Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gareeballah Adam | -- | 2622 | 2022-10-27 13:23:53 | | | |
| 2 | Sirius Huang | Meta information modification | 2622 | 2022-10-28 03:05:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Adam, G.O.; Sharker, S.M.; Ryu, J.H. General Aspects of Carbon Dot and Polymer Composites. Encyclopedia. Available online: https://encyclopedia.pub/entry/31649 (accessed on 08 February 2026).
Adam GO, Sharker SM, Ryu JH. General Aspects of Carbon Dot and Polymer Composites. Encyclopedia. Available at: https://encyclopedia.pub/entry/31649. Accessed February 08, 2026.
Adam, Gareeballah Osman, Shazid Md. Sharker, Ji Hyun Ryu. "General Aspects of Carbon Dot and Polymer Composites" Encyclopedia, https://encyclopedia.pub/entry/31649 (accessed February 08, 2026).
Adam, G.O., Sharker, S.M., & Ryu, J.H. (2022, October 27). General Aspects of Carbon Dot and Polymer Composites. In Encyclopedia. https://encyclopedia.pub/entry/31649
Adam, Gareeballah Osman, et al. "General Aspects of Carbon Dot and Polymer Composites." Encyclopedia. Web. 27 October, 2022.
Copy Citation
Carbon dot-based composite materials have been extensively developed for versatile biomedical applications, such as drug delivery, tissue engineering, bioimaging, biosensors, and photothermal cancer therapy, owing to their excellent mechanical properties, electrical and thermal conductivity, large surface-to-volume ratio, and biocompatibility. For instance, the hydrophobicity and delocalized π-electrons of carbon dots enable insoluble drug loading in carbon composite-based drug delivery carriers. In addition, carbon dot-based materials are suitable for optical and electrochemical biosensor applications owing to their intrinsic properties.
carbon dots
composite materials
polymers
1. Introduction
Carbon dots (CDs) were found during the attempt to purify single-walled carbon nanotubes using electrophoresis [1]. Based on the term dots, CDs can be defined as tiny, carbonized substances having a size of <10 nm, called carbon quantum dots [2]. Various substances can be processed to yield CDs as a starting source, including natural (i.e., watermelon) and synthetic (i.e., citric acid) substances [3][4]. In addition, the following two main strategies have been employed to synthesize CDs: (1) top-down and (2) bottom-up approaches [5][6][7]. CDs have been gaining popularity since they were first discovered owing to their optical, physiochemical, macroscopic, and microscopic properties, as well as electroconductivity, which enables them for use in versatile applications. CDs have been applied in numerous fields including energy conversion, food quality assessment, and material science [8][9]. More importantly, the low toxicity of CDs renders them highly biocompatible; thus, a plethora of biomedical applications have been addressed, including bioimaging, biosensing, cancer and degenerative disease treatment, ion detection, and coronavirus theranostics [9][10]. In addition, CDs have been utilized to convict criminals because they have been used to make efficient materials for detecting forgery and tracing crime scenes [11][12].
CDs and polymer composite materials have also gained significant attention for biochemical, biological, and biomedical applications owing to their facile preparation, cost-effective processing, and biocompatibility [13]. CDs are readily synthesized from a variety of natural and synthetic sources. CD/polymer composites are readily produced by mixing polymer and CDs with/without further treatments (Figure 1a). Although mixing of polymers and nanomaterials is not a novel synthesis process, developing better CD/polymer composite materials that exhibit favorable biocompatibility and optoelectrical properties is an emerging research topic. Polymers with different architectures, such as homo- and co-polymers, hyperbranched polymers, and various polymeric chains, have been employed to anchor and coat CDs to fabricate biocompatible CD/polymer hybrid composite materials. Recent studies have shown that several methods, including ligand exchange between polymers and CDs, grafting polymers to CDs, grafting polymers from CDs, capping polymers onto CDs, and growing CDs within the polymer template, are generally used for biocompatible CD/polymer composite materials [14][15]. As shown in Figure 1b, the facile preparations and remarkable properties of CDs and cross-linked polymeric properties have inspired the potential applications (i.e., drug delivery system, photodynamic therapy, bioimaging, etc.) in biomedical sciences [16].
