| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Phatsawee Jansook | -- | 1809 | 2022-10-28 10:54:01 | | | |
| 2 | Rita Xu | Meta information modification | 1809 | 2022-10-28 11:01:12 | | |
Video Upload Options
Glaucoma is one of the leading causes of irreversible blindness worldwide. It is characterized by progressive optic neuropathy in association with damage to the optic nerve head and, subsequently, visual loss if it is left untreated. Among the drug classes used for the long-term treatment of open-angle glaucoma, prostaglandin analogues (PGAs) are the first-line treatment and are available as marketed eye drop formulations for intraocular pressure (IOP) reduction by increasing the trabecular and uveoscleral outflow. PGAs have low aqueous solubility and are very unstable (i.e., hydrolysis) in aqueous solutions, which may hamper their ocular bioavailability and decrease their chemical stability. Additionally, treatment with PGA in conventional eye drops is associated with adverse effects, such as conjunctival hyperemia and trichiasis. It has been a very challenging for formulation scientists to develop stable aqueous eye drop formulations that increase the PGAs’ solubility and enhance their therapeutic efficacy while simultaneously lowering their ocular side effects.
1. Introduction
| Prostaglandin Analog | Structure | Molecular Weight | Calculated Values a | |
|---|---|---|---|---|
| LogP(o/w) b | Solubility in Water c | |||
| Prostaglandin F2α (pKa 4.76) |
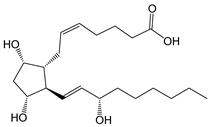 |
354.48 | 2.6 (LogD7.0 0.4) |
30 mg/mL (at pH 7.0) |
| Latanoprost acid | 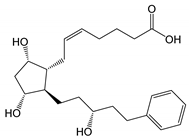 |
390.51 | 2.8 (LogD7.0 0.6) |
7 mg/mL (at pH 7.0) |
| Bimatoprost (Lumigan®) |
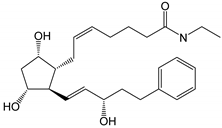 |
415.57 | 2.8 | 40 µg/mL |
| Latanoprost (Xalatan®, Xelpros®) |
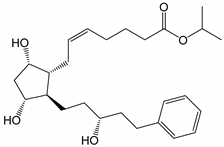 |
432.59 | 4.3 | 6 µg/mL |
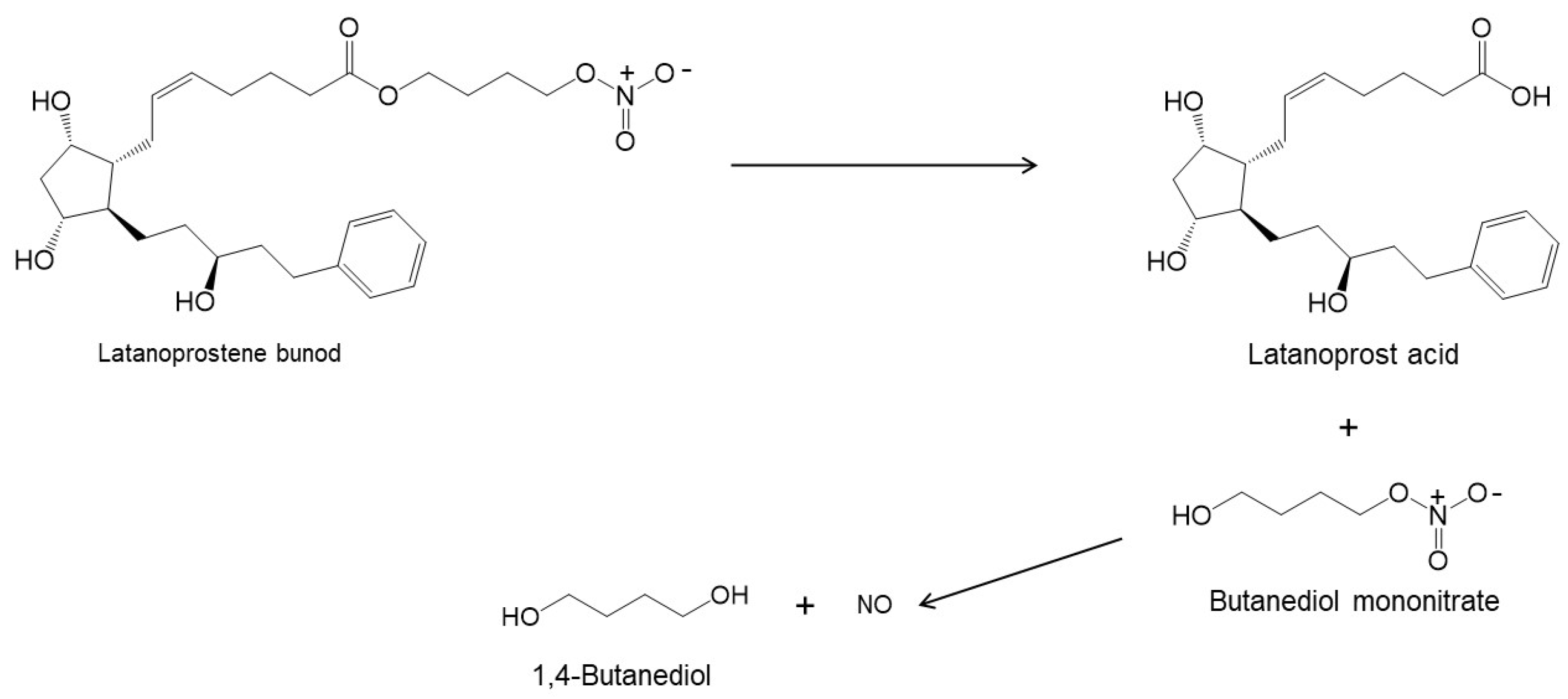
| Latanoprostene bunod (Vyzulta®) |
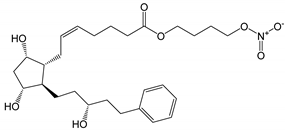 |
507.62 | 4.8 | 1 µg/mL |
| Tafluprost (Taflotan®, Zioptan®) |
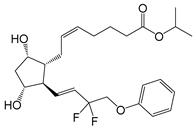 |
452.53 | 3.8 | 10 µg/mL |
| Travoprost (Travatan Z®) |
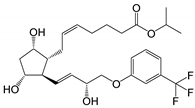 |
500.55 | 4.1 | 4 µg/mL |
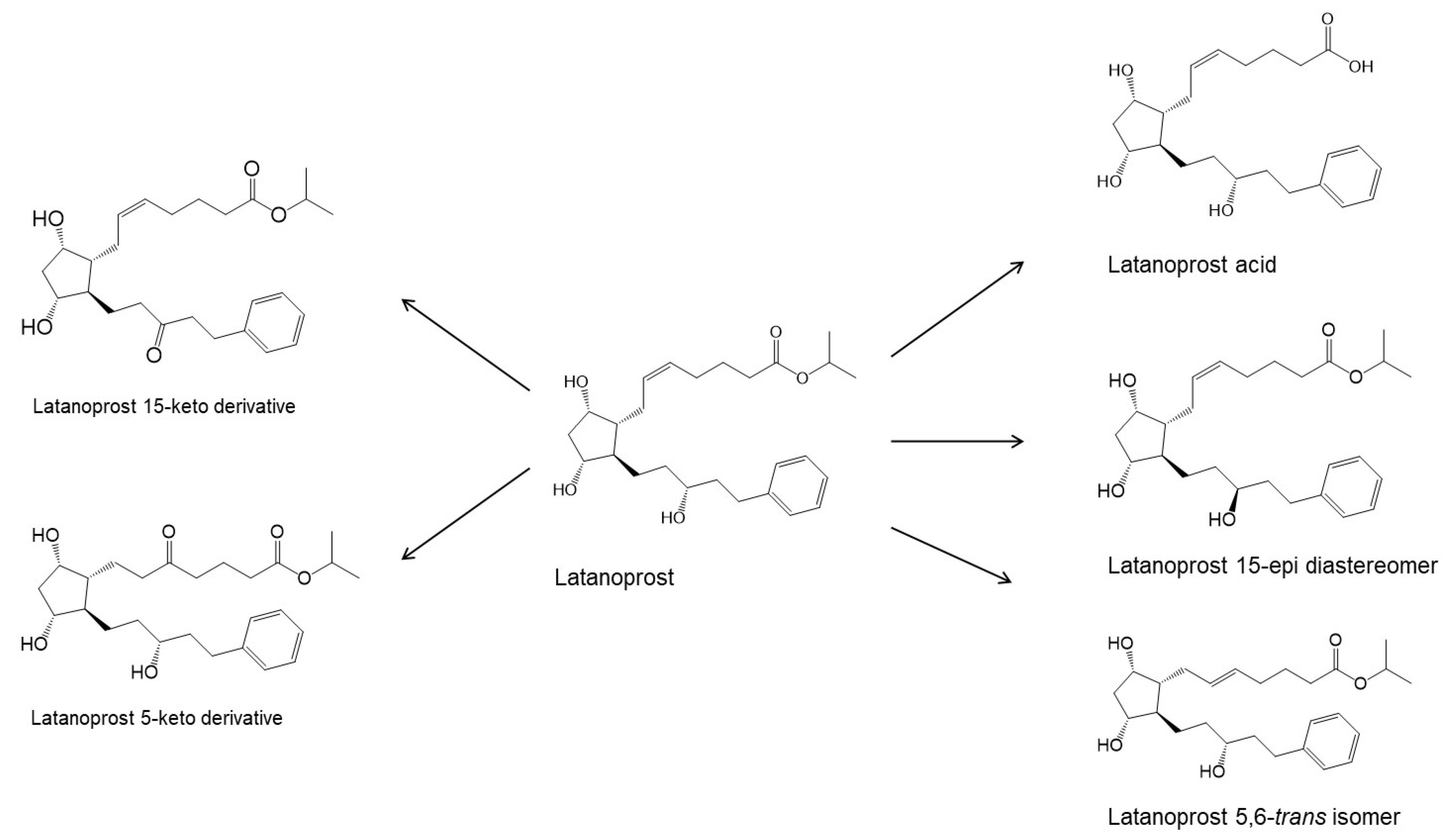
2. Physicochemical Properties and Eye Drop Formulations
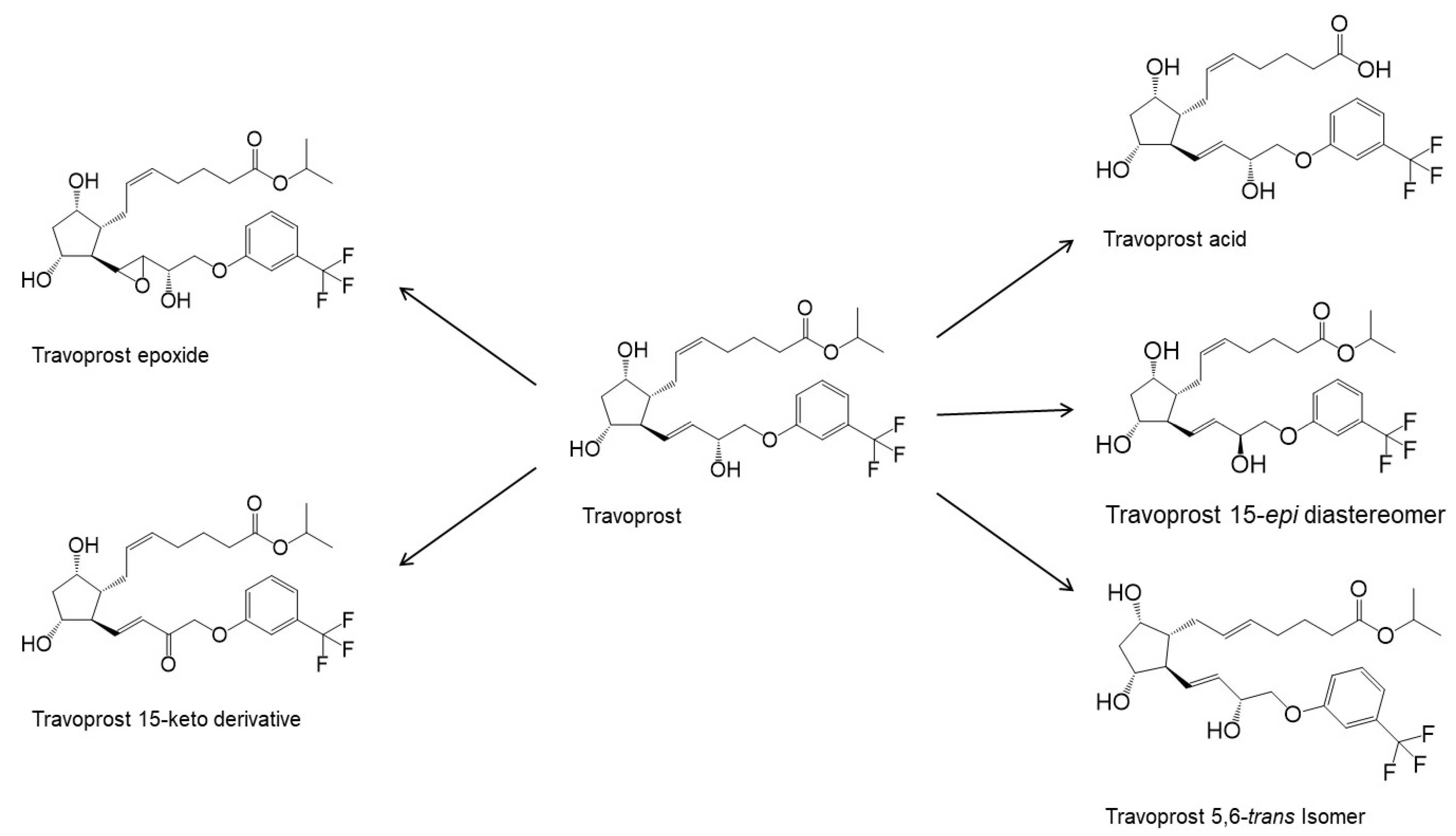
References
- Zukerman, R.; Harris, A.; Vercellin, A.V.; Siesky, B.; Pasquale, L.R.; Ciulla, T.A. Molecular genetics of glaucoma: Subtype and ethnicity considerations. Genes 2021, 12, 55.
