Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zekenova Akzhibek Almatovna and Version 3 by Rita Xu.
One of the global problems is environmental pollution by different biowaste. To solve the problem, biowaste must be recycled. Waste-free technology is also a way of saving exhaustible raw materials. Research on electrochemical energy sources is currently the most dynamically developing area of off-grid energy. Electrochemical capacitors can operate for a long time without changing performance, they have smaller dimensions, high mechanical strength, and a wide operating temperature range. These properties are effective energy-saving devices. Therefore, supercapacitors are widely used in various industries.
- supercapacitor electrodes
- biowaste
- activated carbon
- MnO2
1. Introduction
Currently, there is a growing demand for various energy storage devices, which drives research to develop energy storage systems with a high efficiency at lower costs [1][2][1,2]. Compared to other energy storage devices, supercapacitors can possess a fast charge/discharge feature, long cycling life, excellent reversibility, high power density, and wide temperature range operation. Due to these characteristics, supercapacitors are widely used in consumer electronics that require a burst of power density. It can also be utilized as power supplies for portable devices such as notebooks, smartphones, and computers [2][3][2,3].
Supercapacitors, known as electrochemical capacitors (ECs) or ultracapacitors, are a new electrochemical energy storage system that stores energy by charge accumulation [4]. Supercapacitors are classified into electric double-layer capacitors (EDLC) that are based on non-faradic processes—pseudocapacitors (PC), that are based on faradic processes, and hybrid capacitors (HC), which combines two types of the above charge storage mechanism. Supercapacitors can also be categorized into two types according to the arrangement of electrodes: symmetric capacitors have both anodes and cathodes identical to each other, and asymmetric capacitors are made of two different materials [5].
The choice of raw materials affects the supercapacitor type and the desired electrochemical characteristics.
Figure 1 below classifies the type of supercapacitor according to the electrode materials. Recently, scientists have paid more attention to obtaining electrode materials from non-conventional raw materials. The use of non-conventional raw materials can simultaneously reduce the cost and environmental pollution.
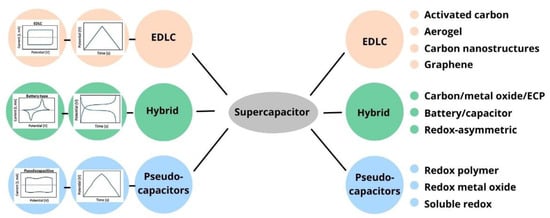
Figure 1. Different classes and subclasses of supercapacitors based on the electrode materials.
As shown in Figure 1, carbon materials, metal oxides, conductive polymers, and various composite materials are used as electrode materials. Different types of carbon materials (such as graphite, graphene, and carbon nanotube) have been applied as a supercapacitor electrode material due to its extraordinary properties, such as high electrochemical stability and high electrical conductivity [6][7][6,7]. However, these electrode materials have their disadvantages. For instance, graphene sheets have a high specific surface area, effective charge storage transport properties, and a wide potential window. They quickly form irreversible agglomerates and restack to their graphite structure, and the determination of intrinsic capacitance becomes very difficult [8]. Despite the superior mechanical and electrical properties of carbon nanotubes (CNTs), one of the main challenges in applications is their difficulty forming homogeneous dispersion, which weakens the electrode’s mechanical, electrical, and chemical properties. Applying these materials is hindered by the prevailing time-consuming and energy-wasting procedures. Because of this, it is preferable to use biowaste-derived carbon materials since they are easily accessible, renewable, and sustainable [7]. Among the above carbon materials, activated carbon (AC) is a popular commercial supercapacitor electrode material due to its low cost, high specific surface area, high electrochemical stability, and high electrical conductivity [6].
The specific capacitance and energy density of EDLCs cannot compete with pseudocapacitors, owing to their inherent electrostatic surface charging mechanism. Therefore, an effective way to prepare high-performance composite electrode materials is combining various functional carbon materials and typical metal oxides or conductive polymers [9]. To date, transition metal oxides have displayed pseudocapacitive behavior with high specific capacitance and multiple oxidation states that make them advantageous for supercapacitor applications [10]. Commonly used transition metal oxides are Fe2O3, MnO2, ZnO, NiCo layered double hydroxides, CoAl layered double hydroxides, and Co3O4; in addition, their composites have been widely studied [11]. Manganese dioxide (MnO2) is an excellent pseudocapacitive electrode material among other advanced transition metal oxides due to the electrochemical abilities, varied range of potentials, natural wide occurrence, environmentally benign property, and theoretical capacitance value (1380 F/g) [12][13][12,13]. MnOx can have a higher capacitance than carbon materials because it possesses faradaic or pseudocapacitance, resulting from fast redox reactions occurring at the interface between metal oxides and electrolytes, in addition to the double-layer capacitance. However, the low electrical conductivity, strong agglomeration, and poor cycle life of metal oxides limit their use in high-capacity storage devices. The use of hybrid active materials consisting of a metal oxide and a carbon host is a promising approach to solve these problems [14].