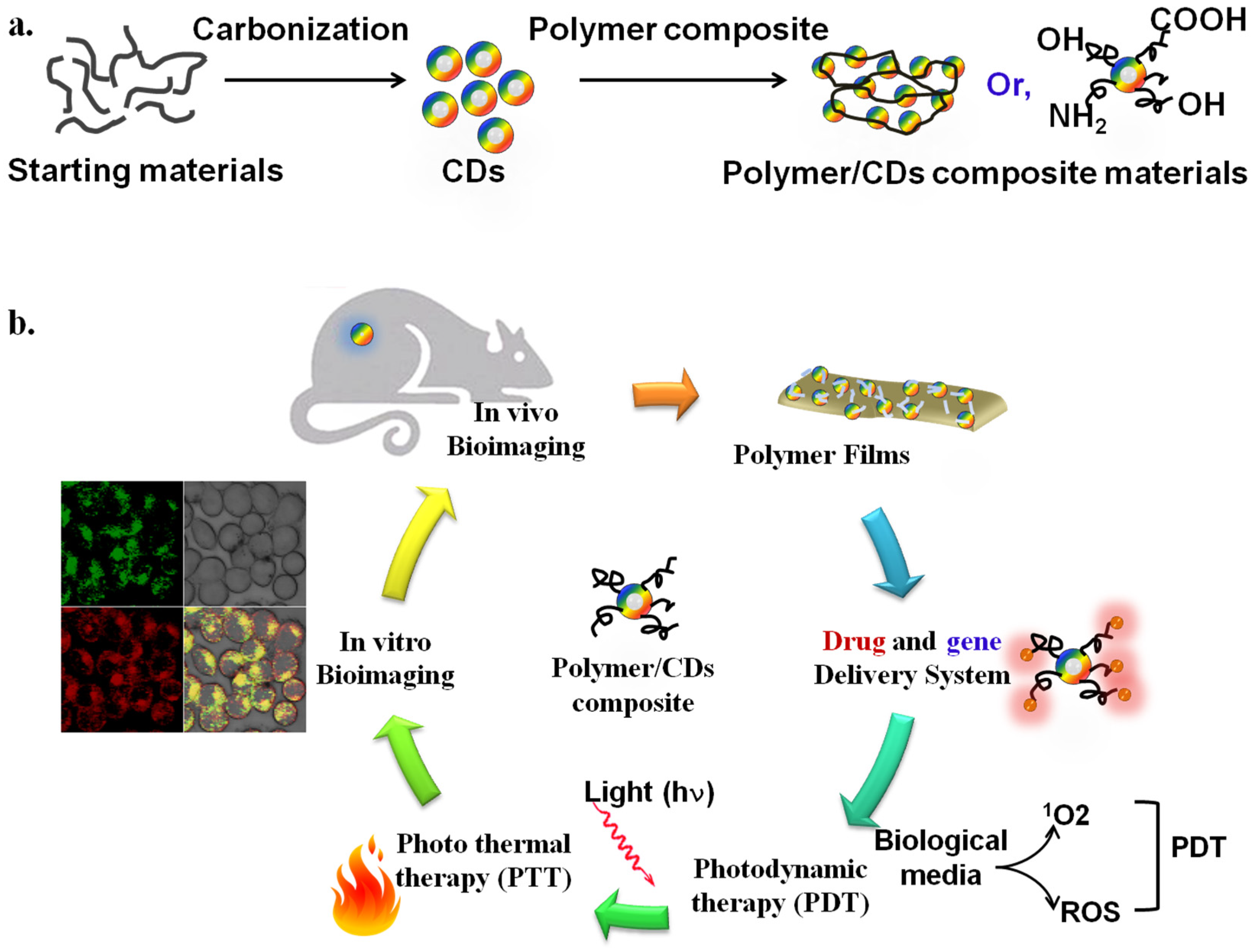
Figure 1. (a) Schematic diagram of the synthesis of CDs from starting materials (first) and fabrications of CD/polymer composite materials (second). (b) The proposed biomedical application of CD/polymer composite materials. The Figure is depicted with slight modification from [16], which is freely accessed, distributed under Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0 (accessed on 14 August 2022)).
2. General Aspects of Carbon Dot/Polymer Composites
The sources of CDs for synthesis are easily obtainable natural carbon and synthetic substances including small organic molecules and polymers. CDs are a type of low-dimensional carbon-dominated nanomaterial, which consists of a sp2/sp3 hybridized carbon skeleton and functional groups. A variety of functional groups (carboxyl, hydroxyl, and amine) enable excellent water solubility and convenience for hybridization with other materials without phase separation. Moreover, a variety of functional groups allow CDs to be easily functionalized with various organic or polymeric molecules and promising nanoparticles [17]. For instance, the ‘ease-to-fabricate’ CD-based polymer films are due to their immediate compatibility in water, resulting in a facile wet mixing procedure of the CD/polymer composite materials [13]. Thus, the hydrophilic CDs and polymers intermingle to form a composite film. The CD/polymer composite films have a high contact angle, which indicates the generation of hydrophobic character on the composite film surface. The surface chemistry and topography of any polymeric film largely depend on its wettability, that is, the less hydrophilic CD/polymer composite.
