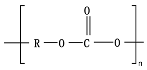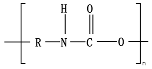1. Mechanical Degradation
Mechanical degradation is the loss of mechanical properties reflected in the polymer’s performance due to the exposure to either a harsh environment or the action of mechanical stresses. Mechanical degradation can occur due to compression, tension, and/or shear forces applied to a polymer. Mechanical factors are not generally predominant during the biodegradation process, but mechanical damage may happen before the action of microorganisms in activating or accelerating the biodegradation process
[1]. Mechanical degradation due to loading in service is common for polymeric materials under mechanical stress, such as for biomaterials in the medical field
[2]. On the other hand, physical forces, such as heating, cooling, wetting, and drying, or surface turbulence induced by air or water, can cause mechanical degradation due to stress cracking
[3]. Mechanical degradation and biotic degradation are correlated, for example, when evaluating the degradation process of mulch films in agriculture settings and compostable films in industrial conditions
[4]. In the scientific literature, it is common to find reports of loss of mechanical properties as an indicator of the ultimate biodegradation process, although these may not be the suitable properties to track for biodegradation but are instead complementary. The diminishing of tensile properties, flexural properties, hardness, and impact resistance are the main outcomes of mechanical degradation
[1][5][6].
Evaluation of mechanical degradation in biodegradable polymers for agricultural films showed that fragmentation increased the biodegradation rate since it increased the surface area available for microbial degradation
[4][7]. Furthermore, the presence of cracks and pores is typical evidence of mechanical degradation. The formation of cavities during mechanical degradation can allow for more water diffusion into the polymer matrix, affecting the hydrolytic abiotic degradation and consequently, the biodegradation process
[8]. In the aquatic environment, such as marine, rivers or lakes, the stress due to the water’s natural dynamic can induce mechanical degradation of biodegradable polymers, as observed for PCL, PHAs, and PLA
[9].
2. Thermal Degradation
Thermal degradation is the consequence of exposing a polymer to heat for an extended period and is called thermo-oxidative degradation in the presence of oxygen (O
2). The first step of thermal degradation is the rupture of macromolecular bonds, resulting in monomeric units or radicals that can react with O
2 to produce peroxide radicals
[5].
For different levels of thermal energy and time exposure, thermal degradation induces different changes in the polymer structure: (1) for temperatures below the glass transition temperature (
Tg), thermal degradation results in physical aging, where the polymer shows a structural rearrangement; (2) for temperatures between
Tg and the melting temperature
(Tm), changes are associated with the loss of dimensions and original shape, crystallization processes and thermal decomposition of low molecular weight (
Mw) additives; (3) for temperatures above
Tm, loss of structure and disordered melt is observed due to loss of structure of the crystalline region; and (4) for temperatures even higher than the decomposition temperature, the material combusts and energy from the material can be recovered
[6].
Thermal degradation occurs throughout the bulk of the polymer and consists of four different reactions that can occur at the same time: (1) chain-end scission or chain depolymerization of C-C bonds that generate volatile products; (2) random chain scission that leads to
Mw reduction; (3) degradation by substituent reactions; and (4) recombination reactions of cyclic and linear oligomers such as in the case of PLA
[5][10].
Thermal degradation is the predominant mechanism at elevated temperatures, since its rate is higher than the rates of hydrolysis, photodegradation, and mechanical degradation. However, at temperatures lower than Tg, it can induce aging of the polymer, improving the efficiency of the biodegradation process.
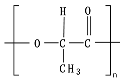
For biodegradable polymers, thermal degradation happens in the range of the melting temperature, which includes temperatures far higher than the range where the biodegradation process mostly occurs (i.e., at mesophilic and thermophilic conditions, 20–60 °C). The
Tm is around 155 °C for PLA and 175 °C for poly(hydroxy butyrate) (PHB), indicating that the thermal degradation will not affect or accelerate the biodegradation process. However, for some thermoplastic polymers such as PCL, the
Tm is around 60 °C, close to the thermophilic range of the composting process so that thermal degradation can play an active role during the biodegradation process
[1]. The energy provided can introduce modifications in the macromolecular structure and enhance the biodegradation process due to increased polymeric chains’ mobility, rearrangement, and the creation of free volume
[1].
