
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vivi Li | -- | 1993 | 2022-10-21 01:47:55 |
Video Upload Options
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of the Earth's crust. In mineralogy, silica (silicon dioxide) SiO2 is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth.
1. General Structure
A silicate mineral is generally an ionic compound whose anions consist predominantly of silicon and oxygen atoms.
In most minerals in the Earth's crust, each silicon atom is the center of an ideal tetrahedron, whose corners are four oxygen atoms covalently bound to it. Two adjacent tetrahedra may share a vertex, meaning that the oxygen atom is a bridge connecting the two silicon atoms. An unpaired vertex represents an ionized oxygen atom, covalently bound to a single silicon atom, that contributes one unit of negative charge to the anion.
Some silicon centers may be replaced by atoms of other elements, still bound to the four corner oxygen corners. If the substituted atom is not normally tetravalent, it usually contributes extra charge to the anion, which then requires extra cations. For example, in the mineral orthoclase [KAlSi3O8]n, the anion is a tridimensional network of tetrahedra in which all oxygen corners are shared. If all tetrahedra had silicon centers, the anion would be just neutral silica [SiO2]n. Replacement of one in every four silicon atoms by an aluminum atom results in the anion [AlSi3O−8]n, whose charge is neutralized by the potassium cations K+.
2. Main Groups
In mineralogy, silicate minerals are classified into seven major groups according to the structure of their silicate anion:[1][2]
| Major group | Structure | Chemical formula | Example |
|---|---|---|---|
| Nesosilicates | isolated silicon tetrahedra | [SiO4]4− | olivine |
| Sorosilicates | double tetrahedra | [Si2O7]6− | epidote, melilite group |
| Cyclosilicates | rings | [SinO3n]2n− | tourmaline group |
| Inosilicates | single chain | [SinO3n]2n− | pyroxene group |
| Inosilicates | double chain | [Si4nO11n]6n− | amphibole group |
| Phyllosilicates | sheets | [Si2nO5n]2n− | micas and clays |
| Tectosilicates | 3D framework | [AlxSiyO(2x+2y)]x− | quartz, feldspars, zeolites |
