the latter, the demand for effective energy storage systems (ESS) today is of crucial importance.
The distributed energy resources (DER) model is another ace in the hole that offers more flexible and integrative energy storage mechanisms within the local power grid close to the end user, thus minimizing the demand for power transmission lines usage [2]. An essential solution to the problem of energy accumulation and storage in distributed networks is the use of chemical power sources (CPS).
However, the wide distribution and commercialization of flow batteries are currently hindered by their insufficient energy capacity and power, primarily due to the low energy density of reagents used [3]. To achieve significant capacity, the electrolyte tanks have to be large enough, along with the aqueous electrolyte, which often makes the battery very heavy and suitable only for stationary applications. Hybrid flow batteries (e.g., zinc-bromine, zinc-cerium, zinc-iron, iron-iron), which have a liquid-solid electrochemical reaction, are prone to additional degradation due to dendrite formation and increased resistance, while the common all-liquid systems such as vanadium and polysulfide bromide often require electrolyte conditioning, a process of balancing the electrolyte chemistry using additional hardware or maintenance steps, which is required daily.
The high application potential of RFB ensures continued interest of the scientific community both in (i) studying the properties and principles of their operation to improve commonly proposed systems, and in (ii) the development of energy storage devices with new reagent types or RFB concepts. These modern studies of RFB are primarily aimed at overcoming two fundamental barriers that significantly hinder the development of the direction: (a) insufficient specific power due to small values of exchange currents for heterogeneous redox reactions used in RFB and (b) low energy density compared to the competing CPS, due to insufficient energy capacity of the reagents per unit volume or mass. To solve these issues scientific community proposed a number of concepts, such as the use of hybrid systems, in which one of the half-reactions in the battery is either replaced by a hydrogen oxidation reaction [4][5][6][7] or an electroactive component in a solid state [8][9][10]. In novel fundamental concepts, high-capacity electrolytes are proposed either in specific states, e.g., [7][11][12][13] or in specific forms, e.g., various salts of halates as highly soluble multielectron redox-active components [14].
2. Halogen Hybrid Flow Batteries Perspective Concepts Analysis
2.1. Hybrid Principle Basis
The hybrid device may lose performance in some way, but this change can push a device from completely non-functional to viable in terms of functionality. So, in the case of lithium batteries, the lithium-metal electrode was sacrificed and replaced by lithium-intercalated graphite, which led to a 30% decrease in theoretical energy density, nevertheless making it possible to create a battery with a long-life cycle [15].
In general, a natural consequence of this approach is the free combination of design and chemistry for the cathode and anode half-cells of the electrochemical system in a way to balance the system as much as possible in terms of performance. When doing this optimization, there is no need to stay within the same design or operation principle for the power source: the ability to combine different technologies is the key to obtaining new energy storage devices that simultaneously combine the advantages of capacitors, electrochemical or fuel cells, flow batteries and other technologies.
At present, this approach to the development of new energy sources is used everywhere. Thus, an increase in the energy capacity of a conventional double-layer capacitor with the reversible redox electrochemical processes on the electrode near-surface layer leads to a new type of electrochemical capacitor–supercapacitor [16][17][18][19][20][21]. The combination of various chemistries of galvanic cells has led to the appearance of lithium-sulfur batteries with outstanding performance in terms of specific energy capacity—a value of more than 500 W h has been experimentally achieved [22][23][24][25][26][27][28]. Even higher energy intensity (comparable to the energy intensity of gasoline fuel) is demonstrated by a hybrid of a fuel cell and a lithium-ion battery, a lithium-air battery, where electric current is generated due to oxidation of lithium at the anode and oxygen reduction from the air at the cathode [29][30][31][32][33][34][35][36][37].
Despite a number of difficulties associated with the use of metal anodes, the choice of catalysts and electrolytes, metal-air electrochemical sources are the prominent result of combining the idea of electrolyte circulating through the system and the design of a galvanic cell, for example, for transport applications [38][39][40][41][42][43][44][45][46][47].
The principle of combining various electrochemical approaches can also play a decisive role in solving the problems of traditional fuel cells, where you can sacrifice air oxygen by replacing it with another reagent (whereas the hydrogen reaction is stored at an anode) because such a replacement immediately eliminates the crucial problems of conventional fuel cells: the high cost of the system due to utilization of platinum and other catalysts, and dissolution of cathode catalyst [48][49][50].
However, an adequate choice of new oxidizing agent for a hybrid flow battery is an extremely difficult task, since several requirements must be met simultaneously: electrolyte must have a high energy density and its reduction reaction must be kinetically fast to ensure the high energy efficiency of the device. Additionally, all reagents and products of the cathodic reaction must be nontoxic, stable and non-flammable, preferably liquids or gases, since, e.g., solids, pastes and suspensions are often commercially less preferable in flow battery design.
