
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yann Gibert | -- | 2002 | 2022-10-27 20:43:20 | | | |
| 2 | Peter Tang | + 15 word(s) | 2017 | 2022-10-28 04:59:44 | | |
Video Upload Options
Xmrk is a gene product closely related to the human epidermal growth factor receptor (EGFR), which is associated with a wide variety of pathological conditions, including cancer. Comparative analyses of Xmrk and EGFR signal transduction in melanoma have shown that both utilize signal transducer and activator of transcription 5 (STAT5) signaling to regulate apoptosis and cell proliferation, phosphoinositide 3-kinase (PI3K) to modulate apoptosis, focal adhesion kinase (FAK) to control migration, and the Ras/Raf/MEK/mitogen-activated protein kinase (MAPK) pathway to regulate cell survival, proliferation, and differentiation. Further, Xmrk and EGFR may also modulate similar chemokine, extracellular matrix, oxidative stress, and microRNA signaling pathways in melanoma. In hepatocellular carcinoma (HCC), Xmrk and EGFR signaling utilize STAT5 to regulate cell proliferation, and Xmrk may signal through PI3K and FasR to modulate apoptosis. At the same time, both activate the Ras/Raf/MEK/MAPK pathway to regulate cell proliferation and E-cadherin signaling.
1. Introduction
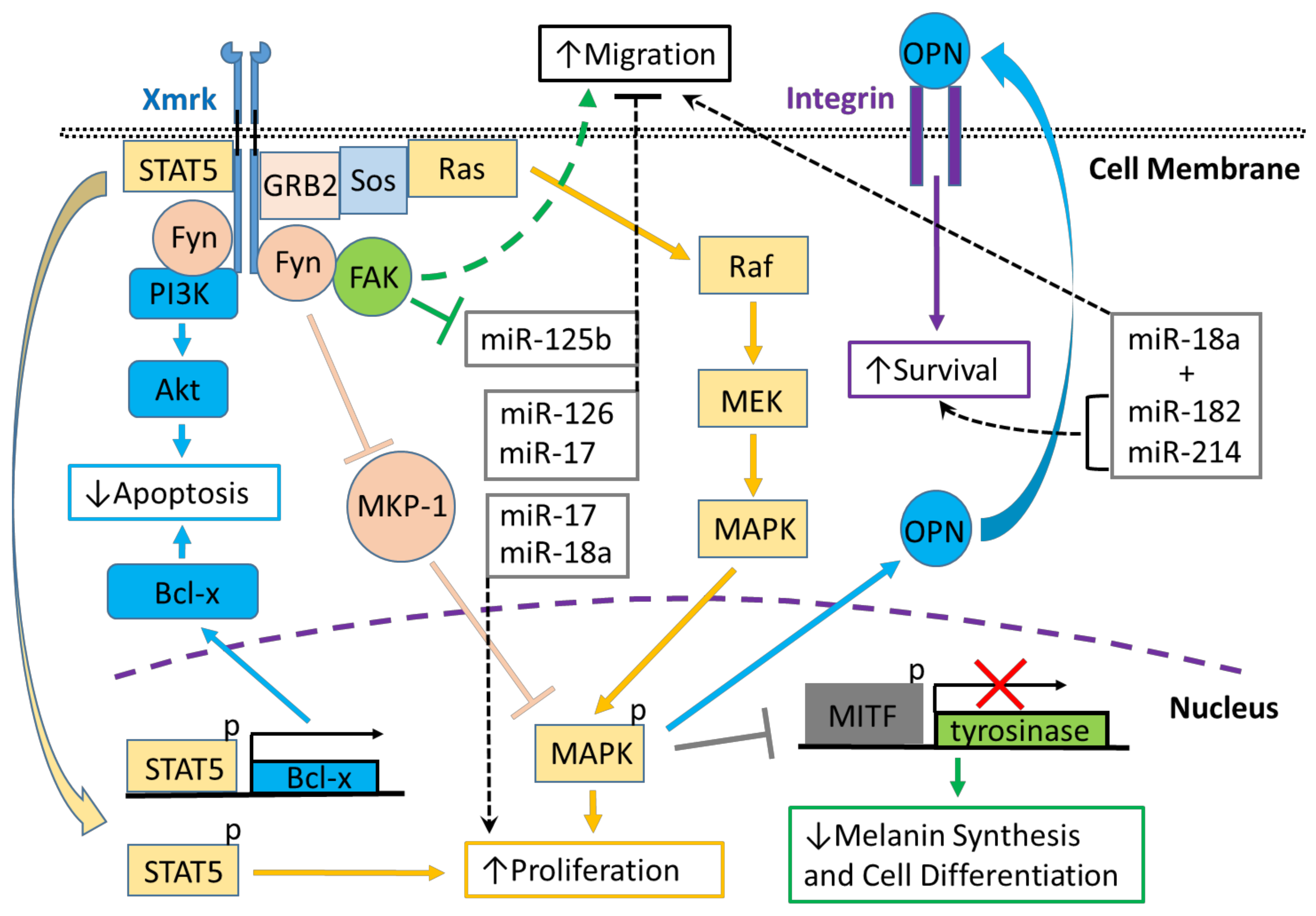
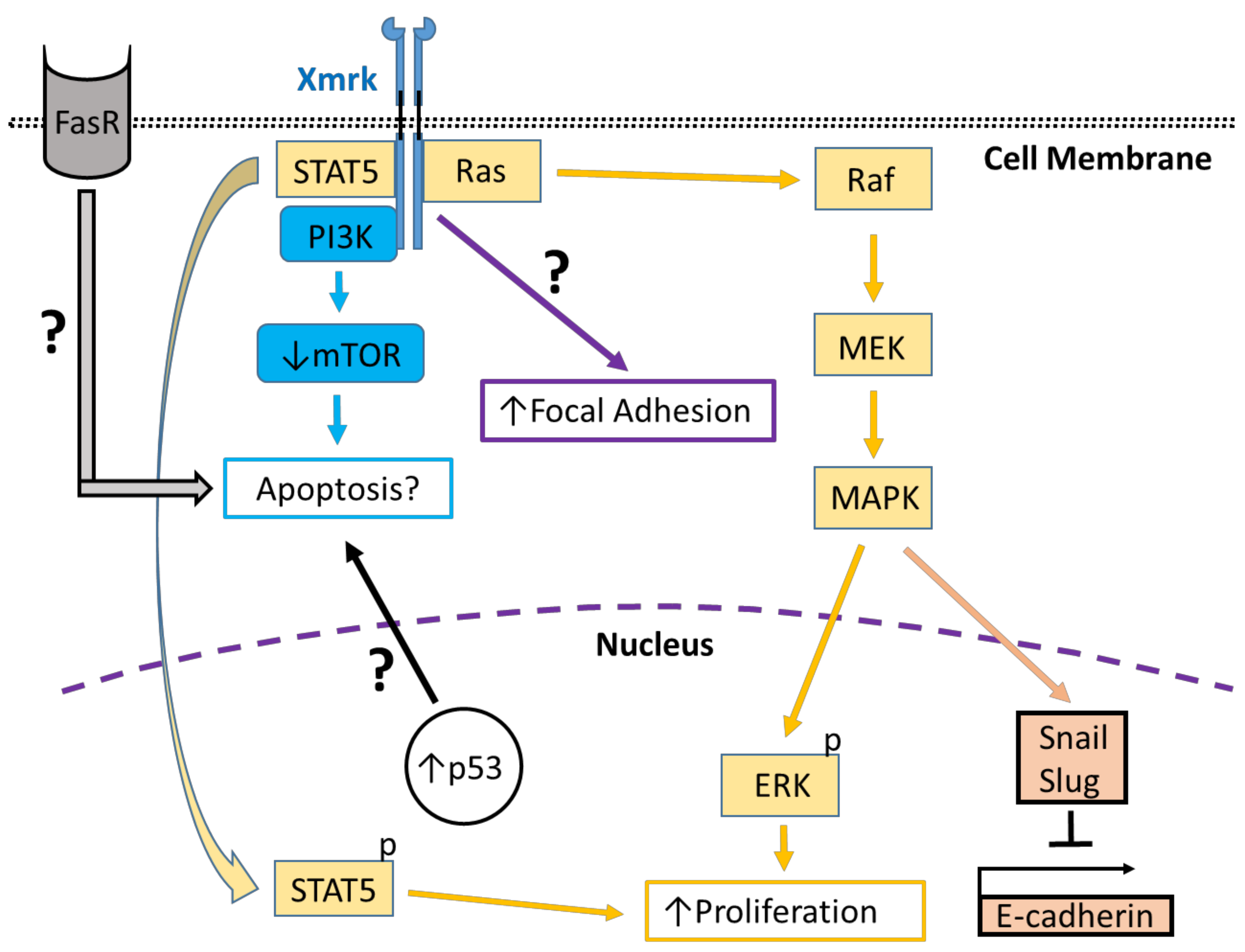
2. Genetic Characterization and Comparison of Xmrk with the Human EGFR
|
Ensembl ID |