Note that tectosilicates can only have additional cations if some of the silicon is replaced by an atom of lower valence such as aluminium. Al for Si substitution is common.
3. Nesosilicates or Orthosilicates
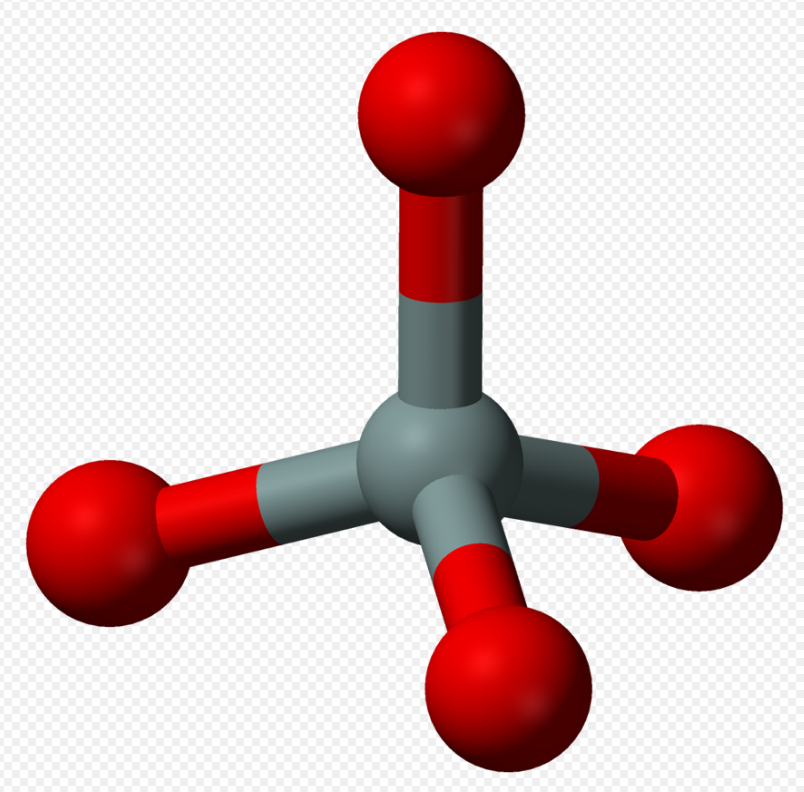

Nesosilicates (from Greek νῆσος nēsos, island), or orthosilicates, have the orthosilicate ion, which constitute isolated (insular) [SiO4]4− tetrahedra that are connected only by interstitial cations. The Nickel–Strunz classification is 09.A –examples include:
- Phenakite group
- Phenakite – Be2SiO4
- Willemite – Zn2SiO4
- Olivine group
- Forsterite – Mg2SiO4
- Fayalite – Fe2SiO4
- Tephroite – Mn2SiO4
- Garnet group
- Pyrope – Mg3Al2(SiO4)3
- Almandine – Fe3Al2(SiO4)3
- Spessartine – Mn3Al2(SiO4)3
- Grossular – Ca3Al2(SiO4)3
- Andradite – Ca3Fe2(SiO4)3
- Uvarovite – Ca3Cr2(SiO4)3
- Hydrogrossular – Ca3Al2Si2O8(SiO4)3−m(OH)4m
- Zircon group
- Zircon – ZrSiO4
- Thorite – (Th,U)SiO4
- Hafnon – (Hf,Zr)SiO4

- Al2SiO5 group
- Andalusite – Al2SiO5
- Kyanite – Al2SiO5
- Sillimanite – Al2SiO5
- Dumortierite – Al6.5–7BO3(SiO4)3(O,OH)3
- Topaz – Al2SiO4(F,OH)2
- Staurolite – Fe2Al9(SiO4)4(O,OH)2
- Humite group – (Mg,Fe)7(SiO4)3(F,OH)2