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56.
- Sali, T. Prostaglandins. In Encyclopedic Reference of Immunotoxicology; Assenmacher, M., Avraham, H.K., Avraham, S., Bala, S., Barnett, J., Basketter, D., Ben-David, Y., Berek, C., Blümel, J., Bolliger, A.P., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 537–540.
- Shaw, J.E.; Ramwell, P.W. Prostaglandins: A general review. Res. Prostaglandins 1971, 1, 1–8.
- Camras, C.B.; Bito, L.Z.; Eakins, K.E. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Investig. Ophthalmol. Vis. Sci. 1977, 16, 1125–1134.
- Russo, A.; Riva, I.; Pizzolante, T.; Noto, F.; Quaranta, L. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: A review. Clin. Ophthalmol. 2008, 2, 897–905.
- Wang, T.; Cao, L.; Jiang, Q.; Zhang, T. Topical medication therapy for glaucoma and ocular hypertension. Front. Pharmacol. 2021, 12, 749858.
- Katsanos, A.; Riva, I.; Bozkurt, B.; Holló, G.; Quaranta, L.; Oddone, F.; Irkec, M.; Dutton, G.N.; Konstas, A.G. A new look at the safety and tolerability of prostaglandin analogue eyedrops in glaucoma and ocular hypertension. Expert Opin. Drug Saf. 2022, 21, 525–539.