3. Photodegradation
Polymers can undergo photodegradation and radiation degradation when exposed to wavelengths in the UV, visible, and infrared (IR) spectrum range or gamma radiation. Photodegradation may occur in the absence of O
2 (photolysis) and the presence of O
2 (photooxidative degradation), leading to rearrangement, chain scission, and cross-linking. The degree of photodegradation is associated with the wavelengths found in sunlight: infrared (IR) radiation, visible light, and UV radiation. The radiation reaching the earth’s surface is in the wavelength of 295 to 2500 nm, corresponding from UV-C to IR
[11].
Polymers that absorb high energy in the UV range are susceptible to oxidation and cleavage due to electron activation at higher energies
[12][13]. Photodegradation can break the polymer chains, produce radicals, change the physical and optical properties, generate a yellowing effect, induce loss of mechanical properties, and reduce the
Mw, leading to a useless material
[5][14]. Photooxidative degradation in polymers can be induced by UV radiation with or without the action of a catalyst, and the increase in temperature can accelerate the process.
In photolysis, light absorption leads directly to the formation of chemical reactions that cause degradation. For polyesters and polyamides, the photolysis mechanism implies two photolytic reactions, Norrish I and Norrish II
[15].
In semicrystalline polymers, the scission is mostly produced in the amorphous fraction and generates two end chains that can restructure and increase crystallinity as degradation continues. The termination step of photooxidative degradation collects free radicals to create inert products. The combination of free radicals can be natural or assisted using stabilizers in the polymer. A review of this process can be found elsewhere
[5][14][16].
Photodegradation can lead to Norrish reactions, and/or crosslinking reactions, or oxidative reactions. The products of the Norrish reactions transform the polymer by photoionization (Norrish I) and chain scission (Norrish II)
[6]. Studies on poly(
l-lactide) (PLLA) and PCL have shown that photodegradation followed a Norrish II reaction
[17][18]. Furthermore, crosslinking was observed for PBAT
[19]; when PBAT films were exposed to solar radiation, the loss of integrity and mechanical degradation observed was due to chain scission and crosslinking
[19][20].
On one hand, photodegradation can induce chain scission that can contribute to the biodegradation process. On the other hand, photodegradation can induce crosslinking, limiting the mobility of polymer chains and the access of water into the bulk’s polymer, reducing the activity of microorganisms, decreasing the rate of the biodegradation process. Enzymatic degradation of PLLA has been reported to be affected by UV treatment due to a dual effect of C=C double bonds formation and reduction in
Mw that also affected the chemical hydrolysis of PLLA films
[18]. A similar effect was reported by Jeon and Kim
[21], where for a short UV treatment, the initial
Mw was the dominant effect. However, at higher times of treatment, the crosslinking was probably dominant and reduced the biodegradation of PLA.
4. Ozone Degradation
The effect of atmospheric ozone on polymers is an increase in the aging rate, leading to a reduction in
Mw and loss of performance in mechanical and O
2 barrier properties
[22].
Poly(vinyl alcohol) (PVOH) has been shown to be degraded by the action of ozone. The associated mechanism starts with the oxidation of the -CHOH- group, which leads to ketonic groups. Fourier transform infrared (FTIR) spectroscopy has shown that the final product is a PVOH oligomer with several ketonic groups along the main oligomer backbone and carboxylic end groups
[23]. Abiotic degradation by the action of ozone, as reported by Cataldo et al., resulted in a loss of original PVOH crystallinity, accelerating the biotic degradation process
[23]. However, for PLA, an increase in crystallinity was reported by Olewnik-Kruszkowska et al., and the amorphous region was the most affected during ozone degradation
[24]. Furthermore, changes in the polymer matrix surface due to ozone exposure were observed as an increase in the surface roughness
[24][25]. Roughness can be beneficial for biofilm formation during biotic degradation. Overall, ozone degradation affects the bulk and surface structural properties of the polymer as observed for crystallinity and surface roughness, and consequently, it affects the biodegradation rate.
5. Hydrolytic Degradation
Chemical hydrolytic degradation is one of the main abiotic degradation mechanisms for biodegradable polymers, especially for aliphatic and aliphatic/aromatic polyesters.
