Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zahra Mohtashami | -- | 1809 | 2022-10-27 05:53:21 | | | |
| 2 | Rita Xu | Meta information modification | 1809 | 2022-10-27 08:34:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mohtashami, Z.; Singh, M.K.; Salimiaghdam, N.; Ozgul, M.; Kenney, M.C. Mitochondrial Derived Peptides. Encyclopedia. Available online: https://encyclopedia.pub/entry/31486 (accessed on 07 February 2026).
Mohtashami Z, Singh MK, Salimiaghdam N, Ozgul M, Kenney MC. Mitochondrial Derived Peptides. Encyclopedia. Available at: https://encyclopedia.pub/entry/31486. Accessed February 07, 2026.
Mohtashami, Zahra, Mithalesh K. Singh, Nasim Salimiaghdam, Mustafa Ozgul, M. Cristina Kenney. "Mitochondrial Derived Peptides" Encyclopedia, https://encyclopedia.pub/entry/31486 (accessed February 07, 2026).
Mohtashami, Z., Singh, M.K., Salimiaghdam, N., Ozgul, M., & Kenney, M.C. (2022, October 27). Mitochondrial Derived Peptides. In Encyclopedia. https://encyclopedia.pub/entry/31486
Mohtashami, Zahra, et al. "Mitochondrial Derived Peptides." Encyclopedia. Web. 27 October, 2022.
Copy Citation
Mitochondrial-derived peptides (MDPs) are translated peptides encoded by short open reading frames (sORFs) within known mitochondrial (mt) DNA genes.
MOTS-c
mitochondrial derived peptides
mitochondrial dysfunction
1. Introduction
Metabolism is a crucial biological function that consists of catabolic and anabolic reactions in living cells [1]. Multiple interrelated metabolic processes such as glycolysis, citric acid cycle, oxidative phosphorylation, fatty acid-oxidation, and gluconeogenesis provide energy for cells to grow, reproduce, and preserve their structures [2][3].
The mitochondria, complex organelles with endosymbiotic origins in early eukaryotic cells, convert most energy via oxidative phosphorylation (OXPHOS), the citric acid cycle, and fatty acid oxidation [1]. Mitochondria are involved in amino acid, lipid, nucleotide, apoptotic, calcium, and retrograde signaling [4][5]. The fact that mitochondria have their own genome, mitochondrial DNA (mtDNA), supports this notion [4].
Mitochondria are dynamic organelles that are responsible for metabolism and the conversion of energy-storing molecules, such as ATP, for the function of the cell [4]. However, mitochondria communicate via reactive oxygen species (ROS), Ca2+, and cytochrome C [5][6][7]. Given their importance, it is not unexpected that mitochondria are sensitive to intrinsic stressors, including mutation and deletion of mtDNA [8], a lack or excess of energetic substrates [9], an increase in ROS levels [10], and stressor extrinsic agents such as toxins, viruses, bacteria, and ultraviolet rays [11]. Chemicals can change mitochondrial function and dynamics, causing aging, neurological illness, diabetes, and cancer [8]. Furthermore, it is believed that mitochondria are substantially capable of locally generating systemic reactions [4]. According to new research, mitochondria have an enlarged genetic impact with the discovery of the Mitochondrial Derived Peptides (MDPs). Humanin (HN), small HN-like peptides (SHLPs), and mitochondrial open Reading frame (ORF) of the twelve S-c (MOTS-c) are MDPs that can modulate cellular metabolism and provide cytoprotection, shattering paradigms with respect to the previously recognized mitochondrial activity [4][5].
Few studies have examined the mitochondrial responses under controlled stress, such as physical stress. There are considerable data demonstrating that stress events are involved in the regulation of this novel class of peptides [4][5]. Aging is characterized by gradual loss of (mitochondrial) metabolic balance, elevated ROS levels and eventually diminished physical capability (Figure 1) [6][7]. Indeed, aging is a substantial risk factor for a variety of chronic non-infectious diseases [8][9][10].