- Norbergite – Mg3(SiO4)(F,OH)2
- Chondrodite – Mg5(SiO4)2(F,OH)2
- Humite – Mg7(SiO4)3(F,OH)2
- Clinohumite – Mg9(SiO4)4(F,OH)2
- Datolite – CaBSiO4(OH)
- Titanite – CaTiSiO5
- Chloritoid – (Fe,Mg,Mn)2Al4Si2O10(OH)4
- Mullite (aka Porcelainite) – Al6Si2O13
4. Sorosilicates
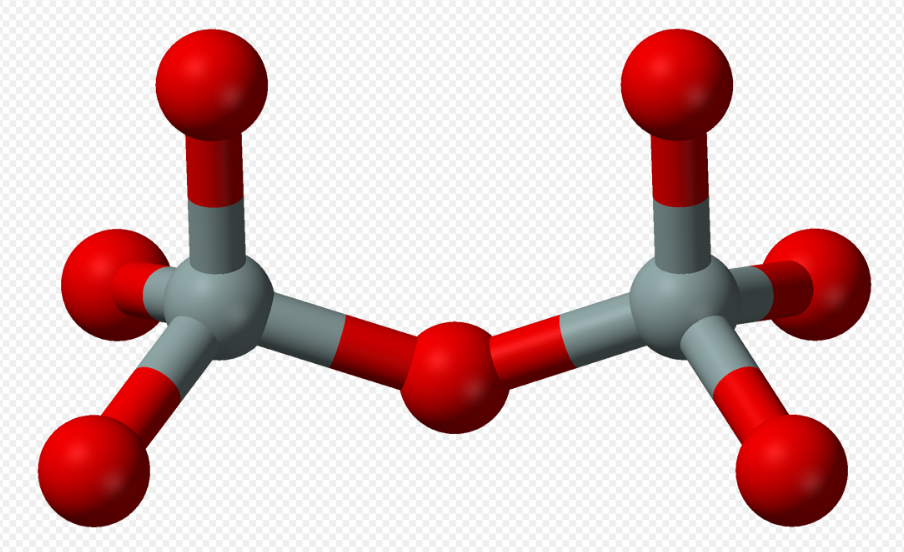

Sorosilicates (from Greek σωρός sōros, heap, mound) have isolated pyrosilicate anions Si2O6−7, consisting of double tetrahedra with a shared oxygen vertex—a silicon:oxygen ratio of 2:7. The Nickel–Strunz classification is 09.B. Examples include:
- Hemimorphite (calamine) – Zn4(Si2O7)(OH)2·H2O
- Lawsonite – CaAl2(Si2O7)(OH)2·H2O
- Axinite – (Ca,Fe,Mn)3Al2(BO3)(Si4O12)(OH)
- Ilvaite – CaFeII2FeIIIO(Si2O7)(OH)
- Epidote group (has both (SiO4)4− and (Si2O7)6− groups)
- Epidote – Ca2(Al,Fe)3O(SiO4)(Si2O7)(OH)
- Zoisite – Ca2Al3O(SiO4)(Si2O7)(OH)
- Tanzanite – Ca2Al3O(SiO4)(Si2O7)(OH)
- Clinozoisite – Ca2Al3O(SiO4)(Si2O7)(OH)
- Allanite – Ca(Ce,La,Y,Ca)Al2(FeII,FeIII)O(SiO4)(Si2O7)(OH)
- Dollaseite-(Ce) – CaCeMg2AlSi3O11F(OH)
- Vesuvianite (idocrase) – Ca10(Mg,Fe)2Al4(SiO4)5(Si2O7)2(OH)4
5. Cyclosilicates



