
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xinjian Zhang | -- | 2053 | 2022-10-24 15:06:57 | | | |
| 2 | Vivi Li | + 1 word(s) | 2054 | 2022-10-27 04:12:19 | | |
Video Upload Options
Dopamine regulates emotional behaviors, including rewarding and aversive behaviors, through the mesolimbic dopaminergic pathway, which projects dopamine neurons from the ventral tegmental area to the nucleus accumbens (NAc). Protein phosphorylation is critical for intracellular signaling pathways and physiological functions, which are regulated by neurotransmitters in the brain. Previous studies have demonstrated that dopamine stimulated the phosphorylation of intracellular substrates, such as receptors, ion channels, and transcription factors, to regulate neuronal excitability and synaptic plasticity through dopamine receptors. Recent advances in proteomics techniques have clarified the mechanisms through which dopamine controls rewarding and aversive behaviors through signal pathways in the NAc.
1. Introduction
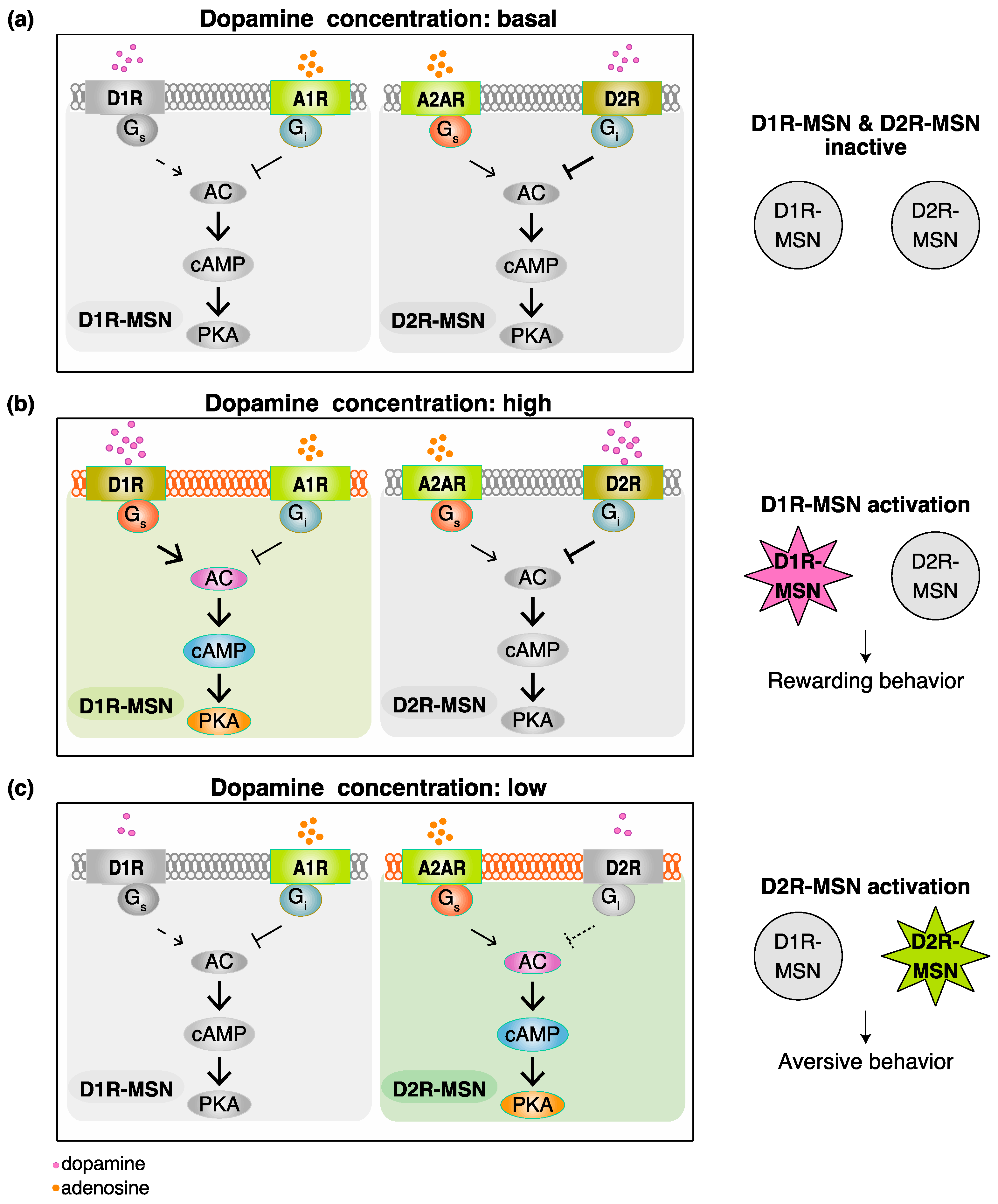