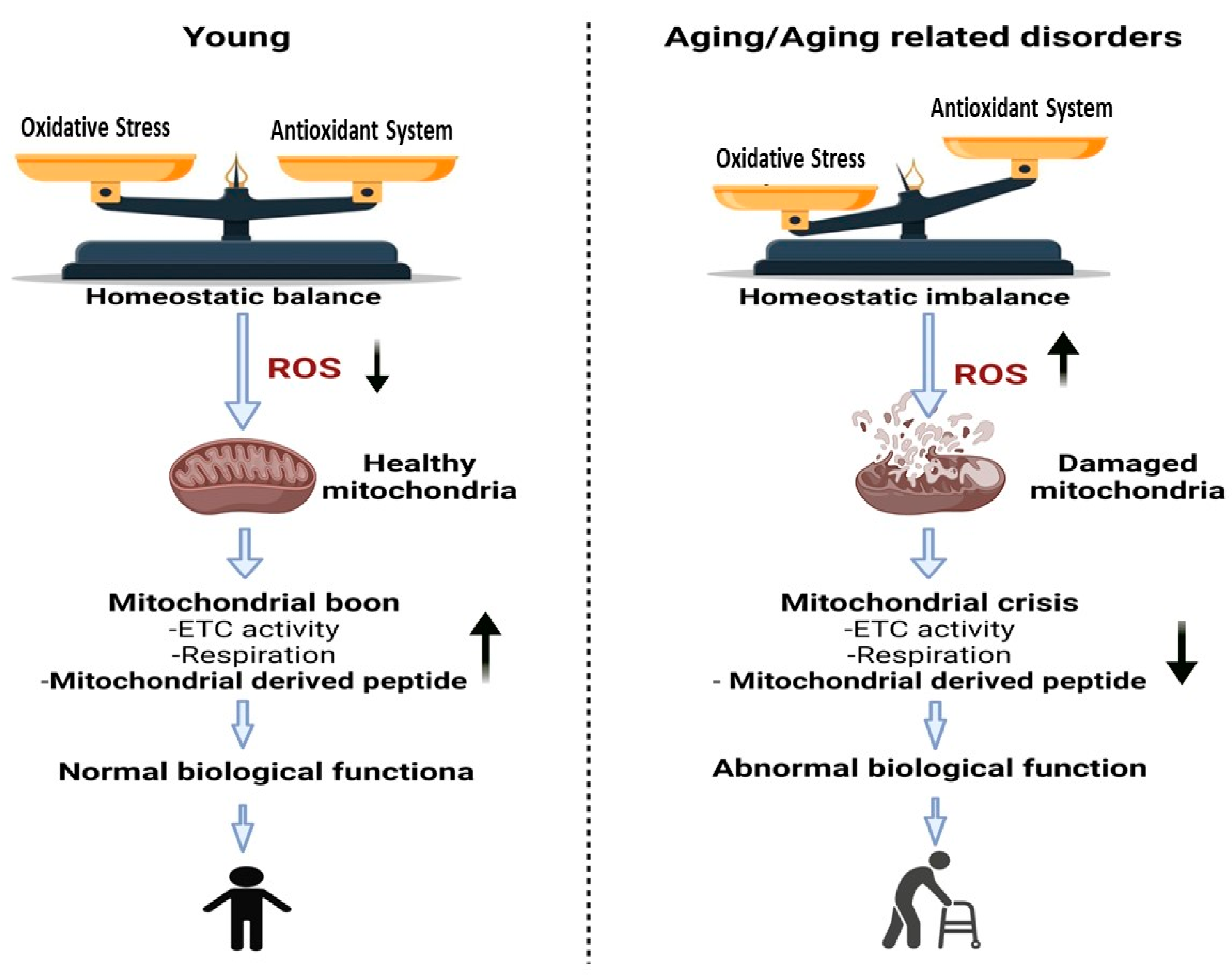
Figure 1. Changes that occur in mitochondria due to aging or aging related disorders are associated with a reduction in the function of mitochondria. Due to the accumulation of mutations and the oxidative damage generated by reactive oxygen species (ROS), the mitochondrial DNA volume, integrity, and functionality all decline with advanced. Left Panel represents young individuals with balanced homeostasis leading to normal biological functions of tissues. Right Panel represents greater levels of oxidative stress that lead to increased ROS and mitochondrial dysfunction along with abnormal biological functions.
2. What Are Mitochondrial Derived Peptides (MDPs)?
Mitochondrial-derived peptides (MDPs) have cytoprotective roles in preserving mitochondrial function and cell viability under stress conditions [4][5][6][7]. The mammalian mtDNA encodes 13 mRNAs, 22 tRNAs, and 2 rRNAs (12S & 16S rRNA) which are structural components of the electron transport chain [8][9]. To date, eight MDPs have been identified, all of which are transcribed from sORFs found in mtDNA genes that encode from the 12S rRNA and 16S rRNA transcripts [4].
The 16S ribosomal RNA gene is 1559 nucleotides in length, found within the MT-RNR2 gene and spans mtDNA nucleotide pairs (nps) 1671–3229 [10]. The 16S rRNA region encodes for Humanin, the first well-studied MDP and Small Humanin-Like Peptides (SHLPs) [11].