Cyclosilicates (from Greek κύκλος kuklos, circle), or ring silicates, have three or more tetrahedra linked in a ring. The general formula is (SixO3x)2x−, where one or more silicon atoms can be replaced by other 4-coordinated atom. The silicon:oxygen ratio is 1:3. Double rings have the formula (Si2xO6x)2x−. The Nickel–Strunz classification is 09.C. Possible ring sizes include:
-
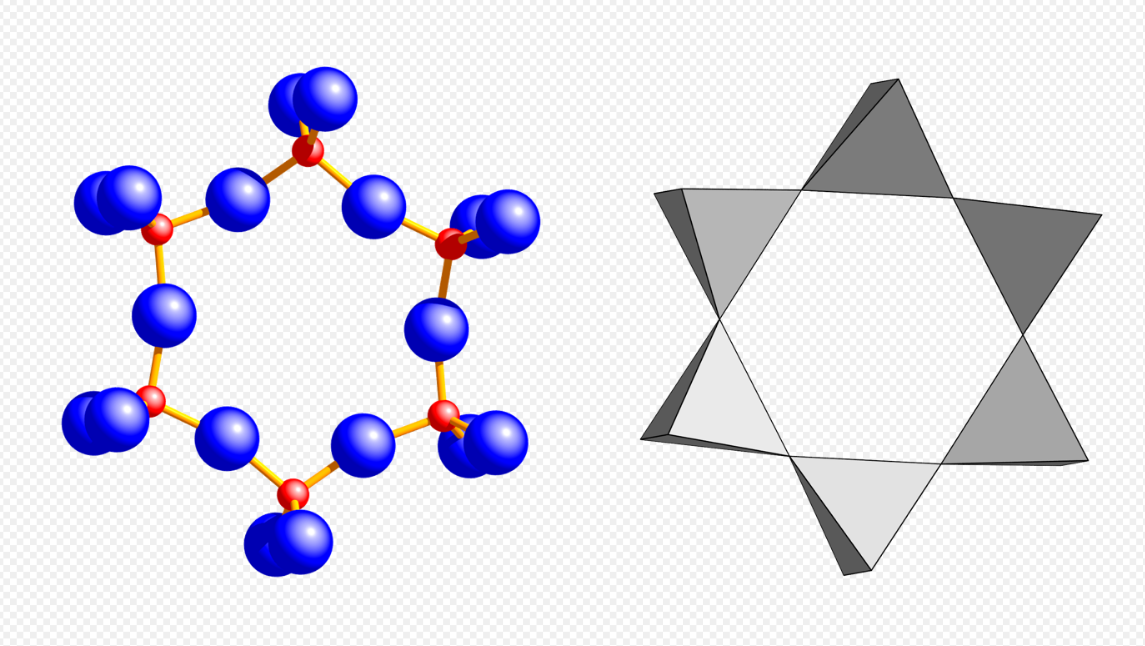
6 units [Si6O18], beryl (red: Si, blue: O). https://handwiki.org/wiki/index.php?curid=1485628
-
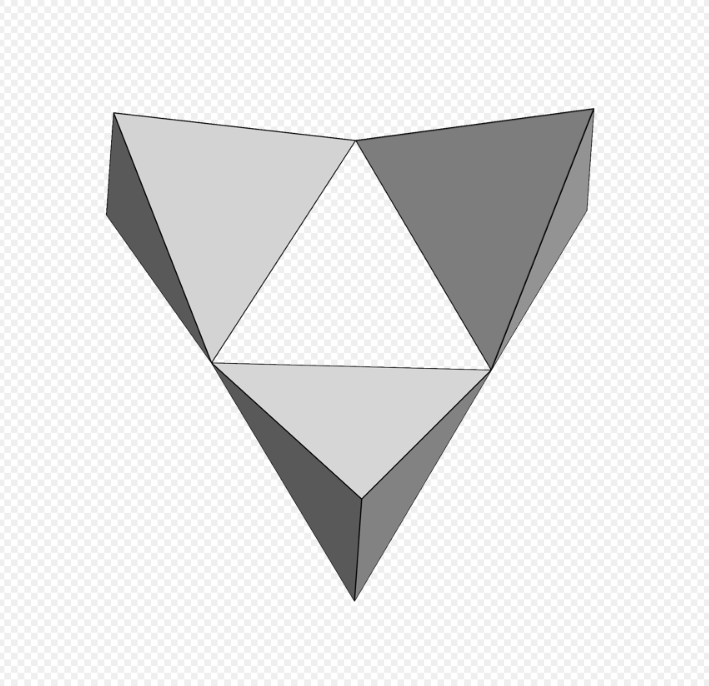
3 units [Si3O9], benitoite. https://handwiki.org/wiki/index.php?curid=1415149
-
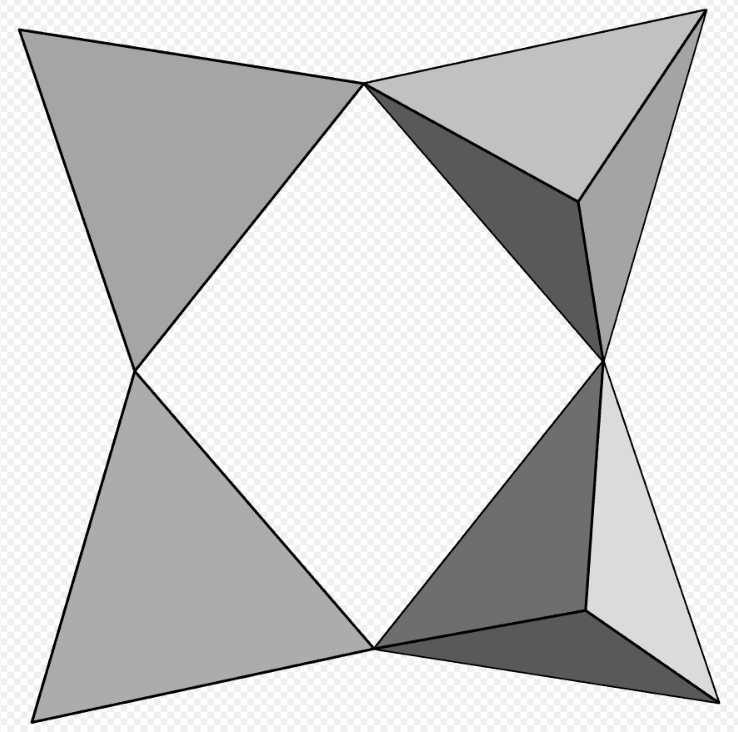
4 units [Si4O12], papagoite. https://handwiki.org/wiki/index.php?curid=1256622
-
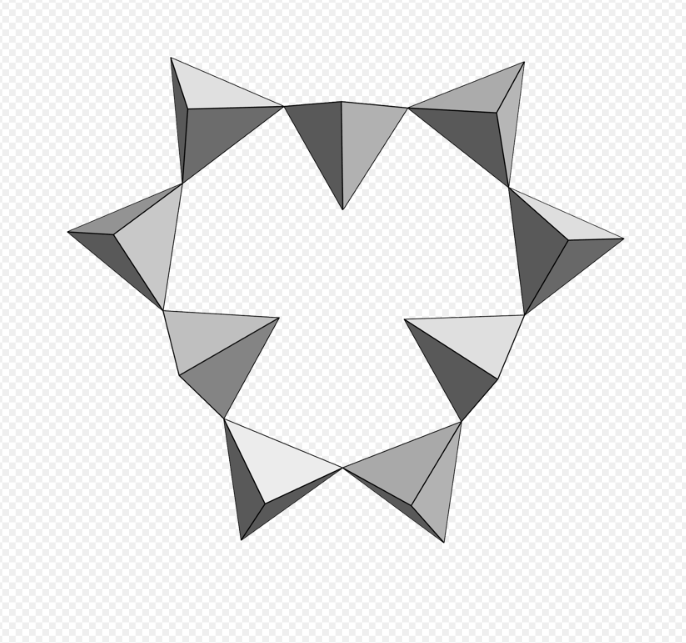
9 units [Si9O27], eudialyte. https://handwiki.org/wiki/index.php?curid=1594971
-
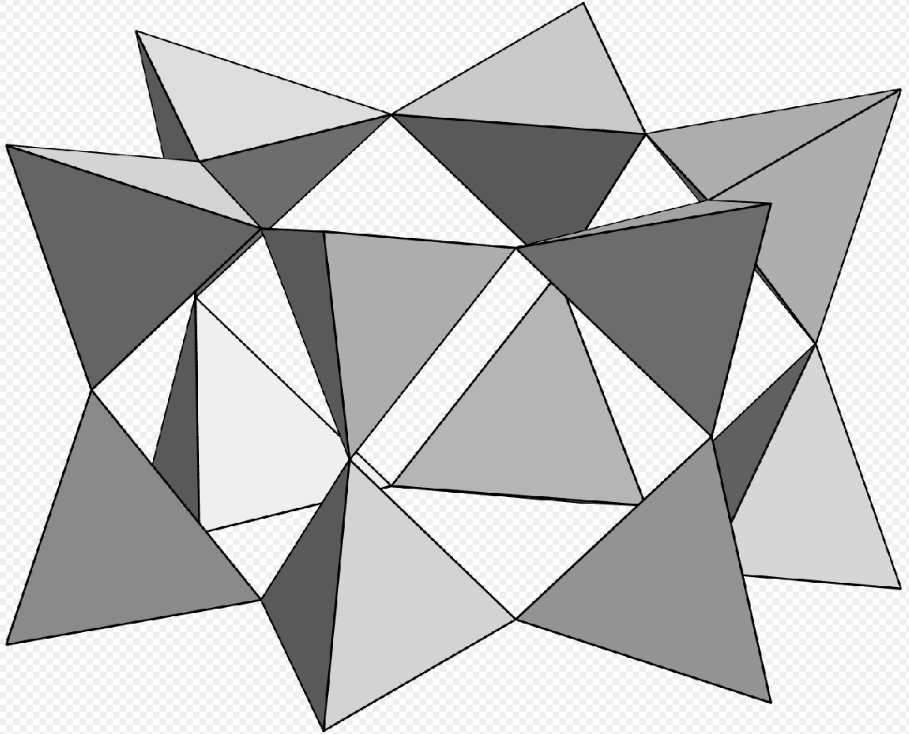
6 units, double ring [Si6O18], milarite. https://handwiki.org/wiki/index.php?curid=1104046
Some example minerals are:
- 3-member single ring
- Benitoite – BaTi(Si3O9)
- 4-member single ring
- Papagoite – CaCuAlSi2O6(OH)3.
