Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zekenova Akzhibek Almatovna | -- | 2180 | 2022-10-28 11:01:40 | | | |
| 2 | Rita Xu | -4 word(s) | 2176 | 2022-10-28 11:55:36 | | | | |
| 3 | Rita Xu | Meta information modification | 2176 | 2022-10-28 12:03:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zekenova, A.; Nazhipkyzy, M.; Li, W.; Kalybayeva, A.; Zhumanova, G.; Zubova, O. Carbon–Manganese Dioxide Composite in Supercapacitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/31787 (accessed on 08 February 2026).
Zekenova A, Nazhipkyzy M, Li W, Kalybayeva A, Zhumanova G, Zubova O. Carbon–Manganese Dioxide Composite in Supercapacitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/31787. Accessed February 08, 2026.
Zekenova, Akzhibek, Meruyert Nazhipkyzy, Wanlu Li, Akmaral Kalybayeva, Guldarikha Zhumanova, Olga Zubova. "Carbon–Manganese Dioxide Composite in Supercapacitors" Encyclopedia, https://encyclopedia.pub/entry/31787 (accessed February 08, 2026).
Zekenova, A., Nazhipkyzy, M., Li, W., Kalybayeva, A., Zhumanova, G., & Zubova, O. (2022, October 28). Carbon–Manganese Dioxide Composite in Supercapacitors. In Encyclopedia. https://encyclopedia.pub/entry/31787
Zekenova, Akzhibek, et al. "Carbon–Manganese Dioxide Composite in Supercapacitors." Encyclopedia. Web. 28 October, 2022.
Copy Citation
One of the global problems is environmental pollution by different biowaste. To solve the problem, biowaste must be recycled. Waste-free technology is also a way of saving exhaustible raw materials. Research on electrochemical energy sources is currently the most dynamically developing area of off-grid energy. Electrochemical capacitors can operate for a long time without changing performance, they have smaller dimensions, high mechanical strength, and a wide operating temperature range. These properties are effective energy-saving devices. Therefore, supercapacitors are widely used in various industries.
supercapacitor electrodes
biowaste
activated carbon
MnO2
1. Introduction
Currently, there is a growing demand for various energy storage devices, which drives research to develop energy storage systems with a high efficiency at lower costs [1][2]. Compared to other energy storage devices, supercapacitors can possess a fast charge/discharge feature, long cycling life, excellent reversibility, high power density, and wide temperature range operation. Due to these characteristics, supercapacitors are widely used in consumer electronics that require a burst of power density. It can also be utilized as power supplies for portable devices such as notebooks, smartphones, and computers [2][3].
Supercapacitors, known as electrochemical capacitors (ECs) or ultracapacitors, are a new electrochemical energy storage system that stores energy by charge accumulation [4]. Supercapacitors are classified into electric double-layer capacitors (EDLC) that are based on non-faradic processes—pseudocapacitors (PC), that are based on faradic processes, and hybrid capacitors (HC), which combines two types of the above charge storage mechanism. Supercapacitors can also be categorized into two types according to the arrangement of electrodes: symmetric capacitors have both anodes and cathodes identical to each other, and asymmetric capacitors are made of two different materials [5].
The choice of raw materials affects the supercapacitor type and the desired electrochemical characteristics.
Figure 1 below classifies the type of supercapacitor according to the electrode materials. Recently, scientists have paid more attention to obtaining electrode materials from non-conventional raw materials. The use of non-conventional raw materials can simultaneously reduce the cost and environmental pollution.