2.2. Halogen Hybrid Flow Batteries
Considering the above-mentioned requisites one can recall the hydrogen-halogen hybrid flow batteries, primarily due to rapid, reversible kinetics, which leads to excellent system performance and the use of inexpensive reagents [10][51][52][53][54]. In such a system a halogen oxidizer X2 (for example, Br2, Cl2, or I2) is used in the form of an aqueous solution and hydrogen (H2) is used as a reducing agent. The potential difference between the halogen and hydrogen electrodes V is equal to the EMF (from 0.54 V for I2 to 1.4 V for Cl2). It should be noted that F2, which is not considered here, has the largest potential difference relative to the standard hydrogen electrode (3.05 V), however, due to technological difficulties in the separation of gaseous fluorine from a mixture of gases, its use is unreasonable due to energy considerations and the problem of materials’ compatibility with this reagent.
In this battery the following reactions undergo on electrodes (from left to right—charge mode, from right to left—discharge):
The protons are transferred from the anode through the membrane into the catholyte (solution in contact with the cathode), whose composition changes inside the discharge unit from X2 to HX, and the HX solution enters the corresponding reservoir.
The most promising halogens that can be used as oxidizing agents in such a system are Cl2 and Br2 because the standard potentials of the corresponding redox couples for these substances are the highest (1.36 V and 1.09 V, respectively, for Cl2/Cl− and for Br2/Br−). Recently, the interest of researchers has mainly shifted towards the hydrogen-bromine system due to its higher specific power with voltage efficiency of more than 90%, as well as a higher oxidant solubility in an aqueous solution.
A serious disadvantage of hydrogen-halogen systems based on chlorine and bromine is the high toxicity and corrosiveness of the halogen oxidizer, whose concentrated solution must be stored in a tank. Another limitation for these systems is the risk of the catalytic layer degradation on the negative electrode due to halogen crossover through the separator, which should not only provide high selectivity but also have low internal resistance to minimize voltage losses.
The transition from traditional vanadium-based redox flow batteries to hydrogen-bromine hybrid chemistry has led to a significant increase in the power density of flow systems. To date, specific power values of 1.4–1.5 W cm2 have been experimentally achieved for the H2/Br2 system [55][56], while for vanadium redox flow batteries, the highest peak power density for laboratory-scale devices reaches 1.3 W cm−2 [57][58][59][60]. The researchers develop new concepts and models: Dr. Wlodarczyk recently explored the bromine cathode thermodynamics for hydrogen–bromine chemistry in concentrated solutions [61]. Dr. Ronen proposed the perspective concept of the hydrogen-bromine flow battery, which does not need separation of the cathode and anode half-cells via proton exchange membrane. He also completed a thorough analysis of the catholyte complexation reaction in homogeneous conditions vs. the efficiency of such a flow battery system [62][63]. Recently Dr. Hou reported a chlorine redox flow battery that reversibly performs electrolysis of NaCl aqueous solution until Cl2 is generated and transformed into the carbonaceous tetrachloride (CCl4) [64]. Dr. Fisher and some other scientific groups thoroughly investigated the complexation of bromine with different chemical agents (BCA) in aqueous electrolytes of halogen-containing flow systems. The problem of bromine vapor pressure reduction and halogen storage in the insoluble matter or solid material form is of interest in these works [65][66][67]. As a result, they showed that bromine can be stored in the fused salt of very high concentrations up to 13.6 M, rushing theoretical energy capacity up to 730 A h L−1 [65][66][67]. Recently this group also measured current densities in the diffusion-limited regime and corresponding current distribution mappings vs. the flow rate values for the H2-Br2 RFB [68].
Despite the recent developments in the H-Br2 battery, the solubility of bromine in water under normal conditions is about 0.21 mol l−1 [56]. Hydrogen-bromine systems usually use a mixture of bromine dissolved in water and hydrobromic acid (up to 8 mol l−1) [56], which makes it possible to achieve an energy capacity of about 170 W h kg−1 [69], much higher than most common vanadium flow batteries energy density of 20 to 50 W h kg−1 [70][71][72][73], nevertheless still much lower than, for example, for lithium-ion batteries [74]. Thus, it can be concluded that, despite promising performance in terms of power density, both vanadium-based compounds and halogens show insufficient solubility in water, which leads to limitations on the value of specific energy capacity.
2.3. Halate Hybrid Flow Batteries
The solution to the problem of the insufficient energy capacity of hydrogen-halogen flow batteries was proposed in 2015 by Yu.V. Tolmachev in [14]—to abandon the use of dissolved halogens in favor of a new family of aqueous multielectron oxidants—solutions of halogen oxoacids.