2.1. Synthesis of Carbon Dot/Polymer Composites
Although CD sources and syntheses have been extensively reviewed, it is noteworthy to briefly highlight the sources and preparation as a prelude to the topic. CDs can be derived from the following two main sources: (1) natural sources, such as carrots, lemons, and funnel seeds, and (2) synthetic sources, such as, phenylalanine and citric acid [4][16]. More studies are needed to determine whether the source of CDs affects their effectiveness and properties. Top-down and bottom-up strategies were employed to prepare the CDs (Figure 2). While the top-down approach involves breaking down the carbon material structures (e.g., chemical oxidation process, plasma reactor, microwave, and laser ablation), the bottom-up process involves the carbonizations of tiny molecules to synthesize CDs (e.g., hydrothermal method, solvothermal method, carbonization, and thermal decomposition) [4][18][19][20][21][22]. CD/polymer hybrid composite materials are prepared by several methods such as mixing, crosslinking reactions, polymerizations, and thermal treatments [23][24][25][26][27]. For instance, simple mixing of CD and polymer solutions [24], incorporation of CD into crosslinked polymer networks [25], and interfacial polymerizations with/without the polymers on the thin-film membranes [26][27] are used to prepare CD/polymer hybrid composite materials.
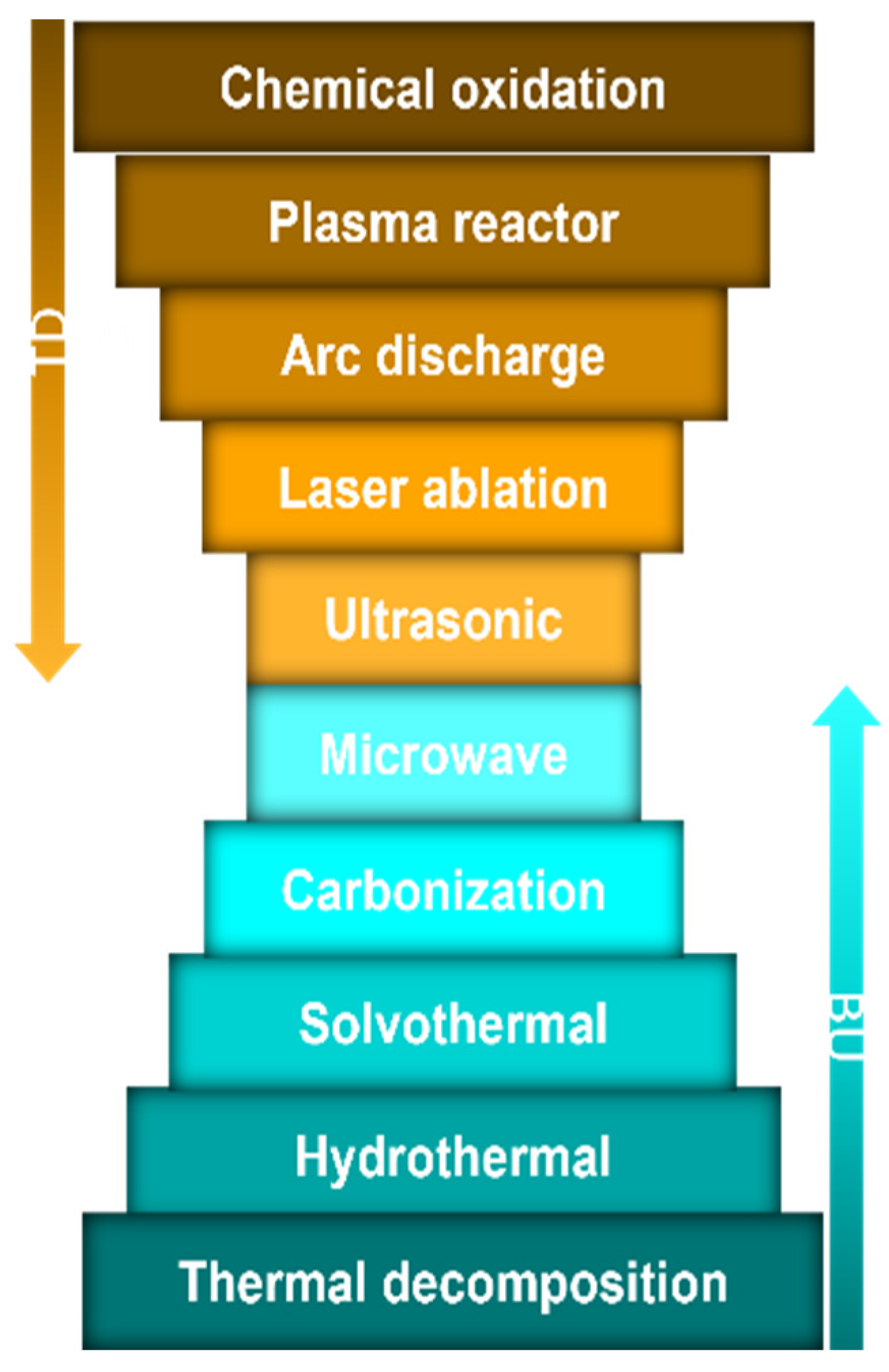
Figure 2. Synthesis of carbon dots: top-down (TD) and bottom-up (BU) approaches.