- 6-member single ring
- Beryl – Be3Al2(Si6O18)
- Bazzite – Be3Sc2(Si6O18)
- Sugilite – KNa2(Fe,Mn,Al)2Li3Si12O30
- Tourmaline – (Na,Ca)(Al,Li,Mg)3−(Al,Fe,Mn)6(Si6O18)(BO3)3(OH)4
- Pezzottaite – Cs(Be2Li)Al2Si6O18
- Osumilite – (K,Na)(Fe,Mg)2(Al,Fe)3(Si,Al)12O30
- Cordierite – (Mg, Fe)2Al4Si5O18
- Sekaninaite – (Fe+2, Mg)2Al4Si5O18
- 9-member single ring
- Eudialyte – Na15Ca6(Fe,Mn)3Zr3SiO(O,OH,H2O)3(Si3O9)2(Si9O27)2(OH,Cl)2
- 6-member double ring
- Milarite – K2Ca4Al2Be4(Si24O60)H2O
Note that the ring in axinite contains two B and four Si tetrahedra and is highly distorted compared to the other 6-member ring cyclosilicates.
6. Inosilicates
Inosilicates (from Greek ἴς is [genitive: ἰνός inos], fibre), or chain silicates, have interlocking chains of silicate tetrahedra with either SiO3, 1:3 ratio, for single chains or Si4O11, 4:11 ratio, for double chains. The Nickel–Strunz classification is 09.D – examples include:
6.1. Single Chain Inosilicates
- Pyroxene group
- Enstatite – orthoferrosilite series
- Enstatite – MgSiO3
- Ferrosilite – FeSiO3
- Pigeonite – Ca0.25(Mg,Fe)1.75Si2O6
- Diopside – hedenbergite series
- Diopside – CaMgSi2O6
- Hedenbergite – CaFeSi2O6
- Augite – (Ca,Na)(Mg,Fe,Al)(Si,Al)2O6
- Sodium pyroxene series
- Jadeite – NaAlSi2O6
- Aegirine (or acmite) – NaFeIIISi2O6
- Spodumene – LiAlSi2O6
- Pyroxferroite - (Fe,Ca)SiO3
- Enstatite – orthoferrosilite series
- Pyroxenoid group
- Wollastonite – CaSiO3
- Rhodonite – MnSiO3