Lithium bromate LiBrO3, which should be converted at the cathode to lithium bromide LiBr, serves as a promising object of study in [14]. Due to the very high solubility of both substances in water (according to [14] even at room temperature, the concentration of a saturated solution of lithium bromate is about 9 mol l−1, and bromide—more than 10 mol l−1) and the transfer of 6 electrons for bromate-bromide transition results in very high charge densities, more than 1000 A h kg−1, which are more than an order of magnitude higher than similar indicators for other redox flow batteries systems proposed to date.
Other advantages of this oxidizing agent, mentioned in [14], and demonstrated experimentally in [75], are its long-term chemical stability (at moderately low pH), low toxicity of the reagent and product, the utilization of commodity chemicals, low cost per unit of generated electrical energy (due to both a relatively inexpensive reagent and the absence of expensive catalysts for the cathodic reaction, in particular, noble metals), low self-discharge rate and the absence of fire and explosion hazards.
Additionally, in addition to lithium bromate, the use of commercially available sodium bromates with water solubility up to 2 mol l−1, seems very promising [14].
The reason why the bromate anion (as well as other oxohalogenate ions) did not attract attention for applications in electrochemical energy earlier is its low electrochemical activity: on all the studied electrodes, including noble metals, its direct electroreduction reaction at high-rate proceeds at high overvoltages. For the first time, it has been experimentally confirmed that despite the direct electroreduction of bromate anion via heterogeneous reactions cannot be performed without the huge cathode overvoltage, nevertheless, it can be carried out via redox mediator autocatalysis (EC″ mechanism) through a combination of the following bromine reduction reactions:
which takes place reversibly even on electrodes without expensive catalysts, with the process of comproportionation in the electrolyte bulk
which proceeds at sufficiently high acidity of the solution.
The results of theoretical and experimental studies [76][77][78][79] confirmed a number of extremely interesting and unexpected effects: the presence of an anomalous dependence of current density on the intensity of the convective mixing of the solution (which is uncharacteristic of any other known electrochemical mechanisms) combined with an anomalously high cathodic current density of order ~ A cm−2 in bromate solutions of the molar concentration range.
Successful confirmation of the developed analytical model predictions with experimental rotating disk electrode data for several solutions of bromate anions in sulfuric and phosphoric acids in the molar range of concentrations is presented in the work [80].
Thus, the quantitative agreement of analytical predictions and experimental data allows researchers to make the following fundamental conclusions on the kinetics of chemical processe:
-
The high reversibility of the electrochemical step (5) of the EC″ mechanism for the bromine/bromide redox couple used in the system, even on carbon electrodes. That is a prerequisite for high current density, sufficient voltage for under load conditions and the specific power of the system;
-
Irreversibility of the chemical step (5) of the EC″ mechanism due to sufficiently high solution acidity (acid volume concentration approximately in the molar range);
-
The first order of the comproportionation reaction (5) with respect to the anions of bromate and bromide, the second order—with respect to the activity of protons:
-
where aH = 10−pH proton activity (in the form of hydroxonium ions), fBrO3 and fBr are activity coefficients of the bromate and the bromide anions, correspondingly.
-
The six-electron nature of the electrochemical process (the Br atom changes its oxidation state from +5, which corresponds to BrO3−, to −1, which corresponds to Br−) during the cycle is a prerequisite for ensuring a high specific energy capacity of the system.
Prof. Cho et al. have performed a numerical study of this redox-mediated bromate-based electrochemical energy system focusing on the transient cell behavior analysis and mass transport analysis of the main reagent, which resulted in strengthening the conclusions made above about the prospects of such approaches [81]. The numerical model provided by Dr. Chinannai and Dr. Ju has also demonstrated the beneficial features of the H2/Br2/BrO3− system, demonstrating the discharge characteristics of an H2/Br2 cell with BrO3− measured under different C-rates [82]. The bromate system was found to have the innovative features to boost cell performance due to its characteristic autocatalytic reaction to keep regenerating enough reactant for the electrochemical reaction, which may lead to a “no-limiting current condition (i.e., no mass transfer loss)” in well-designed cell and operating conditions.