2.2. Characteristics of Carbon Dots
CDs are semi-spherical, nanoscale substances in their physical form. In general, the size of CDs is less than 10 nm. Several strategies can be used to adjust the size, including the filter size, temperature, and reducing agent [28][29][30]. The physiochemical characteristics of CDs slightly differ based on their sources, preparation processes and analytical techniques [28]. For instance, the X-ray crystallography (XRD) profile of CDs obtained from dextrose and nano-biomass dots, prepared by ultrasonic-assisted extraction, are reported to be centered at ~2θ = 26° and 21.5°, respectively [31][32]. CDs can carry a negative or positive charge, with a wide range of zeta potential based on the source of the CDs. For example, CDs prepared from Averrhoa carambola fruit using hydrothermal treatment had zeta potential of −15.21 mV [33]. The organic components were found to vary in content as follows: nitrogen, carbon, and hydrogen contents were approximately 0–20%, 7–49%, and 2–9%, respectively, in a study using various starting materials (polyethyleneimine, N,N-dimethylethylene diamine, diethylenetriamine, ethanolamine, N’-ethylcarbodiimide hydrochloride) and synthesis methods (pyrolysis, microwave, and solvothermal) as reported by Fan et al. [34]. The superficial presence of functional groups (–OH,–COOH, and –NH2), which can be attained by surface passivation techniques, improves the solubility and chemical reactivity of CDs [35].
The absorption spectrum of CDs is very large, covering the entire UV range [2][11][36]. Once excited, CDs display fluorescence, electroluminescence, phosphor-luminescence, and other characteristics [37]. Several explanations of CD photoluminescence have been reported, such as conjugation of the π-domain with a quantum confinement effect originating from the defect/surface state and subdomain state within the graphitic carbon core; the emission may be improved by the surface state, subdomain state, and molecular state crosslink [38]. The understanding of the mechanism behind these luminescent properties remains limited; however, CDs have been utilized in biolabeling and bioimaging owing to their chemiluminescence feature [38].
2.3. Pharmacology of the Carbon Dot—Fate—In the Body
As previously indicated, the majority of CD research has been focused on synthesis, toxicity, and effects. However, pharmacological studies regarding CDs in terms of pharmacokinetics and pharmacodynamics (PKPD) are scarce. The journey of CDs metabolism begins with administration and ends with elimination. The average dose administered differ by studies. Ding and colleagues found that CDs, prepared from citrate by a hydrothermal method, at a concentration of 25–100 µg/mL, inhibited uveal melanoma growth [39]. Furthermore, Phellodendri Cortex Carbonisatus CDs, fabricated by the solvothermal method, showed excellent hemostatic effects in mice at a dose of 1 mg/kg [40][41]. CDs are biotransformed by enzymatic degradation, notably myeloperoxidase, neutrophil, and eosinophil peroxidase enzymes (in the presence of H2O2), which are mainly involved in the metabolism of CDs, as investigated by Martin et al. [42]. In summary, the smaller the size, the lesser the toxicity and more efficient the processing, which includes metabolism, target interaction, and elimination. Ideal CDs have a high specificity to the target, minimal off-site interaction, high cell uptake, are readily excreted or biodegradable, hydrophilic, and have a hydrodynamic size of 6 nm or less. CDs can be excreted by the kidney depending on their size and charge [43].