2. Functional Model for Dopamine D1 Receptors Expressing Medium Spiny Neurons (D1R-MSN) and Dopamine D2 Receptors Expressing Medium Spiny Neurons (D2R-MSN) in Rewarding and Aversive Behaviors
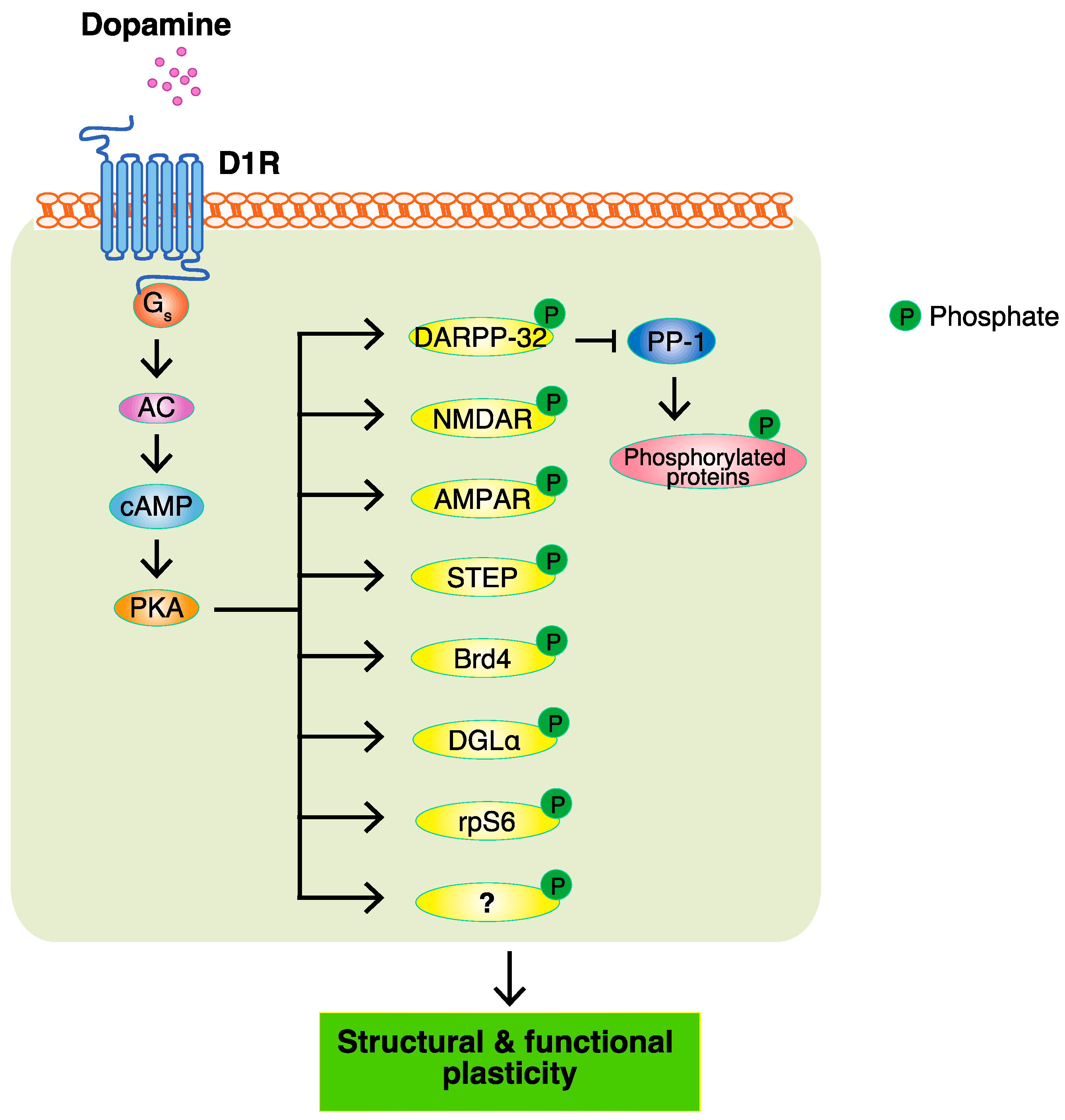
3. PKA and PKA Substrates
4. Phosphoproteomic Analyses Revealed New Phosphorylation Signals Downstream of Dopamine Receptors in Emotional Behaviors
Major efforts have been made to identify the target substrates of PKA so as to understand the modes of action of dopamine, and a few of its substrates, including DARPP-32, GluA1, and NR1, have been reported. The PKA-mediated phosphorylation of DARPP-32 indirectly accumulates phosphoproteins through the inhibition of protein phosphatase PP1. However, it remains unclear about the proteins directly phosphorylated by PKA. To identify the PKA substrates that are responsible for the function of dopamine, a Kinase-Oriented Substrate Screening (KiOSS) method, a novel comprehensive phosphoproteomic analysis, has been developed. To provide information on the phosphorylation signals identified by KiOSS methods, as well as those previously reported in the literature, researchers developed a novel online database, KANPHOS (https://kanphos.neuroinf.jp).
According to previous studies and researchers' KANPHOS data, the stimulation of D1R by dopamine increases intracellular cAMP concentrations through AC, and this is followed by the activation of PKA. PKA phosphorylates Rasgrp2 and Rap1gap to induce the activation of Rap1, which promotes the MAPK pathway. MAPK phosphorylates Kcnq2 to increase membrane excitability. MAPK also phosphorylates Npas4 and MKL2 to facilitate the gene expression, accounting for synaptic plasticity regulated by dopamine. The stimulation of A2AR without the inhibition of D2R also increases intracellular cAMP concentrations through AC, and this is followed by the activation of PKA. The activation of Rap1 induces the MAPK pathway to regulate synaptic plasticity. The stimulation of M1R induces the activation of PKC, and this is followed by the phosphorylation of Kcnq2, which accounts for the increased membrane excitability. Therefore, phosphorylation signals downstream of D1R are involved in D1R-MSN-mediated rewarding behavior, and phosphorylation signals downstream of A2AR and M1R without the inhibition of D2R are involved in D2R-MSN-mediated aversive behavior (Figure 3).