With the uptake of water, susceptible chemical bonds in polymers can undergo chain scission, resulting in a reduction in
Mw, loss of mass and mechanical properties, and increased surface area of the polymer, thereby increasing the available sites for attack by enzymatic activity, which is the biotic step initiated by microorganisms
[1][3][26].
Chemical hydrolysis proceeds via two mechanisms when considering the macrostructure: bulk and surface erosion. Depending on the conditions, these mechanisms can occur independently or combined. Bulk erosion is the dominant mechanism when the water diffusion is faster than the hydrolysis reaction rate, and surface erosion is dominant when the water diffusion into the polymer bulk is slower than the hydrolysis reaction rate
[2][3][27].
When bulk erosion is the dominant mechanism, the
Mw of the polymer is reduced so that the polymer loses its mechanical properties in a short period. Due to the
Mw reduction and higher mobility of shorter polymeric chain segments, crystallinity may change. Loss of mass and changes in geometric shape take more time. The by-products of bulk erosion are first accumulated; when the polymer chains are short enough and reaching
n-mers size, they can start to diffuse out. When the polymer undergoes surface erosion, the mass loss is mostly from the surface while the bulk remains intact. As degradation advances, mass loss happens faster at the surface, and the polymer reduces in size. When compared with bulk erosion, the mechanical properties and
Mw are preserved for an extended period, and the release of by-products from the surface occurs from the beginning
[2].
The kinetic rate of the chemical hydrolysis—surface or bulk dominant—depends on and can be affected by several factors associated with the polymer itself and the environment. The roles of some factors are discussed in the next sections, and additional information can be found elsewhere
[2][3][27][28].
In terms of environmental factors, an increase in temperature and moisture intensifies the rate of chemical hydrolysis
[29]. Polymer chain mobility increases as the temperature increases. Hence, the susceptibility of hydrolysable bonds to undergo chain scission increases. The chemical potential of water on the surrounding media plays a significant role in the hydrolysis of polymers
[30]. Hydrolysis in acidic or alkaline conditions can occur through different mechanisms so the by-products of the reactions can differ
[31]. Finally, catalysts can increase the rate of the hydrolytic process
[32][33]. In terms of polymer factors, hydrophilic polymers are more susceptible to hydrolytic degradation than hydrophobic polymers
[3].
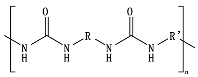
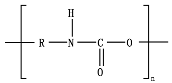
Hydrolysis depends on the presence of hydrolyzable covalent bonds, such as esters, ethers, anhydrides, carbamide (urea), and ester amide (urethane), which increase the rate of chemical hydrolysis
[1][34].
Table 1 compares the half-lives of hydrolyzable bonds in various polymers and shows that poly(anhydride)s are subjected to rapid hydrolysis due to the presence of hydrolyzable bonds of very low half-life. By contrast, polyamides are resistant to hydrolysis due to the resistance of the amide bonds to hydrolysis. The kinetics of the hydrolyzable bond half-life presented in
Table 1 can increase or decrease due to the influence of neighboring groups.
Table 1. Half-lives (time required for 50% hydrolysis) of hydrolysable bonds for different polymers (in water at pH 7 and 25 °C). Adapted from
[35].
The presence of amorphous regions increases the chemical hydrolysis rate due to the easy diffusion of water into the polymer matrix compared to semi- and crystalline polymers, showing well-organized structures where diffusion is limited, even at temperatures higher than
Tg [36][37][38]. So, for polymers with lower or similar values of
Tg than the mesophilic range, the diffusion is mostly controlled by the amorphous region where chemical hydrolysis is dominant.
The macro structure properties, such as the size and shape of the polymer, are factors that condition whether the dominant mechanism will be either surface or bulk erosion. In this way, a material can go from surface to bulk erosion when its thickness is reduced to a value lower than a critical value, which is called the critical sample thickness (
Lcrit)
[28][39].
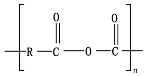
Polyesters, due to the presence of ester groups, are degraded by chemical hydrolysis. Bulk degradation is predominant for aliphatic polyesters, such as poly(glycolic acid) (PGA), PLA, PCL, and PBS. The main stages of the hydrolytic degradation of polyesters undergoing bulk erosion can be summarized as (1) diffusion of water in the polymer matrix (amorphous regions); (2) water reacting with random ester linkage to produce shorter chains; (3) autocatalysis due to the presence of acid chain ends in the medium; and 4) release of water-soluble oligomers and monomers creating a void core and subsequent reduction in
Mw [3][40]. The duration of the chemical hydrolysis process depends mainly on the initial
Mw, crystallinity, temperature, and pH
[5].