Wang et al. investigated the acute toxicity of the CDs prepared from nitric acid [44]. This study concluded that CDs of 5.1 to 51 mg/kg body weight in mice were safe, and no toxic signs or mortality were recorded even after 2 weeks in acute toxicity evaluations. However, subacute doses (0.2 to 20 mg/kg) caused mild changes in liver enzymes and blood lipoprotein profile, indicating a possibility of mild toxic effects; thus, species variation should be considered. In addition, the bioavailability of graphene quantum dots (GQD) in lung tissues has been detected by Fourier-transform infrared spectroscopy (FT-IR) [45]. The intravenously injected GQDs prepared from graphite flakes into the rats showed no significant acute toxicity with 5 and 15 mg/kg body weight, but mild foreign body reactions to the GQD were found. Future studies regarding CDs absorption, distribution, biotransformation, and excretion are essential for understanding the effects of the body on CDs and to enable researchers to design CDs with optimum effects according to the PK characteristics indicated above.
2.4. Carbon Dots and Polymer Hybrid Composite Materials
2.4.1. Features of Carbon Dot/Polymer Composites
CDs and polymer composite materials demonstrate a polymer-carbon hybrid structure rather than a central carbon body structure, contributing to the predominant polymeric surface properties, which is different from the traditional carbonized CDs. Therefore, composite hybrid materials possess superior optical properties compared to conventional carbonized CDs and bare polymers [17][46]. In general, polymers can act as stabilizing agents for high-volume-to-surface nanoparticles such as CDs. Polymer structures tend to diffuse through the nanoparticle surface to compensate for the starving nature of the nanoparticle surface energy. For these CDs, identical phenomena have been encountered in the polymer chains, and desirable synergistic features were presented. The newly formed CD-based hybrid features have higher optical properties, retaining all other polymer features intact. A remarkable advantage of polymer materials is their reliability in the era of functional product development. The durability of polymer materials is sometimes influenced by improper stress depletion, lack of uniformity in reinforcement, and thermal stability. Under these conditions, CDs play a crucial role in remediating inherent demerits. Physical interactions, H-bonding, polar–polar anchoring, polymeric entanglement, and surface passivation allow CDs to achieve better physisorption-aided anchoring of polymer chains onto the surfaces of CDs [13][47]. The polymeric hybridization of CDs mainly includes the following three features: (1) abundant functional groups and attachment of polymer chains, (2) predefined polydispersity in structures and properties influenced by the polymeric conjugation process, and (3) superior optical properties generated by the process of surface passivation. CDs with tunable optical properties have been extensively developed. For example, nitrogen-doped CDs emit bright blue fluorescence when subjected to UV excitation [48]. In addition, CDs showed red fluorescence when rhodamine B was added as a donor molecule, resulting in these unique optical tuning properties to the transfer of nano-radioactive energy from excited donor to acceptor [48]. Furthermore, oxygen-containing CDs obtained from lemon juice via the hydrothermal method showed bright blue-green emissions when excited with UV [49]. CDs (1.8 nm) synthetized from carbon tetrachloride by hydride-reducing agents showed a wide absorption band at 270 nm. By increasing the mean diameter of CDs, a distinct shift was found in wavelength absorption to 304 and 306 nm [29].
Carbon dot/polymer composites are distinguished from a molecular state, where the surface state is the synergistic hybridization of a polymeric backbone. The energy gap of the surface state, where electrons and holes (e/h) pair and recombine to emit fluorescence, corresponds to the extent of the π-electron and surface chemistry. New sub-levels can be created by surface passivation or other effective modification methods, thus roughly controlling the photoluminescence (PL). Moreover, several studies have demonstrated that most of the fluorescence of the CD/polymer composite originates from the surface state and highlights its potential to change the photoluminescence (PL) properties [50][51][52].
Bare CDs usually exhibit light absorption in the UV–vis region, which has an extremely strong UV absorption. In the CD/polymer composite structure, light absorption results from multiple hybrid structures in the polymer/carbon nanoparticles. The π–π* transition is typically attributed to the aromatic carbon structure, and the n–π* transition is assigned to the functional chemical groups with lone pairs of electrons, including the amino-based chromospheres. Molecular state-connected structures possessing a band gap similar to that of organic fluorescent molecules may also generate specific absorption peaks. The absorption in the longer-wavelength region is assigned to the energy level transition from the angstrom (A°)-sized conjugated π-structure. The derivatives of the aforementioned structure connected with various chemical groups (such as –OH,–COOH, and –NH2) can alter the absorption features of the CD/polymer composite structure. The compact structure within the CD/polymer composite materials enhances the interactions among the groups, changing the energy bandgap and resulting in variations in the absorption [17][53].