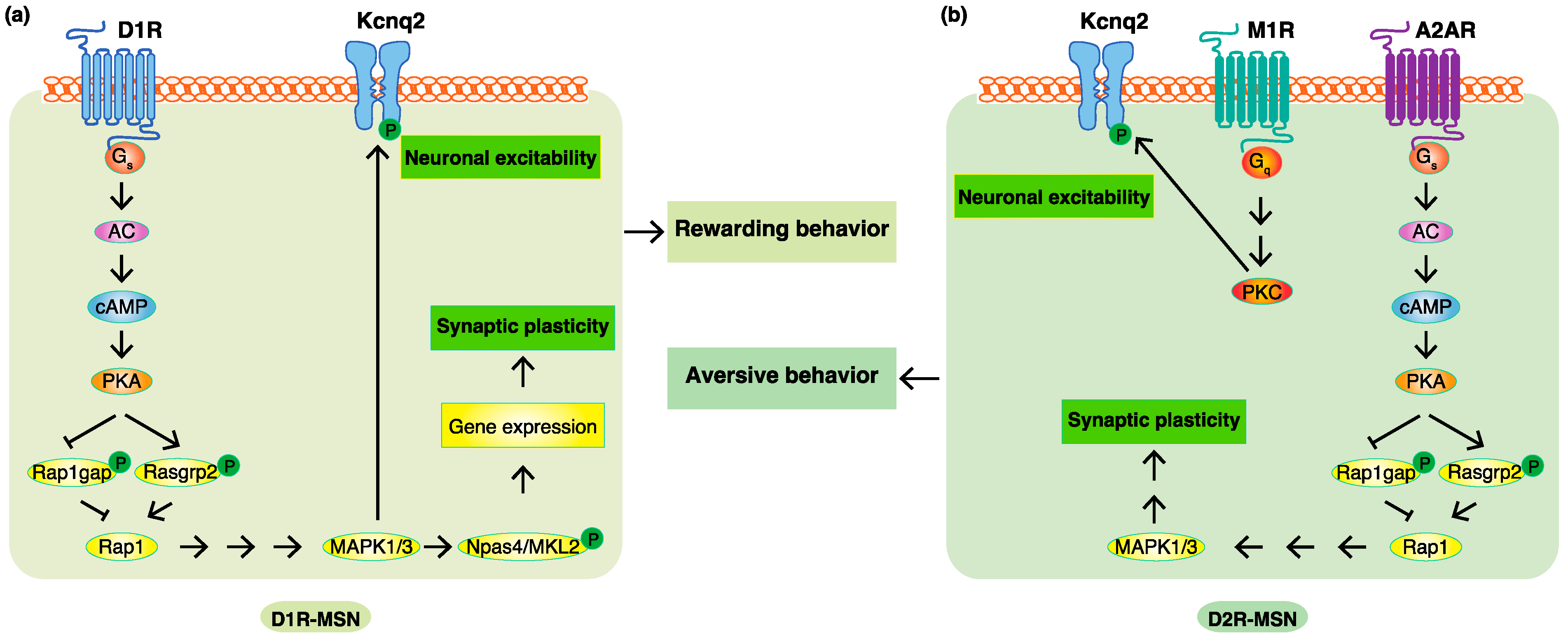
Figure 3. Phosphoproteomic analyses revealed new phosphorylation signals downstream of do-pamine receptors in emotional behaviors. (a) In D1R-MSN, the stimulation of D1R by dopamine increased intracellular cAMP concentrations through AC, and this was followed by the activation of PKA. PKA phosphorylates Rasgrp2 and Rap1gap to induce the activation of Rap1, which pro-motes the MAPK pathway. MAPK phosphorylates Kcnq2 to increase membrane excitability and phosphorylates Npas4 and MKL2 to facilitate the expression of the genes accounting for synaptic plasticity, consequently leading to rewarding behavior. (b) In D2R-MSN, a decreased dopamine concentration cancels the suppressive effects of D2R. The stimulation of A2AR without the inhibi-tion of D2R increases intracellular cAMP concentrations through AC, and this is followed by the activation of PKA. PKA phosphorylates Rasgrp2 and Rap1gap to induce the activation of Rap1, which promotes the MAPK pathway involved in synaptic plasticity. Acetylcholine/M1R signaling activates PKC to promote the phosphorylation of Kcnq2, which is involved in membrane excita-bility. These changes consequently lead to aversive behavior.
References
- Yeragani, V.K.; Tancer, M.; Chokka, P.; Baker, G.B. Arvid Carlsson, and the story of dopamine. Indian J. Psychiatry 2010, 52, 87.
- Perez-Fernandez, J.; Barandela, M.; Jimenez-Lopez, C. The Dopaminergic Control of Movement-Evolutionary Considerations. Int. J. Mol. Sci. 2021, 22, 11284.
- Castillo Diaz, F.; Caffino, L.; Fumagalli, F. Bidirectional role of dopamine in learning and memory-active forgetting. Neurosci. Biobehav. Rev. 2021, 131, 953–963.
- Watabe-Uchida, M.; Eshel, N.; Uchida, N. Neural Circuitry of Reward Prediction Error. Annu. Rev. Neurosci. 2017, 40, 373–394.