- Pectolite – NaCa2(Si3O8)(OH)
6.2. Double Chain Inosilicates
- Amphibole group
- Anthophyllite – (Mg,Fe)7Si8O22(OH)2
- Cummingtonite series
- Cummingtonite – Fe2Mg5Si8O22(OH)2
- Grunerite – Fe7Si8O22(OH)2
- Tremolite series
- Tremolite – Ca2Mg5Si8O22(OH)2
- Actinolite – Ca2(Mg,Fe)5Si8O22(OH)2
- Hornblende – (Ca,Na)2–3(Mg,Fe,Al)5Si6(Al,Si)2O22(OH)2
- Sodium amphibole group
- Glaucophane – Na2Mg3Al2Si8O22(OH)2
- Riebeckite (asbestos) – Na2FeII3FeIII2Si8O22(OH)2
- Arfvedsonite – Na3(Fe,Mg)4FeSi8O22(OH)2
- Inosilicate, pyroxene family, with 2-periodic single chain (Si2O6), diopside. https://handwiki.org/wiki/index.php?curid=1932185
Inosilicate, clinoamphibole, with 2-periodic double chains (Si4O11), tremolite. https://handwiki.org/wiki/index.php?curid=1259122
- Inosilicate, unbranched 3-periodic single chain of wollastonite. https://handwiki.org/wiki/index.php?curid=1328126
-
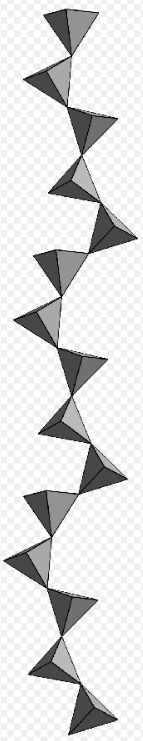
Inosilicate with 5-periodic single chain, rhodonite. https://handwiki.org/wiki/index.php?curid=1388024
-
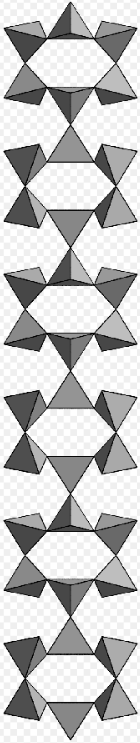
Inosilicate with cyclic branched 8-periodic chain, pellyite. https://handwiki.org/wiki/index.php?curid=1839329
7. Phyllosilicates
Phyllosilicates (from Greek φύλλον phyllon, leaf), or sheet silicates, form parallel sheets of silicate tetrahedra with Si2O5 or a 2:5 ratio. The Nickel–Strunz classification is 09.E. All phyllosilicate minerals are hydrated, with either water or hydroxyl groups attached.