2. Raw Materials
The raw material is essential for obtaining high-quality electrodes. To synthesize activated carbon, the carbon content must be high in the raw material because it will affect the yield of the product. It is also crucial to choose raw materials with low impurities or easily removable impurities. The raw material composition should not contain any toxic compounds for safety reasons. Two types of raw materials are used to obtain activated carbon: non-conventional and conventional activated carbon. Traditional raw materials include wood, peat, bituminous coal, lignite, petroleum coke, and pitch [15][16][17][18][19][20][21][22][15,16,17,18,19,20,21,22]. Table 1 lists characteristics of some carbon-based supercapacitor electrodes from fossil fuels, such as, petroleum, coke, coal, and pitch. Despite the high quality of the obtained carbon, the non-conventional raw materials have some disadvantages, namely, the decreasing availability of fossil fuels, the growing global energy demand, and increased awareness of the environmental impacts of fossil fuel combustion [22]. Therefore, scientists have recently obtained electrode materials from renewable raw materials, mainly waste [23]. To not recycle household waste pollutes the environment and is still an actual problem. In addition to industrial pollution, the environment is also polluted by wood products [24]. To tackle this issue and save non-renewable resources, it is essential to use different types of waste.Table 1. Fossil fuels obtained activated carbons for supercapacitor electrodes.
| Raw Material | S | BET | (m | 2 | /g) | Current Density (A/g) |
Specific Capacitance (F/g) |
Types of Test Cells | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Petroleum coke | 2964 | 0.05 | 220 | Two-electrode cell with symmetrical electrodes. |
[25] | ||||
| Coal | 1032 | 0.5 | 108 | Three-electrode cell with Hg/HgO as the reference electrode, AC as the working | [26] | ||||
| Pitch | 3145 | 0.05 | 272 | electrode and platinum plate as the counter electrode. Two-electrode cell with symmetrical electrodes. |
[27] |
Non-Conventional Biowaste Raw Materials
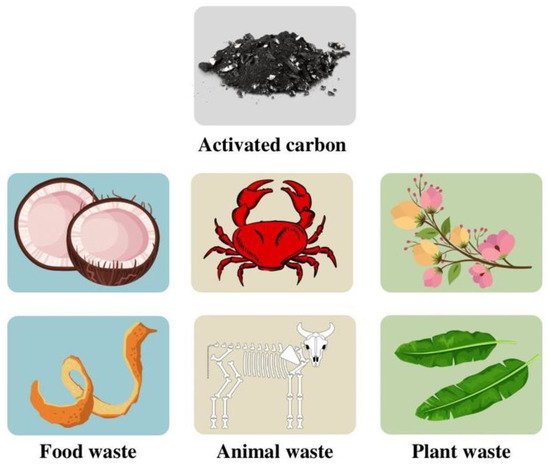
Figure 2. The types of biowaste-derived activated carbon.
Table 2. Biowaste-derived activated carbon and AC-based electrodes for supercapacitor.
| Raw Material | S | BET | (m | 2 | /g) | Current Density (A/g) |
Specific Capacitance (F/g) |
Types of Test Cells | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Plant waste | |||||||||
| Sugarcane bagasse | 725 | 0.2 | 265 | Three-electrode cell (working (AC), reference (saturated calomel electrode), a counter (Pt wire). | [52] | ||||
| Elm flower | 2048.6 | 20 | 216 | Two-electrode cell with symmetrical electrodes. |
[41] | ||||
| Kapok flower | 1904.1 | 1 | 286.8 | Three-electrode cell (counter (Pt wire), reference electrode (Ag/AgCl), working (AC) cell. | [42] | ||||
| Sakura petals | 1433.8 | 0.2 | 265.8 | Three-electrode working (AC), counter (Pt sheet), reference (a saturated calomel electrode). | [53] | ||||
| Lignin | 1425 | 10 mV/s | 140.9 | Three-electrode cell (working (AC), counter (Pt), and references (Ag/AgCl)). | [54] | ||||
| Food waste | |||||||||
| Garlic peel | 3325.2 | 1 | 424.42 | Two-electrode cell with symmetrical electrodes. |
[35] | ||||
| Onion peel | 3150 | 0.5 | 169 | Three-electrode cell (working (AC), counter (glassy carbon), and references (Ag/AgCl)). | [33] | ||||
| Pomelo peel | 1582 | 0.5 | 180 | Three-electrode cell (reference (Hg/HgO), a counter (graphite sheet), working (porous carbon)). | [ |
Table 3. Cycling stability of activated carbon and AC-MnO2 composite.
| Material | Capacitance Retention, % | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice husk-derived carbon | 98.5 (after 5000 charge–discharge cycles) | [58] | [107] | |||||||||||
| 34 | ||||||||||||||
| Rice husk-derived carbon/MnO | 2 | 80.2 (after 5000 charge–discharge cycles) | [58] | [107] | ||||||||||
| ] | ||||||||||||||
| Three-electrode cell (working (porous carbon), counter (Pt strip), and reference (saturated calomel electrode). | ||||||||||||||
| [ | ||||||||||||||
| 36 | ||||||||||||||
| Bamboo-based activated carbon | 99.98 (after 1000 charge–discharge cycles) | ] | ||||||||||||
| [ | 59 | ] | [104] | |||||||||||
| Bamboo-based activated carbon/MnO | 2 | 89.29 (after 1000 charge–discharge cycles) | [59] | [104] | Orange peel | 1391 | 0.5 | 407 | Animal waste | |||||
| Crab shell | 3442 | 0.2 | 280.6 | Two-electrode cell with symmetrical electrodes. |
[37] | |||||||||
| Pork bone | 1260 | 0.5 | 263 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] | |||||||||
| Blackfish bone | 1202 | 0.5 | 302 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] | |||||||||
| Eel bone | 1163 | 0.5 | 264 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