The 12S rRNA gene (MT-RNR1 gene) is 954 nps, spanning from 648 to 1601 nps, which represents approximately 6% of total mtDNA. This 12S rRNA region encodes for MOTS-c (mitochondrial open reading frame of the 12S rRNA type-c), the most recently identified MDP. The discovery of HN, SHLPs and MOTS-c peptides has led to novel areas of research because of their origin from the mitochondrial genome, and subsequent revelations that these peptides play critical functions of neuroprotection, metabolism, signaling and inhibition of apoptosis. Beside some common overlapping functions, each MDP has its own exclusive role causing different response [5]. MOTS-c role in various pathophysiological conditions is described in (Figure 2).
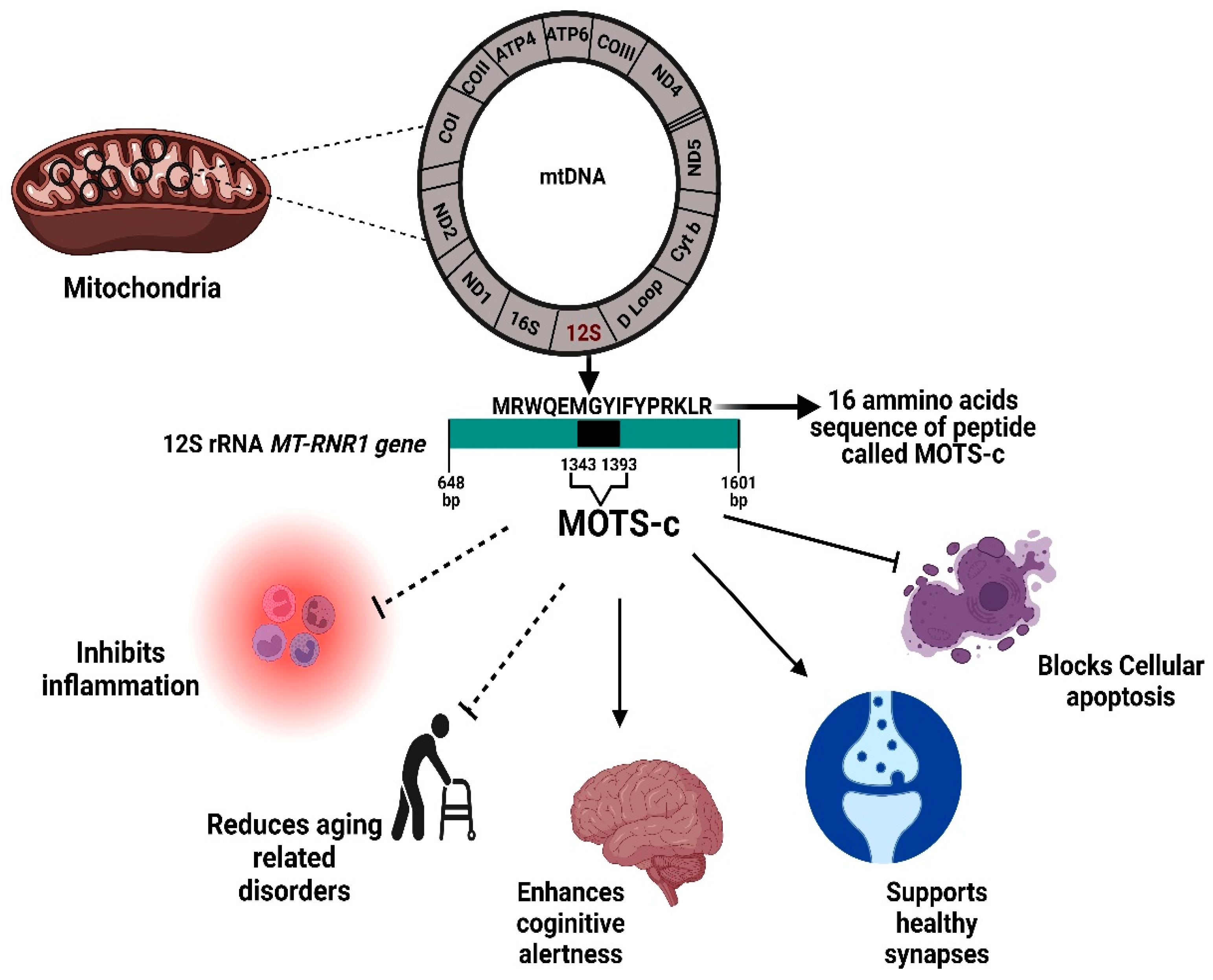
Figure 2. Physiological significance of the MOTS-c protein. MOTS-c is encoded from a region within the 12S rRNA MT-RNR1 gene. The MOTS-c protein has both inhibitory effects (inflammation, age-related disorder, apoptosis) and also promotes healthy functioning in brain and other tissues. bp, base pair.