Thorough research by Dr. Modestov’s group showed for 50 cm2 membrane-electrode assembly of hydrogen-bromate flow battery 0.74 W cm−2 power density was reached at the cell voltage 0.7 V while catholyte utilization rate was 0.93 in a single pass through membrane-electrode assembly, see Figure 1 and Figure 2 [75][83]. As a result, based on the high-grade assessments one can conclude that such a system can be of interest to break through the challenging issues in conventional batteries

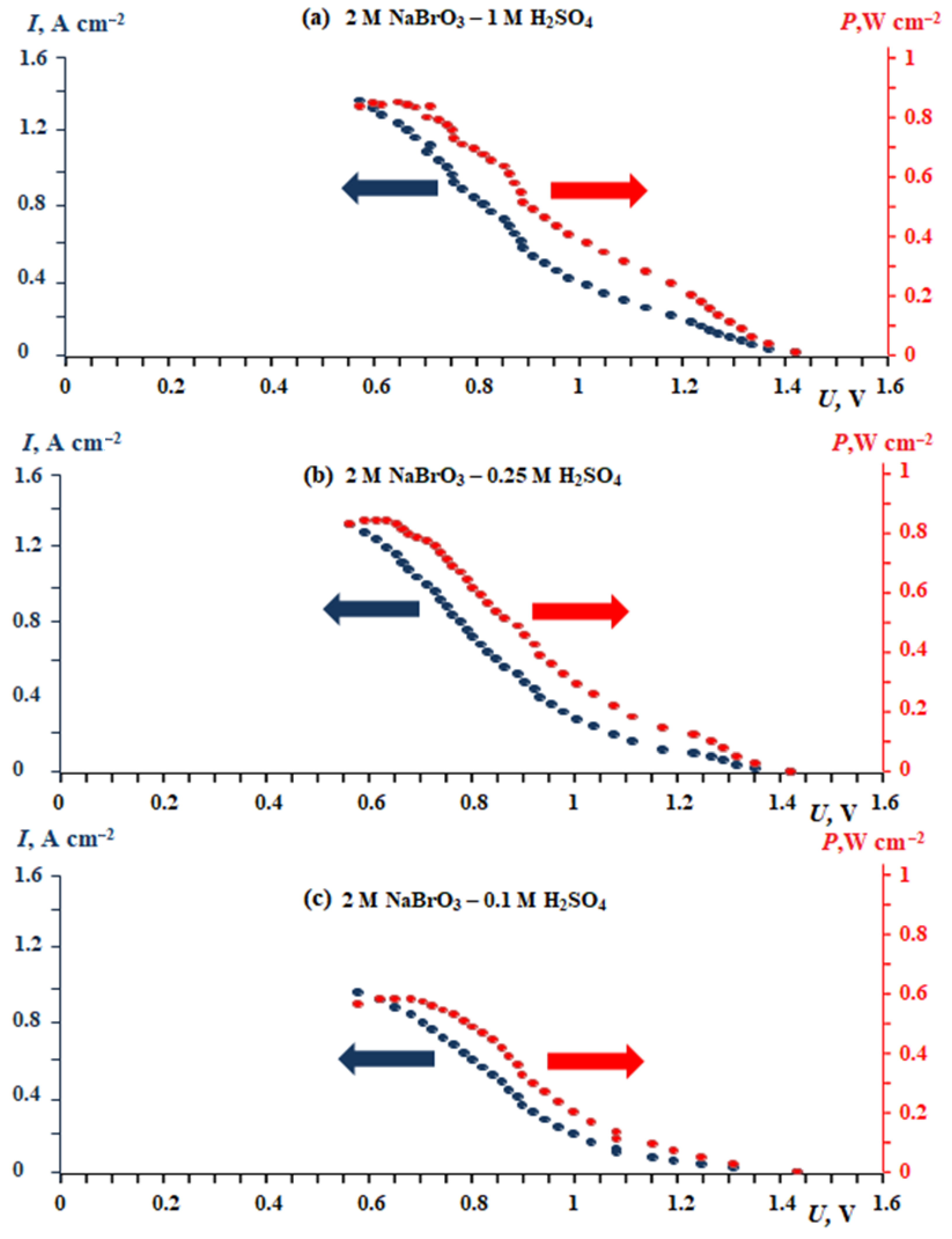
Figure 2. Dependence of current density and specific power vs. voltage for a laboratory membrane electrode assembly prototype (see Figure 1a) with an active surface area of 50 cm2 at different acidic concentrations of the bulk solution: (a) 1 mol L−1 H2SO4, (b) 0.25 mol L−1 H2SO4, (c) 0.1 mol L−1 H2SO4.
References
- Desa, U.N. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 8 September 2022).
- Nikam, V.; Kalkhambkar, V. A Review on Control Strategies for Microgrids with Distributed Energy Resources, Energy Storage Systems, and Electric Vehicles. Int. Trans. Electr. Energy Syst. 2021, 31, 1–26.
- Zigouras, P. 1000 Mile Battery. Available online: http://www.epc-corporation.com/ (accessed on 8 September 2022).
- Tang, L.; Lu, W.; Zhang, H.; Li, X. Progress and Perspective of the Cathode Materials towards Bromine-Based Flow Batteries. Energy Mater. Adv. 2022, 2022, 9850712.
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.V.; Wycisk, R.J.; Pintauro, P.N.; Bates, M.; Mukerjee, S.; Tucker, M.C.; Weber, A.Z. Advanced Hydrogen-Bromine Flow Batteries with Improved Efficiency, Durability and Cost. J. Electrochem. Soc. 2016, 163, A5049–A5056.