2.4.2. Photoluminescent Feature of Carbon Dot and Polymer Composites
The photoluminescence (PL) origin of CDs has been extensively studied; the mechanism generally includes intrinsic state emission (quantum size effect) and surface-defect state emission. The fluorescence origin of the CD/polymer composite structure resembles that of certain fluorescent polymer materials. Standard fluorescent polymer materials include conjugated polymers and polymers that are linked to fluorescent molecules [52]. The fluorescence source may be related to the heteroatom-containing bonds (C-O, C-N, N-O), called sub-fluorophores. Although these sub-fluorophore band gaps are not suitable for visible emission, the configuration of the polymer chains enables interactions among the chemical groups. Therefore, the band gap decreases, resulting in the observed fluorescence emission. Moreover, compared to small-molecule precursors, synthesis conditions such as pH, time, and temperature have a lower impact on the emission wavelength and chemical structure of CD/polymer composite materials [35][46][50].
In addition, the polymer chain can stabilize the CDs. Considering their large surface area, CDs are susceptible to coalesce to form larger aggregates, which enables better fluorescence and sensing behaviors. Polymeric materials act as stabilizers that adsorb onto the surfaces of CDs, resulting from CD/polymer nanocomposites. CD-polymer nanocomposites are currently sufficient to be developed in the field of material science. Thus, CD/polymer composites can be utilized for nanocomposite gel fabrications. Previous studies have demonstrated that carbon dot/polymer composites possess superior physio-mechanical properties compared to conventional polymeric gels [13][54]. Furthermore, the hybrid CD/polymer composite film can be used as a highly swellable, rigid, thermally stable, and photo-responsive material.
Typical polymer passivation agents include polyethylene glycol (PEG), polyethyleneimine (PEI), and similar derivatives. Surface-passivated CDs can simultaneously be modified with different chemical functional groups, including hydroxyl, carbonyl, and carboxyl groups, on the surface defect sites. The doping of organic or polymeric compounds has been used in these methods. Different doping methods, such as N-doping, P-doping, S-doping, and Si-doping, have confirmed the effectiveness of these methods [55][56][57]. For example, N-doped CDs demonstrated an average diameter of 2.5 nm in the n high resolution transmission electron microscopy (HRTEM) image analysis and broad and robust PL spectra over bare CDs [56].
2.4.3. Integrative Feature of Carbon Dot and Polymer Composites
The selection of the polymer and conjugation strategy can retain the required functional groups, such as -COOH and -OH groups, for the secondary loading of bioactive molecules [28]. Integrated CD/polymer composites with bioactive molecules have been demonstrated to possess reliable biocompatibility, bioimaging functions, anticancer effects, and combined drug tracing in vivo. Diverse applications can be achieved with abundant reactive groups, such as drug tracing, optical temperature sensing, magnetic/near-infrared (NIR)-thermally responsive drug delivery, enhanced photosensitivity, and other biomedical sciences, by integrating fluorescent CD/polymer composites with functional compounds. As a result, the major polymeric characteristics of CD/polymer composites result in more changeable properties, promoting their applications in several emerging fields [50].
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317.
- Monte-Filho, S.S.; Andrade, S.I.E.; Lima, M.B.; Araujo, M.C.U. Synthesis of highly fluorescent carbon dots from lemon and onion juices for determination of riboflavin in multivitamin/mineral supplements. J. Pharm. Anal. 2019, 9, 209–216.
- Chu, K.W.; Lee, S.L.; Chang, C.J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689.
- Tadesse, A.; Belachew, N.; Hagos, M.; Basavaiah, K. Synthesis of fluorescent nitrogen and phosphorous co-doped carbon quantum dots for sensing of iron, cell imaging and antioxidant activities. J. Fluoresc. 2021, 31, 763–774.
- Doring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light Sci. Appl. 2022, 11, 75.
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475.
- Cao, L.; Fernando, K.A.S.; Liang, W.X.; Seilkop, A.; Veca, L.M.; Sun, Y.P.; Bunker, C.E. Carbon dots for energy conversion applications. J. Appl. Phys. 2019, 125, 220903.
- Su, W.; Wu, H.; Xu, H.M.; Zhang, Y.; Li, Y.C.; Li, X.H.; Fan, L.N. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836.