2.1. MOTS-c: Origin, History, and Structure
After the discovery of humanin (HN) in 2001, researchers went on in 2015 to identify another new mitochondrial derived peptide (MDP) known as MOTS-c [4]. MOTS-c is in a variety of tissues, co-localizes to mitochondria, and is found in plasma of rodents and humans. MOTS-c has important cellular functions as well as a possible hormonal role [4][12].
In order to replicate complementary DNAs (cDNAs) used to map the region containing 12S rRNA, human myeloblasts were stimulated by interferon [13]. Careful analyses of the sORFs within the human 12S rRNA revealed one consisting of 51 base pairs which is translated into a 16 amino acid sequence of peptide (MRWQEMGYIFYPRKLR) termed as MOTS-c [14]. It was ultimately demonstrated that the MOTS-c peptide was not of nuclear DNA origin (possibly a nuclear mitochondria DNA transfer, NUMT), but rather completely homologous to the mtDNA genome [4].
The mitochondrial genome evolves at a faster rate than the nuclear genome, owing to a greater mutation rate and clonal propagation, which can result in sequence alterations between closely related species [10][15][16]. However, due to a significant positive selection force, some regions of 12S and 16S rRNA are largely maintained across species [17]. MOTS-initial c’s 11 amino acid residues (for a total of 16 amino acids) are highly conserved across 14 mammalian species [4][17]. Notably, “dwarf” sORFs that encode for peptides of 20 amino acids are less conserved [18], which could explain why MOTS-c is not conserved in some lower eukaryotes such as C. elegans and Drosophila melanogaster [19][20].
2.2. Molecular Mechanisms and Pathways of MOTS-C
Mitochondrially derived peptides (MDPs) are retrograde signaling molecules. These peptides regulate mitochondrial bioenergetics and metabolism, which in turn alter systemic insulin sensitivity and glucose homeostasis. Furthermore, Kim et al. demonstrated that MOTS-c, a mitochondrial-encoded peptide, may dynamically translocate to the nucleus in response to metabolic stress and modulate adaptive nuclear gene expression [21]. Humanin and MOTS-c are the two most commonly studied MDPs. Humanin receptors include the seven transmembrane G-protein-coupled receptor formyl-peptide receptor-like-1 (FPRL1) and a trimeric receptor that includes the ciliary neurotrophic factor receptor (CNTFR), the cytokine receptor WSX-1, and the transmembrane glycoprotein gp130 (CNTFR/WSX-1/gp130) [22]. While to date there have not been any cellular receptors described for the MOTS-c peptide. MOTS-c release in the blood is also termed as “mitochondria hormone” or “mitokine” [23]. Its circulation is regulated by the folate cycle and signaling via cAMP and AMPK [4]. MOTS-c expression is age-dependent [24].
MOTS-c is an important regulator for energy balance and is highly associated with amino acid, carbohydrates, and lipid metabolism. In mammalian cells, it is encoded from the mitochondrial DNA and under stress conditions, it then translocates to the nucleus, which is accompanied by higher ROS production [21]. The MOTS-c nuclear translocation is 5′-adenosine monophosphate-activated protein kinase (AMPK) dependent [23][25]. MOTS-c triggers the activation of AMPK and accumulation of 5-aminomidazole-4-carboxamide ribonucleotide (AICAR), a known AMPK activator, by inhibiting the folate cycle and de novo purine biosynthesis [7][26].
AMPK is the major sensor and key regulator of cellular metabolism based on energy availability [27]. Upon rise in the ATP:ADP or ATP:AMP ratios, AMPK is activated and alters the metabolism toward catabolism induction and anabolism suppression by phosphorylation of crucial proteins in various pathways, including mTOR complex 1 (mTORC1) [28][29].
Additionally, during stress, AMPK activates Peroxisome proliferator-activated receptor Gamma Co-activator-1α (PGC-1α) via direct phosphorylation [30][31]. The PGC-1α regulates expression of antioxidants in mitochondria and is a key factor in mito-nuclear communication. It may interact with Nuclear Factor, Erythroid -1 and -2 (NRF-1/2) to block mitochondrial oxidative stress, promote the clearance of damaged mitochondria and enhance mitochondrial biogenesis [32].