- Engelhard, B.; Finkelstein, J.; Cox, J.; Fleming, W.; Jang, H.J.; Ornelas, S.; Koay, S.A.; Thiberge, S.Y.; Daw, N.D.; Tank, D.W. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 2019, 570, 509–513.
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126.
- Weinstein, J.J.; Chohan, M.O.; Slifstein, M.; Kegeles, L.S.; Moore, H.; Abi-Dargham, A. Pathway-Specific Dopamine Ab-normalities in Schizophrenia. Biol. Psychiatry 2017, 81, 31–42.
- Wise, R.A.; Robble, M.A. Dopamine and Addiction. Annu. Rev. Psychol. 2020, 71, 79–106.
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83.
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59.
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046.
- Mitchell, U.H.; Obray, J.D.; Hunsaker, E.; Garcia, B.T.; Clarke, T.J.; Hope, S.; Steffensen, S.C. Peripheral dopamine in rest-less legs syndrome. Front. Neurol. 2018, 9, 155.
- Torrisi, S.A.; Leggio, G.M.; Drago, F.; Salomone, S. Therapeutic challenges of post-traumatic stress disorder: Focus on the dopaminergic system. Front. Pharmacol. 2019, 10, 404.
- Lammel, S.; Lim, B.K.; Ran, C.; Huang, K.W.; Betley, M.J.; Tye, K.M.; Deisseroth, K.; Malenka, R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature 2012, 491, 212–217.
- Sun, F.; Zeng, J.; Jing, M.; Zhou, J.; Feng, J.; Owen, S.F.; Luo, Y.; Li, F.; Wang, H.; Yamaguchi, T.; et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174, 481–496.e419.
- Rehman, I.; Mahabadi, N.; Sanvictores, T.; Rehman, C.I. Classical conditioning. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2017.
- Flagel, S.B.; Clark, J.J.; Robinson, T.E.; Mayo, L.; Czuj, A.; Willuhn, I.; Akers, C.A.; Clinton, S.M.; Phillips, P.E.; Akil, H. A selective role for dopamine in stimulus-reward learning. Nature 2011, 469, 53–57.
- Van Zessen, R.; Flores-Dourojeanni, J.P.; Eekel, T.; van den Reijen, S.; Lodder, B.; Omrani, A.; Smidt, M.P.; Ramakers, G.M.J.; van der Plasse, G.; Stuber, G.D.; et al. Cue and Reward Evoked Dopamine Activity Is Necessary for Maintaining Learned Pavlovian Associations. J. Neurosci. 2021, 41, 5004–5014.
- Morrens, J.; Aydin, C.; Janse van Rensburg, A.; Esquivelzeta Rabell, J.; Haesler, S. Cue-Evoked Dopamine Promotes Con-ditioned Responding during Learning. Neuron 2020, 106, 142–153.e147.
- Danjo, T.; Yoshimi, K.; Funabiki, K.; Yawata, S.; Nakanishi, S. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2014, 111, 6455–6460.
- Tan, K.R.; Yvon, C.; Turiault, M.; Mirzabekov, J.J.; Doehner, J.; Labouebe, G.; Deisseroth, K.; Tye, K.M.; Luscher, C. GABA neurons of the VTA drive conditioned place aversion. Neuron 2012, 73, 1173–1183.
- Bhatia, A.; Saadabadi, A. Biochemistry, Dopamine Receptors. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by β-arrestins. Science 2005, 308, 512–517.
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.P.; Feng, B.; et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493.
- Donthamsetti, P.; Gallo, E.F.; Buck, D.C.; Stahl, E.L.; Zhu, Y.; Lane, J.R.; Bohn, L.M.; Neve, K.A.; Kellendonk, C.; Javitch, J.A. Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol. Psychiatry 2020, 25, 2086–2100.
- Xu, L.; Nan, J.; Lan, Y. The nucleus accumbens: A common target in the comorbidity of depression and addiction. Front. Neural Circuits 2020, 14, 37.
- Castro, D.C.; Bruchas, M.R. A motivational and neuropeptidergic hub: Anatomical and functional diversity within the nucleus accumbens shell. Neuron 2019, 102, 529–552.