- Davies, S.S.; Ju, W.-K.; Neufeld, A.H.; Abran, D.; Chemtob, S.; Roberts, L.J. Hydrolysis of Bimatoprost (Lumigan) to its free acid by ocular tissue in vitro. J. Ocul. Pharmacol. Ther. 2003, 19, 45–54.
- Moussa, W.G.E.H.; Farhat, R.G.; Nehme, J.C.; Sahyoun, M.A.; Schakal, A.R.; Jalkh, A.E.; Karam, M.P.A.; Azar, G.G. Comparison of efficacy and ocular surface disease index score between bimatoprost, latanoprost, travoprost, and tafluprost in glaucoma patients. J. Ophthalmol. 2018, 1319628.
- Najjar, A.; Najjar, A.; Karaman, R. Newly developed prodrugs and prodrugs in development; an insight of the recent years. Molecules 2020, 25, 884.
- Samaha, D.; Diaconu, V.; Bouchard, J.F.; Desalliers, C.; Dupont, A. Effect of latanoprostene bunod on optic nerve head blood flow. Optom. Vis. Sci. 2022, 99, 172–176.
- Harasymowycz, P.; Royer, C.; Mathurin, K.; Lachaine, J.; Beauchemin, C.; Cui, A.X.; Barbeau, M.; Jobin-Gervais, K.; Mathurin, K.; Lachaine, J.; et al. Short-term efficacy of latanoprostene bunod for the treatment of open-angle glaucoma and ocular hypertension: A systematic literature review and a network meta-analysis. Br. J. Ophthalmol. 2022, 106, 640–647.
- Winkler, N.S.; Fautsch, M.P. Effects of prostaglandin analogues on aqueous humor outflow pathways. J. Ocul. Pharmacol. Ther. 2014, 30, 102–109.