- Xu, J.; Guo, Y.; Gong, T.; Cui, K.; Hou, L.; Yuan, C. B, N co-doped carbon dots based fluorescent test paper and hydrogel for visual and efficient dual ion detection. Inorg. Chem. Commun. 2022, 145, 110047.
- Wei, S.; Li, Y.; Liang, H.; Yen, Y.; Lin, Y.; Chang, H. Photoluminescent carbon nanomaterials for sensing of illicit drugs: Focus. Anal. Sci. 2022, 38, 247–260.
- Guo, J.; Li, H.; Ling, L.; Li, G.; Cheng, R.; Lu, X.; Xie, A.-Q.; Li, Q.; Wang, C.-F.; Chen, S. Green synthesis of carbon dots toward anti-counterfeiting. ACS Sust. Chem. Eng. 2020, 8, 1566–1572.
- Ganguly, S.; Das, P.; Banerjee, S.; Das, N.C. Advancement in science and technology of carbon dot-polymer hybrid composites: A review. Funct. Compos. Struct. 2019, 1, 022001.
- Foubert, A.; Beloglazova, N.V.; Rajkovic, A.; Sas, B.; Madder, A.; Goryacheva, I.Y.; De Saeger, S. Bioconjugation of quantum dots: Review & impact on future application. Trac-Trends Analyt. Chem. 2016, 83, 31–48.
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67.
- Sharker, S.M.; Do, M. Nanoscale carbon-polymer dots for theranostics and biomedical exploration. J. Nanotheranostics 2021, 2, 118–130.
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316.
- Jayanthi, M.; Megarajan, S.; Subramaniyan, S.B.; Kamlekar, R.K.; Anbazhagan, V. A convenient green method to synthesize luminescent carbon dots from edible carrot and its application in bioimaging and preparation of nanocatalyst. J. Mol. Liq. 2019, 278, 175–182.
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.H.; Kumar, P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew. Sust. Energy Rev. 2021, 135, 110391.
- Xie, J.D.; Lai, G.W.; Huq, M.M. Hydrothermal route to graphene quantum dots: Effects of precursor and temperature. Diam. Relat. Mater. 2017, 79, 112–118.
- Ross, S.; Wu, R.S.; Wei, S.C.; Ross, G.M.; Chang, H.T. The analytical and biomedical applications of carbon dots and their future theranostic potential: A review. J. Food Drug Anal. 2020, 28, 677–695.
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92.
- Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon dot/polymer composites with various precursors and their sensing applications: A Review. Coatings 2021, 11, 1100.
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan–carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245.
- Li, Y.; Huang, Z.-Z.; Weng, Y.; Tan, H. Pyrophosphate ion-responsive alginate hydrogel as an effective fluorescent sensing platform for alkaline phosphatase detection. Chem. Commun. 2019, 55, 11450–11453.
- Bi, R.; Zhang, R.; Shen, J.; Liu, Y.-N.; He, M.; You, X.; Su, Y.; Jiang, Z. Graphene quantum dots engineered nanofiltration membrane for ultrafast molecular separation. J. Membr. Sci. 2019, 572, 504–511.
- Yang, W.J.; Shao, D.D.; Zhou, Z.; Xia, Q.C.; Chen, J.; Cao, X.L.; Zheng, T.; Sun, S.P. Carbon quantum dots (CQDs) nanofiltration membranes towards efficient biogas slurry valorization. Chem. Eng. J. 2020, 385, 123993.
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K.K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856.
- Linehan, K.; Doyle, H. Size controlled synthesis of carbon quantum dots using hydride reducing agents. J. Mater. Chem. C 2014, 2, 6025–6031.
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195.
- Siddique, A.B.; Pramanick, A.K.; Chatterjee, S.; Ray, M. Amorphous carbon dots and their remarkable ability to detect 2,4,6-trinitrophenol. Sci. Rep. 2018, 8, 9770.
- Zhao, W.B.; Liu, K.K.; Song, S.Y.; Zhou, R.; Shan, C.X. Fluorescent nano-biomass dots: Ultrasonic-assisted extraction and their application as nanoprobe for Fe3+ detection. Nanoscale Res. Lett. 2019, 14, 130.
- Zulfajri, M.; Dayalan, S.; Li, W.Y.; Chang, C.J.; Chang, Y.P.; Huang, G.G. Nitrogen-doped carbon dots from averrhoa carambola fruit extract as a fluorescent probe for methyl orange. Sensors 2019, 19, 5008.