PLA is an example for chemical hydrolysable polymer degradation. In this sense, the environment to which the material is exposed and factors such as temperature, pH, and moisture play major roles in delaying or speeding up the hydrolytic degradation rate. In an industrial composting process (≈58 °C and ≈60% RH), PLA can absorb water and undergo chemical hydrolytic degradation. However, at lower temperatures, such as in agricultural soil environments (≈25 °C), the rate of chemical hydrolysis is low, increasing the time for the enzymatic hydrolysis process to start. One of the main differences between bulk and surface erosion mechanisms can be recognized in the diffusion of the degradation by-products. During the bulk degradation of polyesters, these hydrolysis-formed oligomer and monomer by-products, such as carboxylic acid and hydroxyl groups, are trapped and accumulated inside the bulk, leading to an autocatalytic degradation that tends to accelerate the degradation kinetics
[27][41]. Burkersroda et al.
[39] reported that the hydrolytic degradation of PLA, evaluated at 37 °C, follows a bulk erosion mechanism for thicknesses between 0.5 and 2 mm, a core-accelerated erosion for thicknesses between 2 and 74 mm, and surface erosion for thicknesses greater than 74 mm. Hoüglund et al.
[42] reported that the hydrolysis of 100% PLLA increased upon the addition of a low percentage of
d-Lactide units due to a reduction in the polymer order structure, showing the effect of tacticity and optical purity on the hydrolytic degradation of PLA. In comparison to PGA hydrolysis, PLA hydrolysis is delayed due to the presence of the methyl group in PLA that blocks the attack of water to interact with the hydrolysable bonds
[2][3]. For more insights, a review of PLA’s hydrolysis has been reported by Tsuji
[28].
PBAT, due to the presence of an aromatic group in its polyester chain, experiences a lower hydrolytic degradation rate than polyester with only aliphatic units as PLA and PGA
[43]. The presence of the aromatic group reduces chain flexibility, provides less susceptible bonds, and creates a steric interference effect to the access of the susceptible ester bonds
[44]. The soft aliphatic domain bonds consisting of 1,4-butanediol and adipic acid monomers (BA) are more susceptible to hydrolysis than the hard aromatic bonds of 1,4-butanediol and terephthalic acid monomers (BT). In this sense, PBAT displays good biodegradability when the aromatic moiety concentration is kept below 55 mol%
[45]. Kijchavengkul et al.
[20] also demonstrated that the increase in crosslinking on PBAT has a detrimental effect not only on chemical hydrolysis but also on enzymatic hydrolysis.
Polymers that undergo surface erosion are desirable when designing surgical medical devices and for drug release, since the retention of mechanical properties and capacity for a controlled release of drugs can be achieved by mass loss without compromising the
Mw. Some examples are polyanhydrides, some poly(ortho esters), and some polycarbonates
[46][47][48].