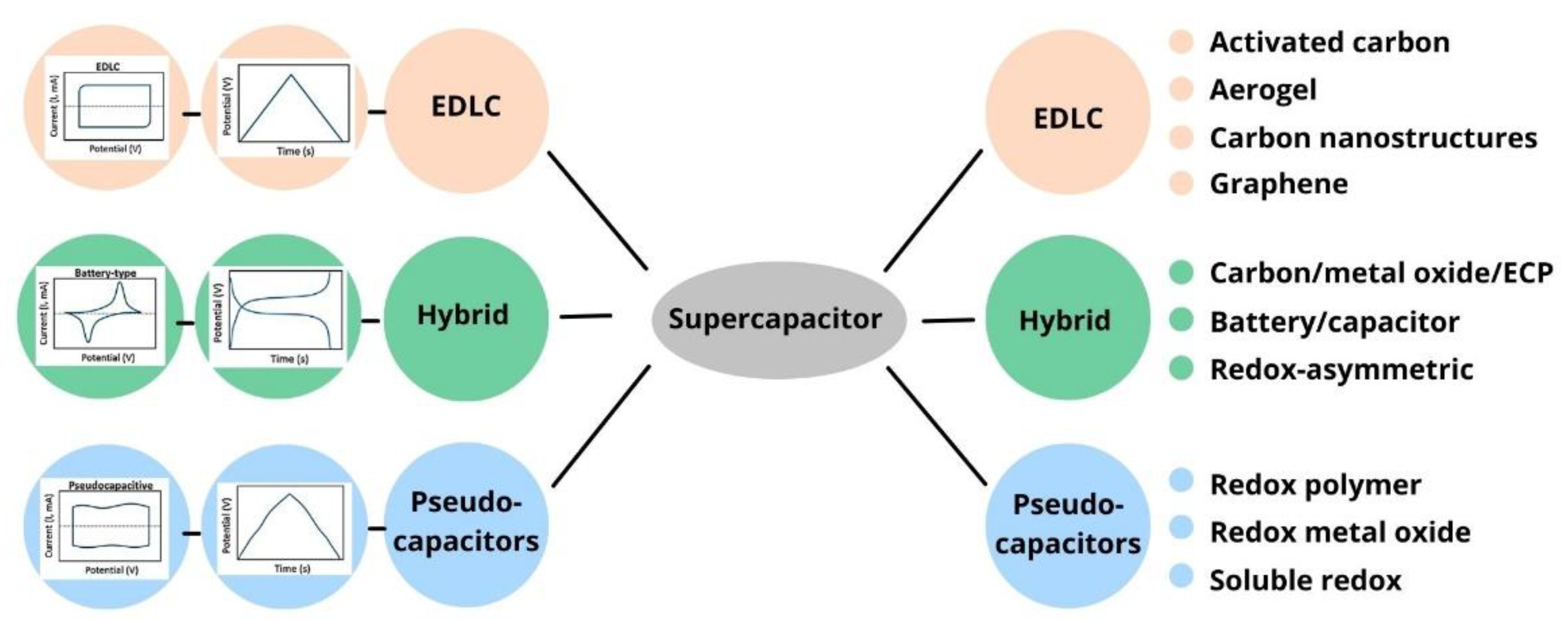
Figure 1. Different classes and subclasses of supercapacitors based on the electrode materials.
As shown in Figure 1, carbon materials, metal oxides, conductive polymers, and various composite materials are used as electrode materials. Different types of carbon materials (such as graphite, graphene, and carbon nanotube) have been applied as a supercapacitor electrode material due to its extraordinary properties, such as high electrochemical stability and high electrical conductivity [6][7]. However, these electrode materials have their disadvantages. For instance, graphene sheets have a high specific surface area, effective charge storage transport properties, and a wide potential window. They quickly form irreversible agglomerates and restack to their graphite structure, and the determination of intrinsic capacitance becomes very difficult [8]. Despite the superior mechanical and electrical properties of carbon nanotubes (CNTs), one of the main challenges in applications is their difficulty forming homogeneous dispersion, which weakens the electrode’s mechanical, electrical, and chemical properties. Applying these materials is hindered by the prevailing time-consuming and energy-wasting procedures. Because of this, it is preferable to use biowaste-derived carbon materials since they are easily accessible, renewable, and sustainable [7]. Among the above carbon materials, activated carbon (AC) is a popular commercial supercapacitor electrode material due to its low cost, high specific surface area, high electrochemical stability, and high electrical conductivity [6].
The specific capacitance and energy density of EDLCs cannot compete with pseudocapacitors, owing to their inherent electrostatic surface charging mechanism. Therefore, an effective way to prepare high-performance composite electrode materials is combining various functional carbon materials and typical metal oxides or conductive polymers [9]. To date, transition metal oxides have displayed pseudocapacitive behavior with high specific capacitance and multiple oxidation states that make them advantageous for supercapacitor applications [10]. Commonly used transition metal oxides are Fe2O3, MnO2, ZnO, NiCo layered double hydroxides, CoAl layered double hydroxides, and Co3O4; in addition, their composites have been widely studied [11]. Manganese dioxide (MnO2) is an excellent pseudocapacitive electrode material among other advanced transition metal oxides due to the electrochemical abilities, varied range of potentials, natural wide occurrence, environmentally benign property, and theoretical capacitance value (1380 F/g) [12][13]. MnOx can have a higher capacitance than carbon materials because it possesses faradaic or pseudocapacitance, resulting from fast redox reactions occurring at the interface between metal oxides and electrolytes, in addition to the double-layer capacitance. However, the low electrical conductivity, strong agglomeration, and poor cycle life of metal oxides limit their use in high-capacity storage devices. The use of hybrid active materials consisting of a metal oxide and a carbon host is a promising approach to solve these problems [14].