- Fan, J.; Claudel, M.; Ronzani, C.; Arezki, Y.; Lebeau, L.; Pons, F. Physicochemical characteristics that affect carbon dot safety: Lessons from a comprehensive study on a nanoparticle library. Int. J. Pharm. 2019, 569, 118521.
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970.
- Mihalache, I.; Radoi, A.; Pascu, R.; Romanitan, C.; Vasile, E.; Kusko, M. Engineering graphene quantum dots for enhanced ultraviolet and visible light p-si nanowire-based photodetector. ACS Appl. Mater. Interfaces 2017, 9, 29234–29247.
- Zhao, B.; Tan, Z. Fluorescent carbon dots: Fantastic electroluminescent materials for light-emitting diodes. Adv. Sci. 2021, 8, 2001977.
- Sun, Z.; Yan, F.; Xu, J.; Zhang, H.; Chen, L. Solvent-controlled synthesis strategy of multicolor emission carbon dots and its applications in sensing and light-emitting devices. Nano Res. 2021, 15, 414–422.
- Ding, Y.; Yu, J.; Chen, X.; Wang, S.; Tu, Z.; Shen, G.; Wang, H.; Jia, R.; Ge, S.; Ruan, J.; et al. Dose-dependent carbon-dot-induced ros promote uveal melanoma cell tumorigenicity via activation of mtor signaling and glutamine metabolism. Adv. Sci. 2021, 8, 2002404.
- Luo, W.K.; Zhang, L.L.; Yang, Z.Y.; Guo, X.H.; Wu, Y.; Zhang, W.; Luo, J.K.; Tang, T.; Wang, Y. Herbal medicine derived carbon dots: Synthesis and applications in therapeutics, bioimaging and sensing. J. Nanobiotechnol. 2021, 19, 320.
- Martin, C.; Jun, G.; Schurhammer, R.; Reina, G.; Chen, P.; Bianco, A.; Menard-Moyon, C. Enzymatic degradation of graphene quantum dots by human peroxidases. Small 2019, 15, e1905405.
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692.
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.H.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon dot therapeutic platforms: Administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small 2022, 18, e2106342.
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 122.
- Tabish, T.A.; Lin, L.; Ali, M.; Jabeen, F.; Ali, M.; Iqbal, R.; Horsell, D.W.; Winyard, P.G.; Zhang, S. Investigating the bioavailability of graphene quantum dots in lung tissues via Fourier transform infrared spectroscopy. Interface Focus 2018, 8, 20170054.
- Song, Y.B.; Zhu, S.J.; Shao, J.R.; Yang, B. Polymer carbon dots-a highlight reviewing their unique structure, bright emission and probable photoluminescence mechanism. J. Polym. Sci. Part A-Polym. Chem. 2017, 55, 610–615.
- Bellani, S.; Bartolotta, A.; Agresti, A.; Calogero, G.; Grancini, G.; Di Carlo, A.; Kymakis, E.; Bonaccorso, F. Solution-processed two-dimensional materials for next-generation photovoltaics. Chem. Soc. Rev. 2021, 50, 11870–11965.
- Muthurasu, A.; Ganesh, V. Tuning optical properties of nitrogen-doped carbon dots through fluorescence resonance energy transfer using Rhodamine B for the ratiometric sensing of mercury ions. Analyt. Methods 2021, 13, 1857–1865.
- He, M.; Zhang, J.; Wang, H.; Kong, Y.; Xiao, Y.; Xu, W. Material and optical properties of fluorescent carbon quantum dots fabricated from lemon juice via hydrothermal reaction. Nanoscale Res. Lett. 2018, 13, 175.
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25.
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957.
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-assisted synthesis of n-doped, multicolor carbon dots toward fluorescent inks, fluorescence sensors, and logic gate operations. Nanomaterials 2022, 12, 312.
- Zhu, S.J.; Song, Y.B.; Zhao, X.H.; Shao, J.R.; Zhang, J.H.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381.
- Bhattacharya, S.; Phatake, R.S.; Nabha Barnea, S.; Zerby, N.; Zhu, J.J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442.
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757.
- Ding, H.; Xiong, H.M. Exploring the blue luminescence origin of nitrogen-doped carbon dots by controlling the water amount in synthesis. RSC Adv. 2015, 5, 66528–66533.
- Liu, C.J.; Zhang, P.; Tian, F.; Li, W.C.; Li, F.; Liu, W.G. One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 2011, 21, 13163–13167.
More
Information
Subjects:
Biochemical Research Methods
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
01 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