In the nucleus, MOTS-c regulates a wide range of genes in response to metabolic dysfunction, including those containing antioxidant response elements (ARE) [33]. It interacts with ARE-regulating stress-responsive transcription factors, such as Nuclear Factor Erythroid 2-Related Factor 2 (NFE2L2/NRF2) [8][25][34]. NFE2L2/NRF2 is a stress-responsive transcription factor that responds to ROS and protect cells under oxidative stress [35]. NRF2/ARE pathway activation plays an antioxidative role in treating acute kidney injury and vascular dysfunction. Notably, NRF2 intersects with AMPK [35] and can regulate MOTS-c-related metabolic pathways. The MOTS-c/NRF2 relationship boosts mitochondrial protection genes, and MOTS-c overexpression increases NRF2 signaling [32]. Different metabolic pathways of MOTS-c are summarized in (Figure 3).
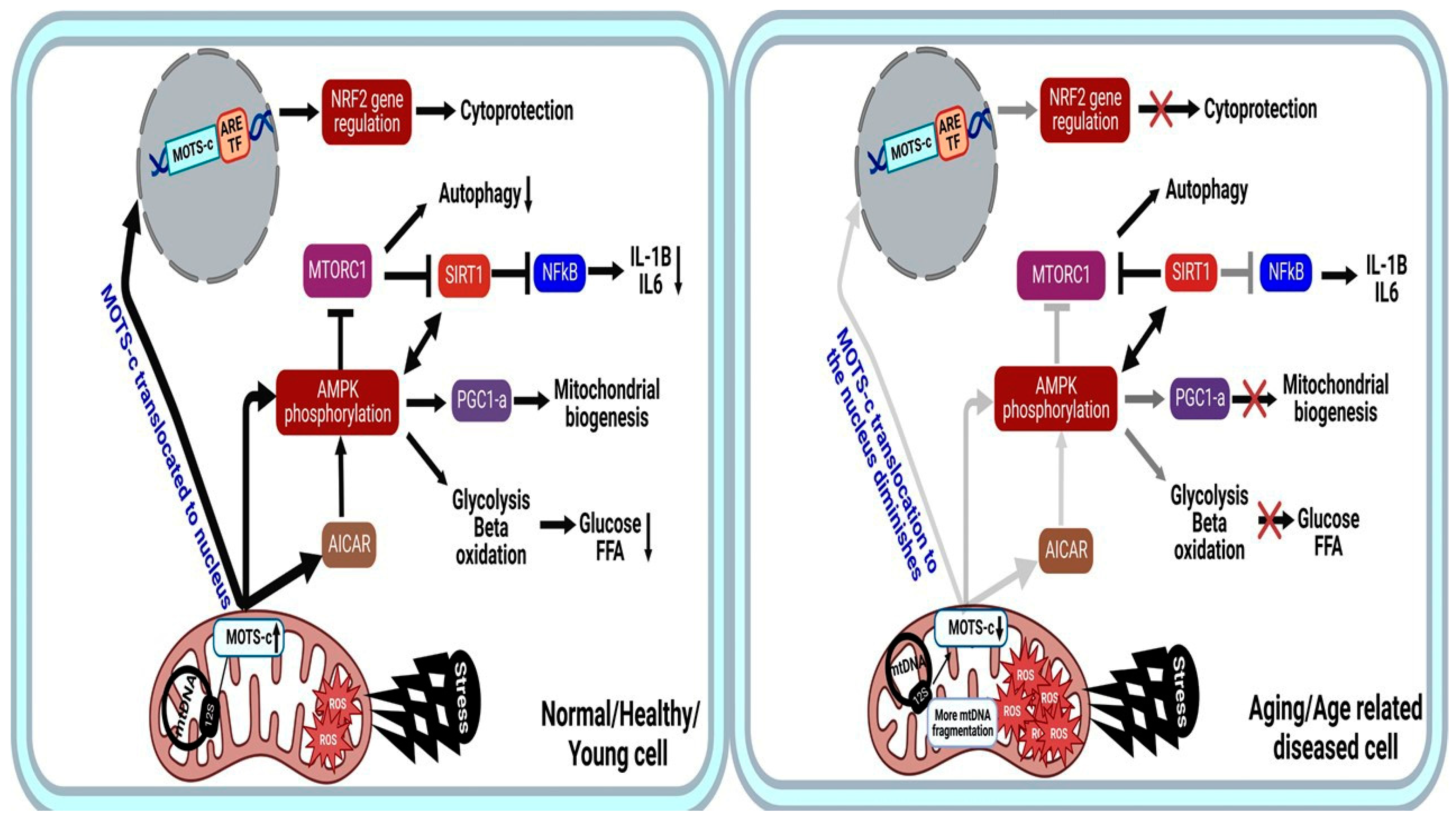
Figure 3. MOTS-c mechanism of action in normal young healthy state (left) vs. Aging or age-related diseases (right). The peptide is capable of interacting with the nuclear genome to provide cryoprotection and has beneficial effects mainly when it comes to the regulation of the metabolisms of AMPK and AICAR. Faded lines indicate less dependent effect or production, Faded line with cross indicate signal is completely lost, and cross indicates signal is lost. AMPK, 5′-Adenosine Monophosphate-activated Protein Kinase; AICAR, 5-AminoImidazole-4-CarboxAmide Ribonucleotide; FFA, Free Fatty Acid; FFA-B, Free Fatty Acid-B oxidation; ARE, Antioxidant Response Elements, TF-Transcription factor.