Transcript |
Length (nt) |
Protein (Uniprot) |
Length (aa) |
|---|---|---|---|---|
|
egfra-201 |
ENSDART00000108964.5 |
1868 |
F1RBY7 |
503 |
|
egfra-202 |
ENSDART00000128514.2 |
1881 |
F1RA48 |
389 |
|
egfra-203 |
ENSDART00000136906.3 |
2437 |
F1R671 |
760 |
|
egfra-205 |
ENSDART00000147261.3 |
3168 |
F1QU74 |
243 |
|
egfra-206 |
ENSDART00000150499.3 |
3007 |
F1Q7X2 |
625 |
|
egfra-207 |
ENSDART00000164152.3 |
6151 |
A0A0R4IFV9 |
1191 |
|
Ensembl ID |
Transcript |
Length (nt) |
Protein (Uniprot) |
Length (aa) |
|---|---|---|---|---|
|
EGFR-201 |
ENST00000275493.7 |
9905 |
P0053-1 |
1210 |
|
EGFR-202 |
ENST00000342916.7 |
2239 |
P00533-4 |
628 |
|
EGFR-203 |
ENST00000344576.6 |
2864 |
P00533-3 |
705 |
|
EGFR-204 |
ENST00000420316.6 |
1570 |
P00533-2 |
405 |
|
EGFR-205 |
ENST00000450046.1 |
691 |
C9JYS6 |
128 |
|
EGFR-206 |
ENST00000454757.6 |
5464 |
E9PFD7 |
1165 |
|
EGFR-207 |
ENST00000455089.5 |
3844 |
Q504U8 |
1091 |
|
NCBI ID |
Transcript |
Length (nt) |
Protein (NCBI) |
Length (aa) |
|
Isoform A |
NM_005228.5 |
9905 |
NP_005219.1 |
1210 |
|
Isoform G |
NM_001346899.2 |
9770 |
NP_001346899.2 |
1165 |
|
Isoform I |
NM_001346941.2 |
9104 |
NP_001333870.1 |
943 |
|
Isoform F |
NM_001346898.2 |
3983 |
NP_001333827.1 |
1136 |
|
Isoform E |
NM_001346897.2 |
3848 |
NP_001333826.1 |
1091 |
|
Isoform D |
NM_201284.2 |
2872 |
NP_958441.1 |
705 |
|
Isoform B |
NM_201282.2 |
2254 |
NP_958439.1 |
628 |
|
Isoform C |
NM_201283.2 |
1575 |