2. Raw Materials
The raw material is essential for obtaining high-quality electrodes. To synthesize activated carbon, the carbon content must be high in the raw material because it will affect the yield of the product. It is also crucial to choose raw materials with low impurities or easily removable impurities. The raw material composition should not contain any toxic compounds for safety reasons.
Two types of raw materials are used to obtain activated carbon: non-conventional and conventional activated carbon. Traditional raw materials include wood, peat, bituminous coal, lignite, petroleum coke, and pitch [15][16][17][18][19][20][21][22]. Table 1 lists characteristics of some carbon-based supercapacitor electrodes from fossil fuels, such as, petroleum, coke, coal, and pitch. Despite the high quality of the obtained carbon, the non-conventional raw materials have some disadvantages, namely, the decreasing availability of fossil fuels, the growing global energy demand, and increased awareness of the environmental impacts of fossil fuel combustion [22]. Therefore, scientists have recently obtained electrode materials from renewable raw materials, mainly waste [23].
To not recycle household waste pollutes the environment and is still an actual problem. In addition to industrial pollution, the environment is also polluted by wood products [24]. To tackle this issue and save non-renewable resources, it is essential to use different types of waste.
Table 1. Fossil fuels obtained activated carbons for supercapacitor electrodes.
| Raw Material | SBET (m2/g) |
Current Density (A/g) |
Specific Capacitance (F/g) |
Types of Test Cells | Reference |
|---|---|---|---|---|---|
| Petroleum coke | 2964 | 0.05 | 220 | Two-electrode cell with symmetrical electrodes. |
[25] |
| Coal | 1032 | 0.5 | 108 | Three-electrode cell with Hg/HgO as the reference electrode, AC as the working | [26] |
| Pitch | 3145 | 0.05 | 272 | electrode and platinum plate as the counter electrode. Two-electrode cell with symmetrical electrodes. |
[27] |
Non-Conventional Biowaste Raw Materials
Recently, renewable biowaste-derived activated carbons have attracted significant attention due to their interesting characteristics of naturally porous or hierarchical structured and heteroatom doping [28]. This biowaste-derived activated carbon has reduced electrochemical properties compared to carbon fibers, commercial activated carbon, CNTs, etc. However, their major advantage is low cost, ease of production, environmental friendliness, and the presence of their inherent functional groups and heteroatoms, which makes them a suitable substitute for carbonaceous materials derived from fossil fuels. Biowaste-derived activated carbon has been identified as a suitable electrode for energy storage devices due to its required pore size, large specific surface area, low equivalent series resistance, and high stability [29].
Non-conventional raw materials are very diverse, and most of them could be easily found in daily lives, such as food waste (coconut shell [30], pecan nutshell waste [31], ginkgo shells [32], onion peel [33], pomelo peel [34], garlic peel [35], orange peel [36], etc.), animal waste (crab shell [37], pork bone [38], blackfish bone [38], eel bone [38], fish bladder [39], etc.), plant waste (Couroupita guianansis [40], Elm flower [41], kapok flower [42], hemp stem [43], pine cones [4], banana leaves [44], etc.). Figure 2 illustrates the types of biowaste-derived activated carbon. Notably, the raw material types increase yearly due to the demand for activated carbon-based supercapacitors.