- Braff, W.A.; Bazant, M.Z.; Buie, C.R. Membrane-Less Hydrogen Bromine Flow Battery. Nat. Commun. 2013, 4, 2346.
- Cho, K.T.; Tucker, M.C.; Weber, A.Z. A Review of Hydrogen/Halogen Flow Cells. Energy Technol. 2016, 4, 655–678.
- Nguyen, T.; Savinell, R.F. Flow Batteries. Electrochem. Soc. Interface 2010, 19, 54–56.
- Khor, A.; Leung, P.; Mohamed, M.R.; Flox, C.; Xu, Q.; An, L.; Wills, R.G.A.; Morante, J.R.; Shah, A.A. Review of Zinc-Based Hybrid Flow Batteries: From Fundamentals to Applications. Mater. Today Energy 2018, 8, 80–108.
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533–11558.
- Yang, F.; Mousavie, S.M.A.; Oh, T.K.; Yang, T.; Lu, Y.; Farley, C.; Bodnar, R.J.; Niu, L.; Qiao, R.; Li, Z. Sodium–Sulfur Flow Battery for Low-Cost Electrical Storage. Adv. Energy Mater. 2018, 8, 1–8.
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986.
- Zhang, X.; Zhang, P.; Chen, H. Organic Multiple Redox Semi-Solid-Liquid Suspension for Li-Based Hybrid Flow Battery. ChemSusChem 2021, 14, 1913–1920.
- Tolmachev, Y.V.; Piatkivskyi, A.; Ryzhov, V.V.; Konev, D.V.; Vorotyntsev, M.A. Energy Cycle Based on a High Specific Energy Aqueous Flow Battery and Its Potential Use for Fully Electric Vehicles and for Direct Solar-to-Chemical Energy Conversion. J. Solid State Electrochem. 2015, 19, 2711–2722.
- Howard, W.F.; Spotnitz, R.M. Theoretical Evaluation of High-Energy Lithium Metal Phosphate Cathode Materials in Li-Ion Batteries. J Power Sources 2007, 165, 887–891.
- Zang, X.; Shen, C.; Kao, E.; Warren, R.; Zhang, R.; Teh, K.S.; Zhong, J.; Wei, M.; Li, B.; Chu, Y.; et al. Titanium Disulfide Coated Carbon Nanotube Hybrid Electrodes Enable High Energy Density Symmetric Pseudocapacitors. Adv. Mater. 2018, 30, 1–8.
- Wang, H.; Xu, Z.; Li, Z.; Cui, K.; Ding, J.; Kohandehghan, A.; Tan, X.; Zahiri, B.; Olsen, B.C.; Holt, C.M.B.; et al. Hybrid Device Employing Three-Dimensional Arrays of MnO in Carbon Nanosheets Bridges Battery-Supercapacitor Divide. Nano Lett. 2014, 14, 1987–1994.
- Choudhary, N.; Li, C.; Chung, H.S.; Moore, J.; Thomas, J.; Jung, Y. High-Performance One-Body Core/Shell Nanowire Supercapacitor Enabled by Conformal Growth of Capacitive 2D WS2 Layers. ACS Nano 2016, 10, 10726–10735.
- Izadi-Najafabadi, A.; Yamada, T.; Futaba, D.N.; Yudasaka, M.; Takagi, H.; Hatori, H. High-Power Supercapacitor Electrodes Nanotube Composite. ACS Nano 2011, 5, 811–819.
- Sun, S.; Zhai, T.; Liang, C.; Savilov, S.V.; Xia, H. Boosted Crystalline/Amorphous Fe2O3-δ Core/Shell Heterostructure for Flexible Solid-State Pseudocapacitors in Large Scale. Nano Energy 2018, 45, 390–397.
- Ahmadi, S.; Bathaee, S.M.T.; Hosseinpour, A.H. Improving Fuel Economy and Performance of a Fuel-Cell Hybrid Electric Vehicle (Fuel-Cell, Battery, and Ultra-Capacitor) Using Optimized Energy Management Strategy. Energy Convers. Manag. 2018, 160, 74–84.
- Pan, H.; Han, K.S.; Engelhard, M.H.; Cao, R.; Chen, J.; Zhang, J.G.; Mueller, K.T.; Shao, Y.; Liu, J. Addressing Passivation in Lithium–Sulfur Battery Under Lean Electrolyte Condition. Adv. Funct. Mater. 2018, 28, 1–7.
- Evers, S.; NaZar, L. New Approaches for High Energy Density Lithium -Sulfur Battery Cathodes. Acc. Chem. Res. 2012, 46, 1135–1143.
- Xu, J.; Zhang, W.; Fan, H.; Cheng, F.; Su, D.; Wang, G. Promoting Lithium Polysulfide/Sulfide Redox Kinetics by the Catalyzing of Zinc Sulfide for High Performance Lithium-Sulfur Battery. Nano Energy 2018, 51, 73–82.