NP_958440.1 |
405 |
|
Isoform H |
NM_001346900.2 |
9676 |
NP_001333829.1 |
1157 |
References
- Yarden, Y. The EGFR family and its ligands in human cancer: Signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37, S3–S8.
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069.
- Komposch, K.; Sibilia, M. EGFR Signaling in Liver Diseases. Int. J. Mol. Sci. 2015, 17, 30.
- Tímár, J.; Vizkeleti, L.; Doma, V.; Barbai, T.; Rásó, E. Genetic progression of malignant melanoma. Cancer Metastasis Rev. 2016, 35, 93–107.
- Barberán, S.; Martín-Durán, J.M.; Cebrià, F. Evolution of the EGFR pathway in Metazoa and its diversification in the planarian Schmidtea mediterranea. Sci. Rep. 2016, 6, 28071.
- Schartl, M. Homology of melanoma-inducing loci in the genus Xiphophorus. Genetics 1990, 126, 1083–1091.
- Schartl, M.; Adam, D. Molecular cloning, structural characterization, and analysis of transcription of the melanoma oncogene of Xiphophorus. Pigment Cell Res. 1992, 2, 173–180.
- Wakamatsu, Y. Establishment of a cell line from the platyfish-swordtail hybrid melanoma. Cancer Res. 1981, 41, 679–680.
- Wittbrodt, J.; Lammers, R.; Malitschek, B.; Ullrich, A.; Schartl, M. The Xmrk receptor tyrosine kinase is activated in Xiphophorus malignant melanoma. EMBO J. 1992, 11, 4239–4246.
- Wellbrock, C.; Fischer, P.; Schartl, M. Receptor tyrosine kinase Xmrk mediates proliferation in Xiphophorus melanoma cells. Int. J. Cancer 1998, 76, 437–442.