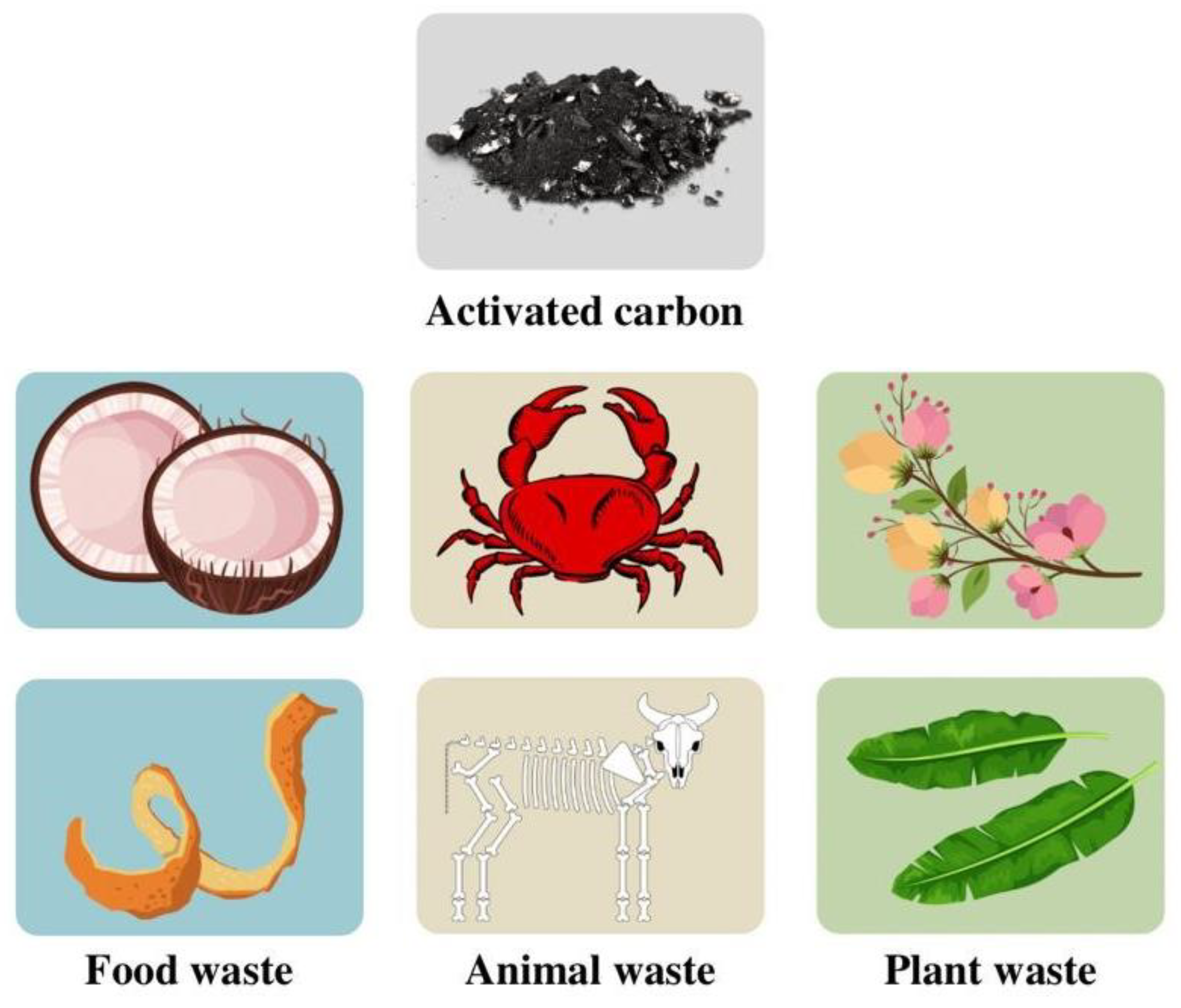
Figure 2. The types of biowaste-derived activated carbon.
Currently, scientists also synthesize activated carbon for the supercapacitor not only from biowaste, for instance, Coca-Cola waste [45], tires [46], tobacco waste [47], plastic bags, and other industrial wastes [48]. Due to the pandemic, the amount of used masks has increased, and scientists in China synthesize carbon from the covers for the supercapacitor electrodes [49].
The properties of biowaste determine the properties of the derived activated carbon, and consequently, the supercapacitor electrode characteristics. Therefore, choosing the proper biowaste organ/tissues is also essential. Biowaste generally consists of organic substances (saccharides, vitamins, fatty acids) and other elements (H, O, N and S). Besides C, O, H, S, and N, some minerals, such as Ca, K, Mg, Na, and Si, may also be contained in the biowaste. The thermal pyrolysis or carbonization process decomposes large quantities of organic matter into H2O and CO2 and forms pores. Other elements can also be removed by thermal conversion to some extent. Therefore, activation is used to obtain porous carbon with a high carbon concentration [50]. The composition may also vary according to the raw material location (country, etc.). For instance, biowaste from saline–alkali land is rich in mineral elements such as, Ca, K, Mg, Na, and Si [6][50].
The raw material is usually pre-treated before carbonation. The biowaste pre-treatment depends on the type of raw material. Currently, there are several types of raw material pretreatment. It includes physical pretreatment (coarse size reduction, chipping, shredding, grinding, milling), biological pretreatment (the action of fungi capable of producing enzymes that can degrade lignin, hemicellulose, and polyphenols), chemical pretreatment (with acids, alkali, organic solvents, and ionic liquids), and physicochemical pretreatment (use of conditions and compounds that affect the physical and chemical properties of biowaste) [51]. In order to make homogeneous and reproducible carbons, the precursors need to be pre-treated through careful grinding and purification [23].