During rest periods, the MOTS-c peptide has a mitochondrial-association and only low quantities of endogenous MOTS-c are found in the nucleus [25]. The distinct MOTS-c nuclear regulatory feature sets it apart from all other MDPs and makes it a promising agent for future research in the fields of diagnosis and treatment of a broad range of metabolic diseases, including aging-related disorders.
2.3. Aging and Longevity: MOTS-c
Aging is a lifelong process that leads to senescence, or a breakdown of biological functions and an incapacity to respond to metabolic stress [33]. Improved mitochondrial fitness and physical capacity aid healthy aging.
MOTS-c levels in 70–81-year-olds drop by nearly 21% compared to 18–30-year-old individuals [36]. MOTS-c shares metabolic pathways with age-modifiers. NAD+, a metabolic cofactor in redox reactions and a critical modulator of cell signalling and survival pathways, diminishes with age. Moreover, NAD+ as a potent sirtuin activator, plays a key role in energy metabolism, cell survival, and aging in model species, therefore, maintaining its level could postpone age-related disorders and, in certain cases, increase longevity [13][37][38][39].
MOTS-c (i) elevates NAD+ levels, (ii) has glycolytic effects via sirtuin 1 (SIRT1) [7], (iii) influences the folate/methionine cycle and (iv) restricts methionine metabolism. Methionine shortage extends mouse lifespan by 45%, lowers visceral fat and age-related diseases, and prevents lens degeneration [40][41].
MOTS-c, whose levels decline with age, has a wide range of health-span consequences. In vivo mice studies showed that intraperitoneal (IP) MOTS-c (15 mg/kg/day) improved the physical performance of mice of different ages (2, 12, 22 and 23.5 months) over a two-week period. This treatment improved the physical capacity and slowed the emergence of age-related deficits [24][42].
While the process of aging is linked to several different factors, including shifts in metabolic control, altered gene expression patterns [43], and high production of ROS [44][45][46], it is unclear exactly how these factors interact to cause aging. Therefore, older age is the biggest risk factor for chronic diseases and functional impairments that limits life expectancy [47]. MOTS-c indications in aging and several age-related diseases are presented schematically in (Figure 4).