- Zhang, S.S. Liquid Electrolyte Lithium/Sulfur Battery: Fundamental Chemistry, Problems, and Solutions. J. Power Sources 2013, 231, 153–162.
- Aurbach, D. Introduction to the Focus Issue on Lithium-Sulfur Batteries: Materials, Mechanisms, Modeling, and Applications. J. Electrochem. Soc. 2018, 165, Y1.
- Du, H.; Li, S.; Qu, H.; Lu, B.; Wang, X.; Chai, J.; Zhang, H.; Ma, J.; Zhang, Z.; Cui, G. Stable Cycling of Lithium-Sulfur Battery Enabled by a Reliable Gel Polymer Electrolyte Rich in Ester Groups. J. Memb. Sci. 2018, 550, 399–406.
- Carbone, L.; Coneglian, T.; Gobet, M.; Munoz, S.; Devany, M.; Greenbaum, S.; Hassoun, J. A Simple Approach for Making a Viable, Safe, and High-Performances Lithium-Sulfur Battery. J. Power Sources 2018, 377, 26–35.
- Liu, Q.; Chang, Z.; Li, Z.; Zhang, X. Flexible Metal—Air Batteries: Progress, Challenges, and Perspectives. Small Methods 2017, 1700231, 1–16.
- Pan, J.; Li, H.; Sun, H.; Zhang, Y.; Wang, L.; Liao, M.; Sun, X. A Lithium—Air Battery Stably Working at High Temperature with High Rate Performance. Small 2017, 1703454, 1–6.
- Zhao, Z.; Huang, J.; Peng, Z. Achilles’ Heel of Li-Air Batteries: Li2CO3. Angew. Chem.-Int. Ed. 2017, 57, 3874–3886.
- Guo, Z.; Li, C.; Liu, J.; Wang, Y.; Xia, Y. A Long-Life Lithium—Air Battery in Ambient Air with a Polymer Electrolyte Containing a Redox Mediator. Angew. Chem. 2017, 129, 7613–7617.
- Wu, F.; Yu, Y. Toward True Lithium-Air Batteries. Joule 2018, 2, 815–817.
- Aurbach, D.; Mccloskey, B.D.; Nazar, L.F.; Bruce, P.G. Underpinning Lithium—Air Batteries. Nat. Publ. Group 2016, 1, 1–11.
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A Lithium-Oxygen Battery with a Long Cycle Life in an Air-like Atmosphere. Nature 2018, 555, 502–506.
- Xu, S.; Yao, Y.; Guo, Y.; Zeng, X.; Lacey, S.D.; Song, H.; Chen, C.; Li, Y.; Dai, J.; Wang, Y.; et al. Textile Inspired Lithium–Oxygen Battery Cathode with Decoupled Oxygen and Electrolyte Pathways. Adv. Mater. 2017, 30, 1704907.
- Torres, A.E.; Balbuena, P.B. Exploring the LiOH Formation Reaction Mechanism in Lithium-Air Batteries. Chem. Mater. 2018, 30, 708–717.
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design Principles for Heteroatom-Doped Carbon Nanomaterials as Highly Efficient Catalysts for Fuel Cells and Metal-Air Batteries. Adv. Mater. 2015, 27, 6834–6840.
- Cui, Z.; Fu, G.; Li, Y.; Goodenough, J.B. Ni3FeN-Supported Fe3Pt Intermetallic Nanoalloy as a High-Performance Bifunctional Catalyst for Metal–Air Batteries. Angew. Chem. 2017, 129, 10033–10037.
- Hardwick, L.J.; León, C.P. de Rechargeable Multi-Valent Metal-Air Batteries. Johns. Matthey Technol. Rev. 2018, 62, 134–149.
- Liu, G.; Kim, J.Y.; Wang, M.; Woo, J.Y.; Wang, L.; Zou, D.; Lee, J.K. Soft, Highly Elastic, and Discharge-Current-Controllable Eutectic Gallium–Indium Liquid Metal–Air Battery Operated at Room Temperature. Adv. Energy Mater. 2018, 8, 1–9.
- Wu, X.; Meng, G.; Liu, W.; Li, T.; Yang, Q.; Sun, X.; Liu, J. Metal-Organic Framework-Derived, Zn-Doped Porous Carbon Polyhedra with Enhanced Activity as Bifunctional Catalysts for Rechargeable Zinc-Air Batteries. Nano Res 2018, 11, 163–173.
- Li, X.; Liu, Z.; Song, L.; Wang, D.; Zhang, Z. Three-Dimensional Graphene Network Supported Ultrathin CeO2 Nanoflakes for Oxygen Reduction Reaction and Rechargeable Metal-Air Batteries. Electrochim. Acta 2018, 263, 561–569.