Table 2 lists characteristics of some carbon-based supercapacitor electrodes from biowaste.
Table 2. Biowaste-derived activated carbon and AC-based electrodes for supercapacitor.
| Raw Material | SBET (m2/g) |
Current Density (A/g) |
Specific Capacitance (F/g) |
Types of Test Cells | Reference |
|---|---|---|---|---|---|
| Plant waste | |||||
| Sugarcane bagasse | 725 | 0.2 | 265 | Three-electrode cell (working (AC), reference (saturated calomel electrode), a counter (Pt wire). | [52] |
| Elm flower | 2048.6 | 20 | 216 | Two-electrode cell with symmetrical electrodes. |
[41] |
| Kapok flower | 1904.1 | 1 | 286.8 | Three-electrode cell (counter (Pt wire), reference electrode (Ag/AgCl), working (AC) cell. | [42] |
| Sakura petals | 1433.8 | 0.2 | 265.8 | Three-electrode working (AC), counter (Pt sheet), reference (a saturated calomel electrode). | [53] |
| Lignin | 1425 | 10 mV/s | 140.9 | Three-electrode cell (working (AC), counter (Pt), and references (Ag/AgCl)). | [54] |
| Food waste | |||||
| Garlic peel | 3325.2 | 1 | 424.42 | Two-electrode cell with symmetrical electrodes. |
[35] |
| Onion peel | 3150 | 0.5 | 169 | Three-electrode cell (working (AC), counter (glassy carbon), and references (Ag/AgCl)). | [33] |
| Pomelo peel | 1582 | 0.5 | 180 | Three-electrode cell (reference (Hg/HgO), a counter (graphite sheet), working (porous carbon)). | [34] |
| Orange peel | 1391 | 0.5 | 407 | Three-electrode cell (working (porous carbon), counter (Pt strip), and reference (saturated calomel electrode). | [36] |
| Animal waste | |||||
| Crab shell | 3442 | 0.2 | 280.6 | Two-electrode cell with symmetrical electrodes. |
[37] |
| Pork bone | 1260 | 0.5 | 263 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
| Blackfish bone | 1202 | 0.5 | 302 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
| Eel bone | 1163 | 0.5 | 264 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
By comparing Table 1 and Table 2, researchers can observe that biowaste materials are excellent for a supercapacitor, and by using biowaste materials, researchers can even obtain a supercapacitor with a comparatively higher capacity than with fossil fuels that have obtained activated carbon [25][26][27][55]. Depending on the method of obtaining them (such as temperature, activation time, activation type, activating agents etc.), different types of biocarbon behave differently as supercapacitors.
3. The Performance of Supercapacitors
The performance of supercapacitors is evaluated on the basis of acceptably high energy densities (>10 Wh kg), substantially greater power density, fast charge discharge process (within seconds), excellent cycle stability (more than 100 times of batteries), low self-discharging, safe operation, and low cost [2]. Advanced electrical double-layer capacitors (EDLCs) contain organic electrolytes that operate at 2.7 V, and reach energy densities around 5–8 Wh/kg or 7–10 Wh/L. A commercial corporation offers a 48 V ultra-capacitor module with 1,000,000 duty cycles or a ten-year DC life and 48 V DC working voltage, and the modules are engineered explicitly for hybrid bus and construction equipment to provide cost-effective solutions. Currently, the industry leaders in SC production are Cellergy, Ioxus, Maxwell Technologies, Murata Manufacturing, Nanoramic Laboratories, Nec Tokin, Nippon Chemi-Con, and Panasonic. Supercapacitor devices from these manufacturers have capacitance in the range of 1200–5000 F, low direct current (DC) equivalent series resistance (ESR) values of less than one mΩ, short-circuit current in the range of 600–2400 A, and per cell energy-storage capabilities in the range of 0.6–3 Wh [56].
Nippon Chemi-Con uses activated carbon with a large specific surface area of around 2000 m2/g for the electrodes, and the raw materials of the activated carbon include pitch, resin, and natural plant materials.
Researchers can say that currently activated carbon composite supercapacitors are not yet widely used commercially, while activated carbon supercapacitors, including from biowaste, are in high demand in the commercial sector.
Owing to the two different working mechanisms, EDLCs have a higher cyclic stability or performance. Since an electrode chemical reaction is involved in pseudocapacitors, irreversible components will accumulate during cycling, leading to deteriorating performance; therefore, the cycling stability of porous carbon will be higher than composites (Table 3) [57].