- Malitschek, B.; Wittbrodt, J.; Fischer, P.; Lammers, R.; Ullrich, A.; Schartl, M. Autocrine stimulation of the Xmrk receptor tyrosine kinase in Xiphophorus melanoma cells and identification of a source for the physiological ligand. J. Biol. Chem. 1994, 269, 10423–10430.
- Winkler, C.; Wittbrodt, J.; Lammers, R.; Ullrich, A.; Schartl, M. Ligand-dependent tumor induction in medakafish embryos by a Xmrk receptor tyrosine kinase transgene. Oncogene 1994, 9, 1517–1525.
- Winnemoeller, D.; Wellbrock, C.; Schartl, M. Activating mutations in the extracellular domain of the melanoma inducing receptor Xmrk are tumorigenic in vivo. Int. J. Cancer 2005, 117, 723–729.
- Laisney, J.A.; Mueller, T.D.; Schartl, M.; Meierjohann, S. Hyperactivation of constitutively dimerized oncogenic EGF receptors by autocrine loops. Oncogene 2013, 32, 2403–2411.
- Lu, Y.; Boswell, M.; Boswell, W.; Kneitz, S.; Hausmann, M.; Klotz, B.; Regneri, J.; Savage, M.; Amores, A.; Postlethwait, J.; et al. Comparison of Xiphophorus and human melanoma transcriptomes reveals conserved pathway interactions. Pigment Cell Melanoma Res. 2018, 31, 496–508.
- Gossen, M.; Freundlieb, S.; Bender, G.; Müller, G.; Hillen, W.; Bujard, H. Transcriptional activation by tetracyclines in mammalian cells. Science 1995, 268, 1766–1769.
- Knopf, F.; Schnabel, K.; Haase, C.; Pfeifer, K.; Anastassiadis, K.; Weidinger, G. Dually inducible TetON systems for tissue-specific conditional gene expression in zebrafish. Proc. Natl. Acad. Sci. USA 2010, 107, 19933–19938.
- Li, Z.; Huang, X.; Zhan, H.; Zeng, Z.; Li, C.; Spitsbergen, J.M.; Meierjohann, S.; Schartl, M.; Gong, Z. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J. Hepatol. 2012, 56, 419–425.
- Li, Z.; Luo, H.; Li, C.; Huo, X.; Yan, C.; Huang, X.; Al-Haddawi, M.; Mathavan, S.; Gong, Z. Transcriptomic analysis of a transgenic zebrafish hepatocellular carcinoma model reveals a prominent role of immune responses in tumour progression and regression. Int. J. Cancer 2014, 135, 1564–1573.
- Zheng, W.; Li, Z.; Nguyen, A.T.; Li, C.; Emelyanov, A.; Gong, Z. Xmrk, kras and myc transgenic zebrafish liver cancer models share molecular signatures with subsets of human hepatocellular carcinoma. PLoS ONE 2014, 9, e91179.
- Li, Z.; Zheng, W.; Li, H.; Li, C.; Gong, Z. Synergistic Induction of Potential Warburg Effect in Zebrafish Hepatocellular Carcinoma by Co-Transgenic Expression of Myc and xmrk Oncogenes. PLoS ONE 2015, 10, e0132319.
- Förnzler, D.; Altschmied, J.; Nanda, I.; Kolb, R.; Baudler, M.; Schmid, M.; Schartl, M. The Xmrk oncogene promoter is derived from a novel amplified locus of unusual organization. Genome Res. 1996, 6, 102–113.
- Dimitrijevic, N.; Winkler, C.; Wellbrock, C.; Gómez, A.; Duschl, J.; Altschmied, J.; Schartl, M. Activation of the Xmrk proto-oncogene of Xiphophorus by overexpression and mutational alterations. J. Biol. Chem. 1997, 272, 131–137.
- Weis, S.; Schartl, M. The macromelanophore locus and the melanoma oncogene Xmrk are separate genetic entities in the genome of Xiphophorus. Genetics 1998, 149, 1909–1920.
- Schartl, M.; Hornung, U.; Gutbrod, H.; Volff, J.N.; Wittbrodt, J. Melanoma loss-of-function mutants in Xiphophorus caused by Xmrk-oncogene deletion and gene disruption by a transposable element. Genetics 1999, 153, 1385–1394.
- Gómez, A.; Volff, J.N.; Hornung, U.; Schartl, M.; Wellbrock, C. Identification of a second egfr gene in Xiphophorus uncovers an expansion of the epidermal growth factor receptor family in fish. Mol. Biol. Evol. 2004, 21, 266–275.