- Wang, Y.J.; Fang, B.; Zhang, D.; Li, A.; Wilkinson, D.P.; Ignaszak, A.; Zhang, L.; Zhang, J. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal–Air Batteries; Springer: Singapore, 2018; Volume 1, ISBN 0123456789.
- Lee, D.U.; Park, M.G.; Cano, Z.P.; Ahn, W.; Chen, Z. Hierarchical Core–Shell Nickel Cobaltite Chestnut-like Structures as Bifunctional Electrocatalyst for Rechargeable Metal–Air Batteries. ChemSusChem 2018, 11, 406–414.
- Han, X.; He, G.; He, Y.; Zhang, J.; Zheng, X.; Li, L.; Zhong, C.; Hu, W.; Deng, Y.; Ma, T.Y. Engineering Catalytic Active Sites on Cobalt Oxide Surface for Enhanced Oxygen Electrocatalysis. Adv. Energy Mater. 2018, 8, 1–13.
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen Electrocatalysts in Metal-Air Batteries: From Aqueous to Nonaqueous Electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786.
- Wu, Z.P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–Realloying Enabled High Durability for Pt–Pd-3d-Transition Metal Nanoparticle Fuel Cell Catalysts. Nat. Commun. 2021, 12, 1–14.
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.J. Ultralow-Loading Platinum-Cobalt Fuel Cell Catalysts Derived from Imidazolate Frameworks. Science 2018, 362, 1276–1281.
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current Progress of Pt and Pt-Based Electrocatalysts Used for Fuel Cells. Sustain. Energy Fuels 2019, 4, 15–30.
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox Flow Batteries: A Review. J Appl. Electrochem. 2011, 41, 1137–1164.
- Dowd, R.P.; Zeets, M.; Van Nguyen, T. Effect of Br2 Complexation on a Hydrogen-Bromine Flow Battery Performance. ECS Meet. Abstr. 2015, 47, 1886.
- Cho, K.T.; Albertus, P.; Battaglia, V.; Kojic, A.; Srinivasan, V.; Weber, A.Z. Optimization and Analysis of High-Power Hydrogen/Bromine-Flow Batteries for Grid-Scale Energy Storage. Energy Technol. 2013, 1, 596–608.
- Xu, Y.; Xie, C.; Li, X. Bromine–Graphite Intercalation Enabled Two-Electron Transfer for a Bromine-Based Flow Battery. Trans. Tianjin Univ. 2022, 28, 186–192.
- Livshits, V.; Ulus, A.; Peled, E. High-Power H2/Br2 Fuel Cell. Electrochem. Commun. 2006, 8, 1358–1362.
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High Performance Hydrogen/Bromine Redox Flow Battery for Grid-Scale Energy Storage. J. Electrochem. Soc. 2012, 159, A1806–A1815.
- Kim, J.; Park, H. Recent Advances in Porous Electrodes for Vanadium Redox Flow Batteries in Grid-Scale Energy Storage Systems: A Mass Transfer Perspective. J. Power Sources 2022, 545, 231904.
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of Material Research and Development for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2013, 101, 27–40.
- Zhang, H.; Lu, W.; Li, X. Progress and Perspectives of Flow Battery Technologies. Electrochem. Energy Rev. 2019, 2, 492–506.
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the All-vanadium Redox Flow Battery for Energy Storage: A Review of Technological, Financial and Policy Aspects. Int. J. Energy Res. 2012, 36, 1105–1120.
- Wlodarczyk, J.K.; Küttinger, M.; Friedrich, A.K.; Schumacher, J.O. Exploring the Thermodynamics of the Bromine Electrode in Concentrated Solutions for Improved Parametrisation of Hydrogen–Bromine Flow Battery Models. J. Power Sources 2021, 508, 230202.
- Ronen, R.; Atlas, I.; Suss, M.E. Theory of Flow Batteries with Fast Homogeneous Chemical Reactions. J. Electrochem. Soc. 2018, 165, A3820–A3827.
- Ronen, R.; Gloukhovski, R.; Suss, M.E. Single-Flow Multiphase Flow Batteries: Experiments. J. Power Sources 2022, 540, 231567.
- Hou, S.; Chen, L.; Fan, X.; Fan, X.; Ji, X.; Wang, B.; Cui, C.; Chen, J.; Yang, C.; Wang, W.; et al. High-Energy and Low-Cost Membrane-Free Chlorine Flow Battery. Nat. Commun. 2022, 13, 1–8.
- Küttinger, M.; Loichet Torres, P.A.; Meyer, E.; Fischer, P. Properties of Bromine Fused Salts Based on Quaternary Ammonium Molecules and Their Relevance for Use in a Hydrogen Bromine Redox Flow Battery. Chem. Eur. J. 2022, 28, e202103491.