Table 3. Cycling stability of activated carbon and AC-MnO2 composite.
| Material | Capacitance Retention, % | Reference |
|---|---|---|
| Rice husk-derived carbon | 98.5 (after 5000 charge–discharge cycles) | [58] |
| Rice husk-derived carbon/MnO2 | 80.2 (after 5000 charge–discharge cycles) | [58] |
| Bamboo-based activated carbon | 99.98 (after 1000 charge–discharge cycles) | [59] |
| Bamboo-based activated carbon/MnO2 | 89.29 (after 1000 charge–discharge cycles) | [59] |
SCs have the following advantages: long cycle life, high specific power, quick charge and discharge, and a wide operating temperature range, but the disadvantages may include low energy density, high dielectric absorption, price, and high self-discharge [10][56].
Due to the advantages of supercapacitors, they are used in the public sector, in automobiles and transportation equipment, in the defense and military industries, in computers and memory backup chips, in medicine and industry, and in power grids for power quality control and battery monitoring [60].
References
- Sun, K.; Zhang, Z.; Peng, H.; Zhao, G.; Ma, G.; Lei, Z. Hybrid symmetric supercapacitor assembled by renewable corn silks based porous carbon and redox-active electrolytes. Mater. Chem. Phys. 2018, 218, 229–238.
- Chang, L.; Hang Hu, Y. Supercapacitors. Compr. Energy Syst. 2018, 2, 663–695.
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825.
- Manyala, N.; Bello, A.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Coniferous pine biomass: A novel insight into sustainable carbon materials for supercapacitors electrode. Mater. Chem. Phys. 2016, 182, 139–147.
- Barik, R.; Ingole, P.P. ScienceDirect Electrochemistry Challenges and prospects of metal sulfide materials for supercapacitors. Curr. Opin. Electrochem. 2020, 21, 327–334.
- Zhao, Y.; Dong, C.; Sheng, L. Heteroatoms-doped Pillared Porous Carbon Architectures with Ultrafast Electron and Ion Transport Capabilities under High Mass Loadings for High-rate Supercapacitors Heteroatoms-doped Pillared Porous Carbon Architectures with Ultrafast Electron and Ion Tra. ACS Sustain. Chem. Eng. 2020, 8, 8664–8674.
- Sim, G.; De Oliveira, H.P.; Larsson, S.H.; Thyrel, M.; Lima, E.C. A Short Review on the Electrochemical Performance of Hierarchical and Nitrogen-Doped Activated Biocarbon-Based Electrodes for Supercapacitors. Nanomaterials 2021, 11, 424.
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445.
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/Carbon Composites for Supercapacitor: Synthesis and Electrochemical Performance. Front. Mater. 2020, 7, 1–16.
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852.
- Ali, S.; Saleh, L.; Hadi, R.; Farhoudian, S.; Nedaei, M. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2020, 857, 158281.
- Wadekar, P.H.; Khose, R.V.; Pethsangave, D.A.; Some, S. Waste-Derived Heteroatom-Doped Activated Carbon/Manganese Dioxide Trio-Composite for Supercapacitor Applications. Energy Technol. 2020, 8, 1901402.
- Guo, W.; Yu, C.; Li, S.; Wang, Z.; Yu, J.; Huang, H.; Qiu, J. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472.
- Yang, D.; Ionescu, M.I. Metal Oxide–Carbon Hybrid Materials for Application in Supercapacitors; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128104644.
- Martin-Sanchez, N.; Sanchez-Montero, M.J.; Izquierdo, C.; Salvador, F. Highlighting the role of surface groups in the gasification of carbonized raw materials of different natures with steam and supercritical water at pressures ranging from 1 to 1000 bar. Carbon N. Y. 2016, 99, 502–513.
- Volperts, A.; Dobele, G.; Zhurinsh, A.; Vervikishko, D.; Shkolnikov, E.; Ozolinsh, J. Wood-based activated carbons for supercapacitor electrodes with a sulfuric acid electrolyte. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 319–326.
- Veksha, A.; Sasaoka, E.; Uddin, M.A. The influence of porosity and surface oxygen groups of peat-based activated carbons on benzene adsorption from dry and humid air. Carbon N. Y. 2009, 47, 2371–2378.
- Xiao, N.; Wei, Y.; Li, H.; Wang, Y.; Bai, J.; Qiu, J. Boosting the sodium storage performance of coal-based carbon materials through structure modification by solvent extraction. Carbon N. Y. 2020, 162, 431–437.