- Hikida, T.; Kimura, K.; Wada, N.; Funabiki, K.; Nakanishi, S. Distinct roles of synaptic transmission in direct and indi-rect striatal pathways to reward and aversive behavior. Neuron 2010, 66, 896–907.
- Hart, A.S.; Rutledge, R.B.; Glimcher, P.W.; Phillips, P.E. Phasic dopamine release in the rat nucleus accumbens symmetri-cally encodes a reward prediction error term. J. Neurosci. 2014, 34, 698–704.
- Liu, C.; Kershberg, L.; Wang, J.; Schneeberger, S.; Kaeser, P.S. Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites. Cell 2018, 172, 706–718.e715.
- Liu, C.; Kaeser, P.S. Mechanisms and regulation of dopamine release. Curr. Opin. Neurobiol. 2019, 57, 46–53.
- Martel, J.C.; Gatti McArthur, S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Con-cepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003.
- Marcellino, D.; Kehr, J.; Agnati, L.F.; Fuxe, K. Increased affinity of dopamine for D(2) -like versus D(1) -like receptors. Relevance for volume transmission in interpreting PET findings. Synapse 2012, 66, 196–203.
- Cadoni, C.; Solinas, M.; Di Chiara, G. Psychostimulant sensitization: Differential changes in accumbal shell and core dopamine. Eur. J. Pharmacol. 2000, 388, 69–76.
- Yuen, J.; Goyal, A.; Rusheen, A.E.; Kouzani, A.Z.; Berk, M.; Kim, J.H.; Tye, S.J.; Blaha, C.D.; Bennet, K.E.; Jang, D.P.; et al. Cocaine-Induced Changes in Tonic Dopamine Concentrations Measured Using Multiple-Cyclic Square Wave Voltammetry in vivo. Front. Pharmacol. 2021, 12, 705254.
- Oleson, E.B.; Gentry, R.N.; Chioma, V.C.; Cheer, J.F. Subsecond dopamine release in the nucleus accumbens predicts con-ditioned punishment and its successful avoidance. J. Neurosci. 2012, 32, 14804–14808.
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625.
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55.
- Zhang, X.; Nagai, T.; Ahammad, R.U.; Kuroda, K.; Nakamuta, S.; Nakano, T.; Yukinawa, N.; Funahashi, Y.; Yamahashi, Y.; Amano, M.; et al. Balance between dopamine and adenosine signals regulates the PKA/Rap1 pathway in striatal me-dium spiny neurons. Neurochem. Int. 2019, 122, 8–18.
- De Jong, J.W.; Afjei, S.A.; Pollak Dorocic, I.; Peck, J.R.; Liu, C.; Kim, C.K.; Tian, L.; Deisseroth, K.; Lammel, S. A Neural Cir-cuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 2019, 101, 133–151.e137.
- Beninger, R.J.; Nakonechny, P.L.; Savina, I. cAMP-dependent protein kinase and reward-related learning: In-tra-accumbens Rp-cAMPS blocks amphetamine-produced place conditioning in rats. Psychopharmacology 2003, 170, 23–32.
- Nagai, T.; Nakamuta, S.; Kuroda, K.; Nakauchi, S.; Nishioka, T.; Takano, T.; Zhang, X.; Tsuboi, D.; Funahashi, Y.; Nakano, T.; et al. Phosphoproteomics of the Dopamine Pathway Enables Discovery of Rap1 Activation as a Reward Signal In Vivo. Neuron 2016, 89, 550–565.
- Lin, Y.H.; Yamahashi, Y.; Kuroda, K.; Faruk, M.O.; Zhang, X.; Yamada, K.; Yamanaka, A.; Nagai, T.; Kaibuchi, K. Ac-cumbal D2R-medium spiny neurons regulate aversive behaviors through PKA-Rap1 pathway. Neurochem. Int. 2021, 143, 104935.
- Greengard, P. The neurobiology of slow synaptic transmission. Science 2001, 294, 1024–1030.