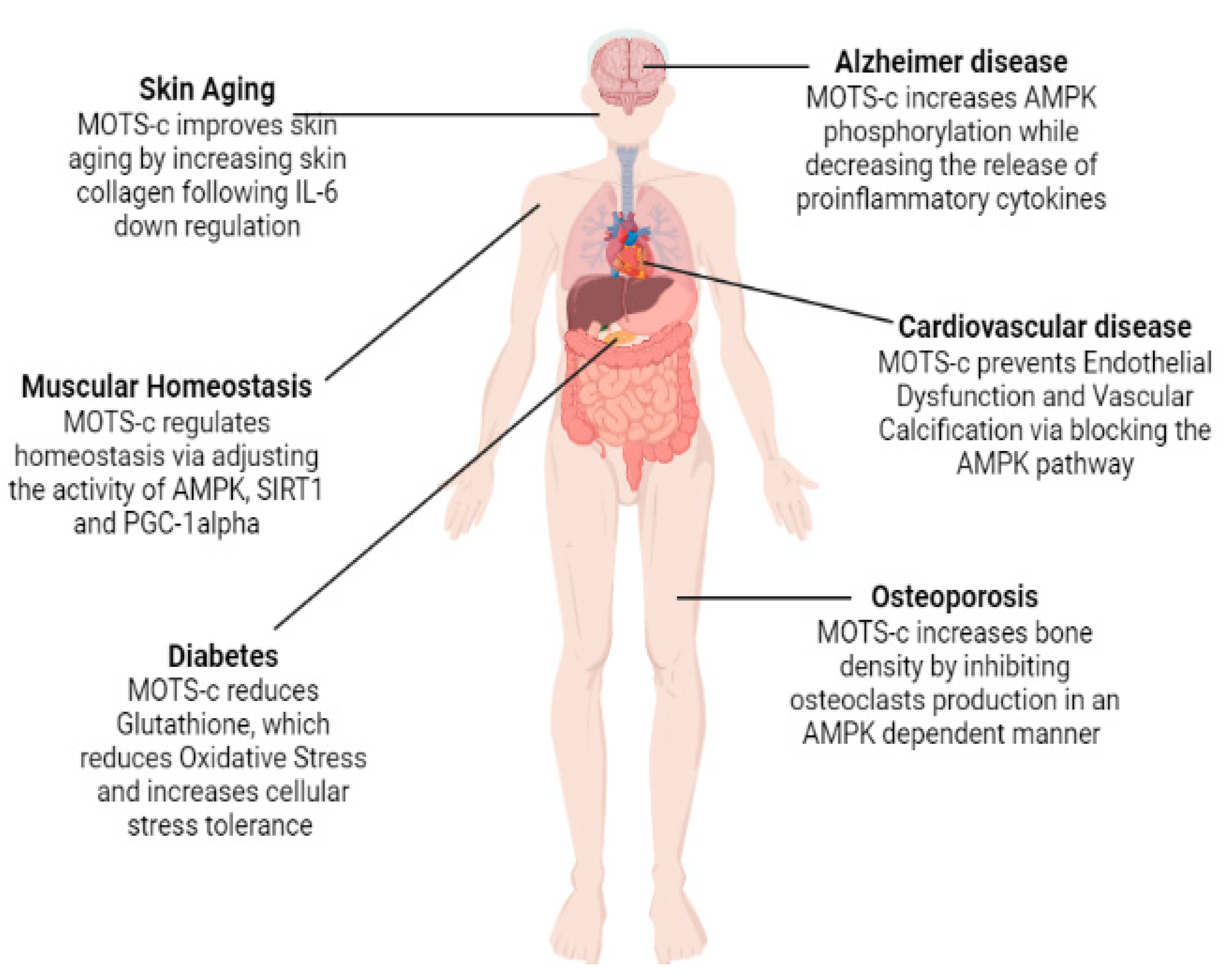
Figure 4. Role of MOTS-c in relation to different age-related disorders.
References
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647.
- DeBerardinis, J.R.; Thompson, C.B. Cellular metabolism and disease: What do metabolic outliers teach us? Cell 2012, 148, 1132–1144.
- Hill, B.G.; Shiva, S.; Ballinger, S.; Zhang, J.; Darley-Usmar, V.M. Bioenergetics and translational metabolism: Implications for genetics, physiology and precision medicine. Biol. Chem. 2019, 401, 3–29.
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454.
- Kim, S.J.; Xiao, J.; Wan, J.; Cohen, P.; Yen, K. Mitochondrially derived peptides as novel regulators of metabolism. J. Physiol. 2017, 595, 6613–6621.
- Yen, K.; Lee, C.; Mehta, H.; Cohen, P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J. Mol. Endocrinol. 2013, 50, R11–R19.
- Mendelsohn, R.A.; Larrick, J.W. Mitochondrial-Derived Peptides Exacerbate Senescence. Rejuvenation Res. 2018, 21, 369–373.
- Kim, S.J.; Miller, B.; Kumagai, H.; Silverstein, A.R.; Flores, M.; Yen, K. Mitochondrial-derived peptides in aging and age-related diseases. Geroscience 2021, 43, 1113–1121.
- Gustafsson, M.C.; Falkenberg, M.; Larsson, N.G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160.
- Galtier, N.; Enard, D.; Radondy, Y.; Bazin, E.; Belkhir, K. Mutation hot spots in mammalian mitochondrial DNA. Genome Res. 2006, 16, 215–222.
- Sreekumar, G.P.; Kannan, R. Mechanisms of protection of retinal pigment epithelial cells from oxidant injury by humanin and other mitochondrial-derived peptides: Implications for age-related macular degeneration. Redox. Biol. 2020, 37, 101663.
- Vaziri, H.; Dessain, S.K.; Eaton, E.N.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159.
- Tsuzuki, T.; Nomiyama, H.; Setoyama, C.; Maeda, S.; Shimada, K. Presence of mitochondrial-DNA-like sequences in the human nuclear DNA. Cell 2001, 107, 149–159.