- Li, C.; Wang, Y.; Xiao, N.; Li, H.; Ji, Y.; Guo, Z.; Liu, C.; Qiu, J. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction. Carbon N. Y. 2019, 151, 46–52.
- Li, G.Q.; Tian, F.H.; Zhang, Y.F.; Ding, J.L.; Fu, Y.L.; Wang, Y.; Zhang, G.J. Bamboo/lignite-based activated carbons produced by steam activation with and without ammonia for SO2 adsorption. Xinxing Tan Cailiao/New Carbon Mater. 2014, 29, 486–492.
- Starck, J.; Burg, P.; Muller, S.; Bimer, J.; Furdin, G.; Fioux, P.; Vix-Guterl, C.; Begin, D.; Faure, P.; Azambre, B. The influence of demineralisation and ammoxidation on the adsorption properties of an activated carbon prepared from a Polish lignite. Carbon N. Y. 2006, 44, 2549–2557.
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565.
- Tabac, S.; Eisenberg, D. Pyrolyze this paper: Can biomass become a source for precise carbon electrodes? Curr. Opin. Electrochem. 2021, 25, 100638.
- Vallero, D. Air Pollutant Emissions. In Fundamentals of Air Pollution; Elsevier: Amsterdam, The Netherlands, 2014; pp. 787–827.
- Tan, M.H.; Zheng, J.T.; Li, P.; Noritatsu, T.; Wu, M.B. Preparation and modification of high performance porous carbons from petroleum coke for use as supercapacitor electrodes. Xinxing Tan Cailiao/New Carbon Mater. 2016, 31, 343–351.
- Lu, Q.; Xu, Y.Y.; Mu, S.J.; Li, W.C. The effect of nitrogen and/or boron doping on the electrochemical performance of non-caking coal-derived activated carbons for use as supercapacitor electrodes. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 442–450.
- Wei, F.; Zhang, H.F.; He, X.J.; Ma, H.; Dong, S.A.; Xie, X.Y. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors. Xinxing Tan Cailiao/New Carbon Mater. 2019, 34, 132–139.
- Tang, W.; Zhang, Y.; Zhong, Y.; Shen, T.; Wang, X.; Xia, X.; Tu, J. Natural biomass-derived carbons for electrochemical energy storage. Mater. Res. Bull. 2017, 88, 234–241.
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon N. Y. 2020, 170, 1–29.
- Liang, Q.; Liu, Y.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Liu, Q. Optimized preparation of activated carbon from coconut shell and municipal sludge. Mater. Chem. Phys. 2020, 241, 122327.
- Martínez-Casillas, D.C.; Mascorro-Gutiérrez, I.; Arreola-Ramos, C.E.; Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Ramos-Sánchez, V.H.; Cuentas-Gallegos, A.K. A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon N. Y. 2019, 148, 403–412.
- Jiang, L.; Yan, J.; Hao, L.; Xue, R.; Sun, G.; Yi, B. High rate performance activated carbons prepared from ginkgo shells for electrochemical supercapacitors. Carbon N. Y. 2013, 56, 146–154.
- Musyoka, N.M.; Mutuma, B.K.; Manyala, N. Onion-derived activated carbons with enhanced surface area for improved hydrogen storage and electrochemical energy application. RSC Adv. 2020, 10, 26928–26936.
- Li, J.; Luo, F.; Lin, T.; Yang, J.; Yang, S.; He, D.; Xiao, D.; Liu, W. Pomelo peel-based N, O-codoped hierarchical porous carbon material for supercapacitor application. Chem. Phys. Lett. 2020, 753, 137597.
- Ji, T.; Han, K.; Teng, Z.; Li, J.; Wang, M.; Zhang, J.; Cao, Y.; Qi, J. Synthesis of Activated Carbon Derived from Garlic Peel and Its Electrochemical Properties. Int. J. Electrochem. Sci. 2021, 16, 150653.
- Ranaweera, C.K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes. J. Carbon Res. Artic. 2017, 3, 25.
- Gao, Y.; Yue, Q.; Gao, B. High surface area and oxygen-enriched activated carbon synthesized from animal cellulose and evaluated in electric double-layer capacitors. RSC Adv. 2015, 5, 31375–31383.
- Niu, L.; Shen, C.; Yan, L.; Zhang, J.; Lin, Y.; Gong, Y.; Li, C.; Sun, C.Q.; Xu, S. Waste bones derived nitrogen–doped carbon with high micropore ratio towards supercapacitor applications. J. Colloid Interface Sci. 2019, 547, 92–101.