6. Biotic Enzymatic Degradation
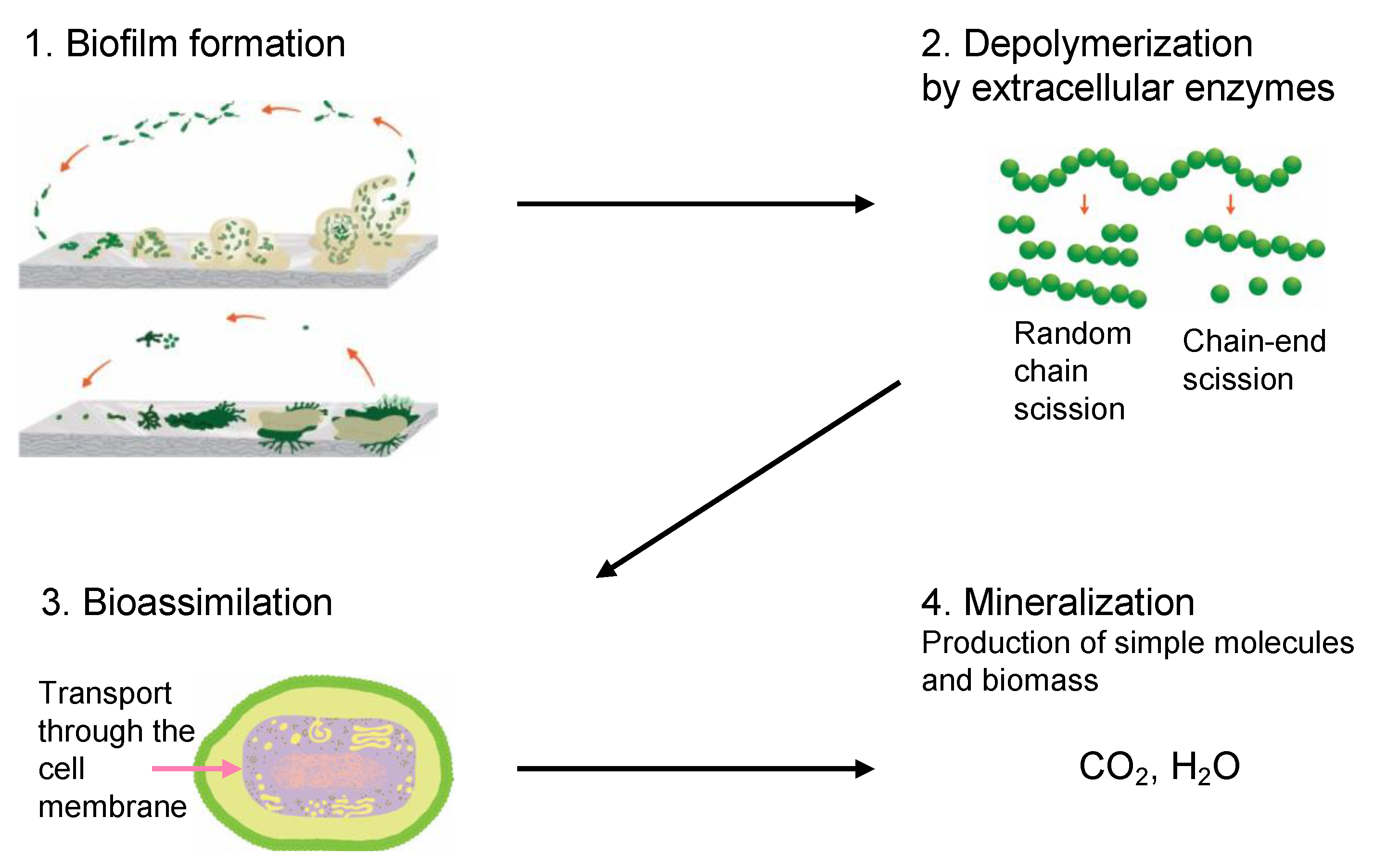
Biotic enzymatic degradation is the mechanism where microorganisms break down organic substances through an enzymatic process. The four main stages of biotic degradation are shown in
Figure 1. The main outcome of biotic degradation is the reduction of the polymer structure to small molecules that are assimilated by the microorganisms as a source of carbon and energy, resulting in final products such as CO
2 and H
2O in aerobic conditions. Microorganisms such as bacteria and fungi are actively involved in the biodegradation process. These microorganisms have their own optimal growth conditions; for this reason, biotic degradation is a complex process where several factors associated with the polymer, microorganisms, and the environment come into play
[29]. The abiotic mechanisms described above, such as photo, hydrolytic, or even mechanical degradation, can enhance the biotic degradation process by increasing the surface area for biofilm formation or by reducing the
Mw [5]. However, the dominant mechanism in the biotic degradation process is related to biotic agents.
Figure 1. The four main stages involved in the biotic degradation process: (
1) biofilm formation—establishment of microbial colonies on the polymer surface through the secretion of extracellular polymeric substances, (
2) depolymerization—breakdown of polymer chains into small molecules such as oligomers, trimers, dimers, and monomers by the action of extracellular enzymes, (
3) bioassimilation—metabolization of low
Mw compounds (dimers, monomers) by transportation through the cell membrane and (
4) mineralization—carbon is biologically oxidized to CO
2 through a series of cycles, releasing energy and water and other compounds. Adapted from
[49].