- Harhay, G.P.; Sonstegard, T.S.; Keele, J.W.; Heaton, M.P.; Clawson, M.L.; Snelling, W.M.; Wiedmann, R.T.; Van Tassell, C.P.; Smith, T.P. Characterization of 954 bovine full-CDS cDNA sequences. BMC Genom. 2005, 6, 166.
- Brown, M.W.; George, M., Jr.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971.
- Stewart, B.J.; Chinnery, P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 2015, 16, 530–542.
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 16612–16616.
- Aspden, J.L.; Eyre-Walker, Y.C.; Phillips, R.J.; Amin, U.; Mumtaz, M.A.; Brocard, M.; Couso, J.P. Extensive translation of small open reading frames revealed by Poly-Ribo-Seq. eLife 2014, 3, e03528.
- Durieux, J.; Wolff, S.; Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 2011, 144, 79–91.
- Woo, K.D.; Shadel, G.S. Mitochondrial stress signals revise an old aging theory. Cell 2011, 144, 11–12.
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018, 28, 516–524.e7.
- Hashimoto, Y.; Kurita, M.; Aiso, S.; Nishimoto, I.; Matsuoka, M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol. Biol. Cell 2009, 20, 2864–2873.
- Lee, C.; Kim, K.H.; Cohen, P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic. Biol. Med. 2016, 100, 182–187.
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 1–11.
- Zarse, K.; Ristow, M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 2015, 21, 355–356.
- Tan, J.X.; Finkel, T. Mitochondria as intracellular signaling platforms in health and disease. J. Cell Biol. 2020, 219, e202002179.
- Crozet, P.; Margalha, L.; Confraria, A.; Rodrigues, A.; Martinho, C.; Adamo, M.; Elias, C.A.; Baena-González, E. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front. Plant Sci. 2014, 5, 190.
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135.
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008, 30, 214–226.
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022.
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125.
- Li, Q.; Lu, H.; Hu, G.; Ye, Z.; Zhai, D.; Yan, Z.; Wang, L.; Xiang, A.; Lu, Z. Earlier changes in mice after D-galactose treatment were improved by mitochondria derived small peptide MOTS-c. Biochem. Biophys. Res Commun. 2019, 513, 439–445.
- Fuku, N.; Pareja-Galeano, H.; Zempo, H.; Alis, R.; Arai, Y.; Lucia, A.; Hirose, N. The mitochondrial-derived peptide MOTS-c: A player in exceptional longevity? Aging Cell 2015, 14, 921–923.
- Sun, H.; Guo, X.; Wang, Z.; Wang, P.; Zhang, Z.; Dong, J.; Zhuang, R.; Zhou, Y.; Ma, G.; Cai, W. Alphalipoic acid prevents oxidative stress and peripheral neuropathy in nab-paclitaxel-treated rats through the Nrf2 signalling pathway. Oxid. Med. Cell Longev. 2019, 2019, 3142732.
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell Biol. 2016, 36, 1931–1942.
- D’Souza, R.F.; Woodhead, J.S.; Hedges, C.P.; Zeng, N.; Wan, J.; Kumagai, H.; Lee, C.; Cohen, P.; Cameron-Smith, D.; Mitchell, C.J.; et al. Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging 2020, 12, 5244–5258.
- Bonkowski, S.M.; Sinclair, D.A. Slowing ageing by design: The rise of NAD (+) and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690.
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471.
- Haigis, C.M.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295.
- Zimmerman, J.A.; Malloy, V.; Krajcik, R.; Orentreich, N. Nutritional control of aging. Exp. Gerontol. 2003, 38, 47–52.
- Miller, R.A.; Buehner, G.; Chang, Y.; Harper, J.M.; Sigler, R.; Smith-Wheelock, M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 2005, 4, 119–125.
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911.
- Shadyab, H.A.; LaCroix, A.Z. Genetic factors associated with longevity: A review of recent findings. Ageing Res. Rev. 2015, 19, 1–7.
- Sergiev, V.P.; Dontsova, O.A.; Berezkin, G.V. Theories of aging: An ever-evolving field. Acta Nat. 2015, 7, 9–18.
- Dai, D.F.; Chiao, Y.A.; Martin, G.M.; Marcinek, D.J.; Basisty, N.; Quarles, E.K.; Rabinovitch, P.S. Mitochondrial-targeted catalase: Extended longevity and the roles in various disease models. Prog. Mol. Biol. Transl. Sci. 2017, 146, 203–241.
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61.
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.5K
Revisions:
2 times
(View History)
Update Date:
27 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