- Sirengo, K.; Jande, Y.A.C.; Kibona, T.E.; Hilonga, A.; Muiva, C.; King’ondu, C.K. Fish bladder-based activated carbon/Co3O4/TiO2 composite electrodes for supercapacitors. Mater. Chem. Phys. 2019, 232, 49–56.
- Elanthamilan, E.; Sriram, B.; Rajkumar, S.; Dhaneshwaran, C.; Nagaraj, N.; Princy Merlin, J.; Vijayan, A.; Wang, S.F. Couroupita guianansis dead flower derived porous activated carbon as efficient supercapacitor electrode material. Mater. Res. Bull. 2019, 112, 390–398.
- Chen, H.; Yu, F.; Wang, G.; Chen, L.; Dai, B.; Peng, S. Nitrogen and Sulfur Self-Doped Activated Carbon Directly Derived from Elm Flower for High-Performance Supercapacitors. ACS Omega 2018, 3, 4724–4732.
- Zheng, L.H.; Chen, M.H.; Liang, S.X.; Lü, Q.F. Oxygen-rich hierarchical porous carbon derived from biomass waste-kapok flower for supercapacitor electrode. Diam. Relat. Mater. 2021, 113, 108267.
- Sun, W.; Lipka, S.M.; Swartz, C.; Williams, D.; Yang, F. Hemp-derived activated carbons for supercapacitors. Carbon N. Y. 2016, 103, 181–192.
- Roy, C.K.; Shah, S.S.; Reaz, A.H.; Sultana, S.; Chowdhury, A.N.; Firoz, S.H.; Zahir, M.H.; Ahmed Qasem, M.A.; Aziz, M.A. Preparation of Hierarchical Porous Activated Carbon from Banana Leaves for High-performance Supercapacitor: Effect of Type of Electrolytes on Performance. Chem.—Asian J. 2020, 16, 296–308.
- Boyjoo, Y.; Cheng, Y.; Zhong, H.; Tian, H.; Pan, J.; Pareek, V.K.; Jiang, S.P.; Lamonier, J.F.; Jaroniec, M.; Liu, J. From waste Coca Cola® to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors. Carbon N. Y. 2017, 116, 490–499.
- Boota, M.; Paranthaman, M.P.; Naskar, A.K.; Li, Y.; Akato, K.; Gogotsi, Y. Waste tire derived carbon-polymer composite paper as pseudocapacitive electrode with long cycle life. ChemSusChem 2015, 8, 3576–3581.
- Chen, H.; Guo, Y.; Wang, F.; Wang, G.; Qi, P.; Guo, X.; Dai, B.; Yu, F. An activated carbon derived from tobacco waste for use as a supercapacitor electrode material. Carbon N. Y. 2018, 130, 848.
- Utetiwabo, W.; Yang, L.; Tufail, M.K.; Zhou, L.; Chen, R.; Lian, Y.; Yang, W. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors. Chin. Chem. Lett. 2020, 31, 1474–1489.
- Hu, X.; Lin, Z. Transforming waste polypropylene face masks into S-doped porous carbon as the cathode electrode for supercapacitors. Ionics 2021, 27, 2169–2179.
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794.
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685.
- Sarkar, S.; Arya, A.; Gaur, U.K.; Gaur, A. Investigations on porous carbon derived from sugarcane bagasse as an electrode material for supercapacitors. Biomass Bioenergy 2020, 142, 105730.
- Ma, F.; Ding, S.; Ren, H.; Liu, Y. Sakura-based activated carbon preparation and its performance in supercapacitor applications. RSC Adv. 2019, 9, 2474–2483.
- Kusuma, H.D.; Rochmadi; Prasetyo, I.; Ariyanto, T. Mesoporous manganese oxide/lignin-derived carbon for high performance of supercapacitor electrodes. Molecules 2021, 26, 7104.
- Wei, L.; Nitta, N.; Yushin, G. Lithographically patterned thin activated carbon films as a new technology platform for on-chip devices. ACS Nano 2013, 7, 6498–6506.
- Şahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674.
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122.
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Synthesis of hierarchically porous MnO2/rice husks derived carbon composite as high-performance electrode material for supercapacitors. Appl. Energy 2016, 178, 260–268.
- Huang, T.; Qiu, Z.; Wu, D.; Hu, Z. Bamboo-based activated carbon @ MnO2 nanocomposites for flexible high-performance supercapacitor electrode materials. Int. J. Electrochem. Sci. 2015, 10, 6312–6323.
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
28 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