- Küttinger, M.; Riasse, R.; Wlodarczyk, J.; Fischer, P.; Tübke, J. Improvement of Safe Bromine Electrolytes and Their Cell Performance in H2/Br2 Flow Batteries Caused by Tuning the Bromine Complexation Equilibrium. J. Power Sources 2022, 520, 230804.
- Wu, W.; Xu, S.; Lin, Z.; Lin, L.; He, R.; Sun, X. A Polybromide Confiner with Selective Bromide Conduction for High Performance Aqueous Zinc-Bromine Batteries. Energy Storage Mater. 2022, 49, 11–18.
- Cantu, B.B.M.; Fischer, P.; Zitoun, D.; Tübke, J.; Pinkwart, K. In Situ Measurement of Localized Current Distribution in H2-br2 Redox Flow Batteries. Energies 2021, 14, 4945.
- Tolmachev, Y.V. Hydrogen-Halogen Electrochemical Cells: A Review of Applications and Technologies. Russ. J. Electrochem. 2014, 50, 301–316.
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in Electrical Energy Storage System: A Critical Review. Prog. Nat. Sci. 2009, 19, 291–312.
- Zeng, Y.K.; Zhao, T.S.; An, L.; Zhou, X.L.; Wei, L. A Comparative Study of All-Vanadium and Iron-Chromium Redox Flow Batteries for Large-Scale Energy Storage. J. Power Sources 2015, 300, 438–443.
- Manohar, A.K.; Kim, K.M.; Plichta, E.; Hendrickson, M.; Rawlings, S.; Narayanan, S.R. A High Efficiency Iron-Chloride Redox Flow Battery for Large-Scale Energy Storage. J. Electrochem. Soc. 2016, 163, A5118–A5125.
- Naresh, R.P.; Surendran, A.; Ragupathy, P.; Dixon, D. Enhanced Electrochemical Performance of Zinc/Bromine Redox Flow Battery with Carbon-Nanostructured Felt Generated by Cobalt Ions. J. Energy Storage 2022, 52, 104913.
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1–24.
- Modestov, A.D.; Konev, D.V.; Tripachev, O.V.; Antipov, A.E.; Tolmachev, Y.V.; Vorotyntsev, M.A. A Hydrogen–Bromate Flow Battery for Air-Deficient Environments. Energy Technol. 2018, 6, 242–245.
- Petrov, M.M.; Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; et al. Redox Flow Batteries: Role in Modern Electric Power Industry and Comparative Characteristics of the Main Types. Russ. Chem. Rev. 2021, 90, 677–702.
- Vorotyntsev, M.A.; Konev, D.V.; Tolmachev, Y.V. Electroreduction of Halogen Oxoanions via Autocatalytic Redox Mediation by Halide Anions: Novel EC" Mechanism. Theory for Stationary 1D Regime. Electrochim. Acta 2015, 173, 779–795.
- Vorotyntsev, M.A.; Antipov, A.E.; Tolmachev, Y.V. One-Dimensional Model of Steady-State Discharge Process in Hydrogen-Bromate Flow Battery. Electrochim. Acta 2016, 222, 1555–1561.
- Vorotyntsev, M.A.; Antipov, A.E. Bromate Electroreduction from Acidic Solution at Spherical Microelectrode under Steady-State Conditions: Theory for the Redox-Mediator Autocatalytic (EC”) Mechanism. Electrochim. Acta 2017, 258, 544–553.
- Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Petrov, M.M.; Pichugov, R.D.; Vorotyntsev, M.A. Bromate Electroreduction from Sulfuric Acid Solution at Rotating Disk Electrode: Experimental Study. Electrochim. Acta 2018, 259, 346–360.
- Cho, K.T.; Razaulla, T. Redox-Mediated Bromate Based Electrochemical Energy System. J. Electrochem. Soc. 2019, 166, A286–A296.
- Chinannai, M.F.; Ju, H. Analysis of Performance Improvement of Hydrogen/Bromine Flow Batteries by Using Bromate Electrolyte. Int. J. Hydrogen Energy 2021, 46, 13760–13774.
- Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Vorotyntsev, M.A. Hydrogen-Bromate Flow Battery: Can One Reach Both High Bromate Utilization and Specific Power? J. Solid State Electrochem. 2019, 23, 3075–3088.
- Pichugov, R.; Konev, D.; Speshilov, I.; Abunaeva, L.; Petrov, M.; Vorotyntsev, M.A. Analysis of the Composition of Bromide Anion Oxidation Products in Aqueous Solutions with Different PH via Rotating Ring-Disk Electrode Method. Membranes 2022, 12, 820.
- Modestov, A.; Kartashova, N.; Pichugov, R.; Petrov, M.; Antipov, A.; Abunaeva, L. Bromine Crossover in Operando Analysis of Proton Exchange Membranes in Hydrogen−Bromate Flow Batteries. Membranes 2022, 12, 815.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
31 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