- Meierjohann, S.; Mueller, T.; Schartl, M.; Buehner, M. A structural model of the extracellular domain of the oncogenic EGFR variant Xmrk. Zebrafish 2006, 3, 359–369.
- Gómez, A.; Wellbrock, C.; Gutbrod, H.; Dimitrijevic, N.; Schartl, M. Ligand-independent dimerization and activation of the oncogenic Xmrk receptor by two mutations in the extracellular domain. J. Biol. Chem. 2001, 276, 3333–3340.
- Altschmied, J.; Ditzel, L.; Schartl, M. Hypomethylation of the Xmrk oncogene promoter in melanoma cells of Xiphophorus. Biol. Chem. 1997, 378, 1457–1466.
- Regneri, J.; Volff, J.N.; Schartl, M. Transcriptional control analyses of the Xiphophorus melanoma oncogene. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 178, 116–127.
- Reiter, J.L.; Threadgill, D.W.; Eley, G.D.; Strunk, K.E.; Danielsen, A.J.; Sinclair, C.S.; Pearsall, R.S.; Green, P.J.; Yee, D.; Lampland, A.L.; et al. Comparative genomic sequence analysis and isolation of human and mouse alternative EGFR transcripts encoding truncated receptor isoforms. Genomics 2001, 71, 1–20.
- Boerner, J.L.; Danielsen, A.; Maihle, N.J. Ligand-independent oncogenic signaling by the epidermal growth factor receptor: V-ErbB as a paradigm. Exp. Cell Res. 2003, 284, 111–121.
- Murphy-Ullrich, J.E. The de-adhesive activity of matricellular proteins: Is intermediate cell adhesion an adaptive state? J. Clin. Investig. 2001, 107, 785–790.
- Ullrich, A.; Coussens, L.; Hayflick, J.S.; Dull, T.J.; Gray, A.; Tam, A.W.; Lee, J.; Yarden, Y.; Libermann, T.A.; Schlessinger, J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984, 309, 418–425.
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52.
- Kim, H.M.; Lee, S.H.; Lim, J.; Yoo, J.; Hwang, D.Y. The epidermal growth factor receptor variant type III mutation frequently found in gliomas induces astrogenesis in human cerebral organoids. Cell Prolif. 2021, 54, e12965.
- Du, Z.; Brown, B.P.; Kim, S.; Ferguson, D.; Pavlick, D.C.; Jayakumaran, G.; Benayed, R.; Gallant, J.N.; Zhang, Y.K.; Yan, Y.; et al. Structure-function analysis of oncogenic EGFR Kinase Domain Duplication reveals insights into activation and a potential approach for therapeutic targeting. Nat. Commun. 2021, 12, 1382.
- Albitar, L.; Pickett, G.; Morgan, M.; Wilken, J.A.; Maihle, N.J.; Leslie, K.K. EGFR isoforms and gene regulation in human endometrial cancer cells. Mol. Cancer 2010, 9, 166.
- Guillaudeau, A.; Durand, K.; Bessette, B.; Chaunavel, A.; Pommepuy, I.; Projetti, F.; Robert, S.; Caire, F.; Rabinovitch-Chable, H.; Labrousse, F. EGFR soluble isoforms and their transcripts are expressed in meningiomas. PLoS ONE 2012, 7, e37204.
- Guillaudeau, A.; Durand, K.; Rabinovitch-Chable, H.; Pommepuy, I.; Mesturoux, L.; Robert, S.; Chaunavel, A.; Moreau, J.J.; Labrousse, F. Adult diffuse gliomas produce mRNA transcripts encoding EGFR isoforms lacking a tyrosine kinase domain. Int. J. Oncol. 2012, 40, 1142–1152.
- Jaillon, O.; Aury, J.M.; Brunet, F.; Petit, J.L.; Stange-Thomann, N.; Mauceli, E.; Bouneau, L.; Fischer, C.; Ozouf-Costaz, C.; Bernot, A.; et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 2004, 431, 946–957.
- Laisney, J.A.; Braasch, I.; Walter, R.B.; Meierjohann, S.; Schartl, M. Lineage-specific co-evolution of the Egf receptor/ligand signaling system. BMC Evol. Biol. 2010, 10, 27.
- Ningappa, M.; So, J.; Glessner, J.; Ashokkumar, C.; Ranganathan, S.; Min, J.; Higgs, B.W.; Sun, Q.; Haberman, K.; Schmitt, L.; et al. The Role of ARF6 in Biliary Atresia. PLoS ONE 2015, 10, e0138381.