- Yoshida, F.; Topliss, J.G. Unified model for the corneal permeability of related and diverse compounds with respect to their physicochemical properties. J. Pharm. Sci. 1996, 85, 819–823.
- Loftsson, T. Topical drug delivery to the retina: Obstacles and routes to success. Expert Opin. Drug Deliv. 2022, 19, 9–21.
- Sripetch, S.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: Thermodynamic considerations. Int. J. Pharm. 2021, 597, 120332.
- Hageman, M.J. Prostaglandin E2. In Chemical Stability of Pharmaceuticals: A Handbook for Pharmacists, 2nd ed.; Connors, K.A., Amidon, G.L., Stella, V.J., Eds.; John Wiley & Sons: New York, NY, USA, 1986; pp. 719–727.
- Oesterling, T.O.; Morozowich, W.; Roseman, T.J. Prostaglandins. J. Pharm. Sci. 1972, 61, 1861–1895.
- Stehle, R.G. Physical chemistry, stability, and handling of prostaglandins E2, F2α, D2, and I2: A critical summary. Methods Enzymol. 1982, 86, 436–458.
- Morgan, P.V.; Proniuk, S.; Blanchard, J.; Noecker, R.J. Effect of temperature and light on the stability of latanoprost and its clinical relevance. J. Glaucoma 2001, 10, 401–405.
- Velpandian, T.; Kotnala, A.; Halder, N.; Ravi, A.K.; Archunan, V.; Sihota, R. Stability of latanoprost in generic formulations using controlled degradation and patient usage simulation studies. Curr. Eye Res. 2015, 40, 561–571.
- Ochiai, A.; Iida, K.; Takabe, H.; Kawamura, E.; Sato, Y.; Kato, Y.; Ohkuma, M.; Danjo, K. Formulation design of latanoprost eye drops to improve the stability at room temperature. J. Pharm. Sci. Technol. 2010, 70, 324–332.
- Sawatdee, S.; Phetmung, H.; Srichana, T. Development of a stable latanoprost solution for use as eye drops. Chiang Mai J. Sci. 2013, 40, 656–668.
- Zhou, X.; Li, X.; Xu, J.; Cheng, Y.; Cao, F. Latanoprost-loaded cyclodextrin microaggregate suspension eye drops for enhanced bioavailability and stability. Eur. J. Pharm. Sci. 2021, 160, 105758.
- Rodriguez-Aller, M.; Guinchard, S.; Guillarme, D.; Pupier, M.; Jeannerat, D.; Rivara-Minten, E.; Veuthey, J.-L.; Gurny, R. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur. J. Pharm. Biopharm. 2015, 95, 203–214.
- Ochiai, A.; Danjo, K. The stabilization mechanism of latanoprost. Int. J. Pharm. 2011, 410, 23–30.
- Airy, S.; Chiou, J.; Nguyen, H.; Jani, R.; Gan, O.; Kabra, B.; Nguyen, H.; Weiner, A. Developmental preformulation studies in the design of travoprost ophthalmic solution 0.004% (TRAVANTAN®). In Proceedings of the American Association of Pharmaceutical Scientists (AAPS) Annual Meeting, Toronto, ON, Canada, 16–29 October 2002.
- Kahook, M.Y. Travoprost Z ophthalmic solution with sofZia: Clinical safety and efficacy. Expert Rev. Ophthalmol. 2007, 2, 363–368.
- Alviset, G.; Corvis, Y.; Hammad, K.; Lemut, J.; Maury, M.; Mignet, N.; Boudy, V. New preservative-free formulation for the enhanced ocular bioavailability of prostaglandin analogues in glaucoma. Pharmaceutics 2022, 14, 453.
- Loftson, T. Drug Stability for Pharmaceutical Scientists; Academic Press: Cambridge, CA, USA, 2014; p. 170.
- Johnson, T.V.; Gupta, P.K.; Vudathala, D.K.; Blair, I.A.; Tanna, A.P. Thermal stability of bimatoprost, latanoprost, and travoprost under simulated daily use. J. Ocul. Pharmacol. Ther. 2011, 27, 51–59.
- Swymer, C.; Neville, M.W. Tafluprost: The first preservative-free prostaglandin to treat open-angle glaucoma and ocular hypertension. Ann. Pharmacother. 2012, 46, 1506–1510.
- Kaufman, P.L. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin. Pharmacother. 2017, 18, 433–444.