Examples include:
- Serpentine subgroup
- Antigorite – Mg3Si2O5(OH)4
- Chrysotile – Mg3Si2O5(OH)4
- Lizardite – Mg3Si2O5(OH)4
- Clay minerals group
- Halloysite – Al2Si2O5(OH)4
- Kaolinite – Al2Si2O5(OH)4
- Illite – (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)]
- Montmorillonite – (Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2O
- Vermiculite – (MgFe,Al)3(Al,Si)4O10(OH)2·4H2O
- Talc – Mg3Si4O10(OH)2
- Sepiolite – Mg4Si6O15(OH)2·6H2O
- Palygorskite (or attapulgite) – (Mg,Al)2Si4O10(OH)·4(H2O)
- Pyrophyllite – Al2Si4O10(OH)2
- Mica group
- Biotite – K(Mg,Fe)3(AlSi3)O10(OH)2
- Fuchsite – K(Al,Cr)2(AlSi3O10)(OH)2
- Muscovite – KAl2(AlSi3)O10(OH)2
- Phlogopite – KMg3(AlSi3)O10(OH)2
- Lepidolite – K(Li,Al)2–3(AlSi3)O10(OH)2
- Margarite – CaAl2(Al2Si2)O10(OH)2
- Glauconite – (K,Na)(Al,Mg,Fe)2(Si,Al)4O10(OH)2
- Chlorite group
- Chlorite – (Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6
-
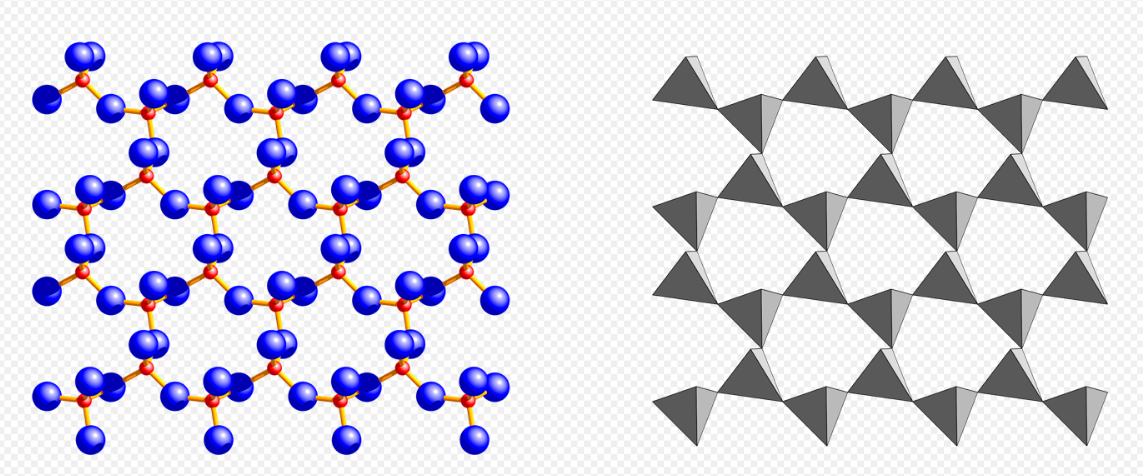
Phyllosilicate, mica group, muscovite (red: Si, blue: O). https://handwiki.org/wiki/index.php?curid=1536194
-
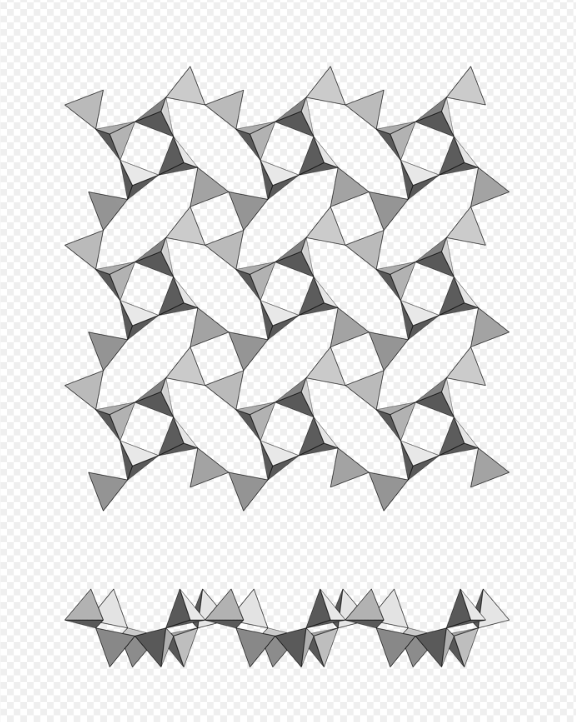
Phyllosilicate, single net of tetrahedra with 4-membered rings, apophyllite-(KF)-apophyllite-(KOH) series. https://handwiki.org/wiki/index.php?curid=1496820
-
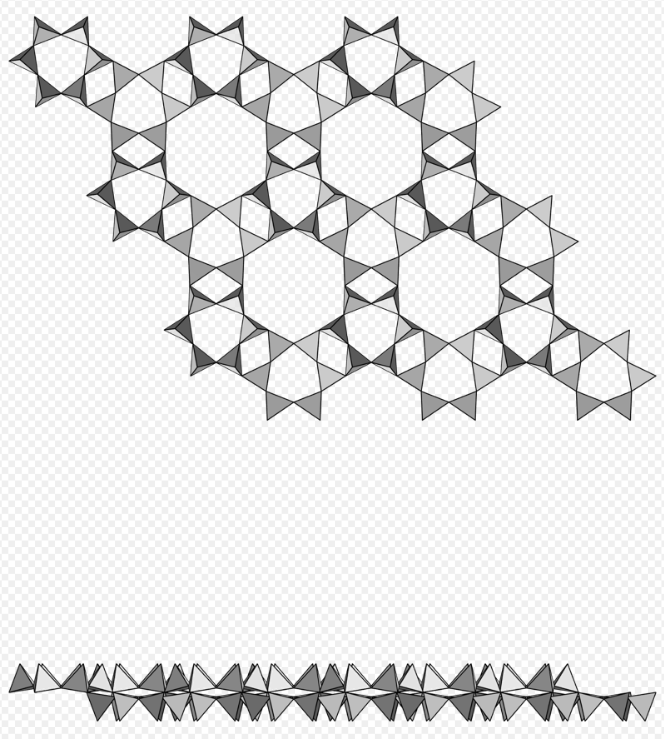
Phyllosilicate, single tetrahedral nets of 6-membered rings, pyrosmalite-(Fe)-pyrosmalite-(Mn) series. https://handwiki.org/wiki/index.php?curid=1244572
-
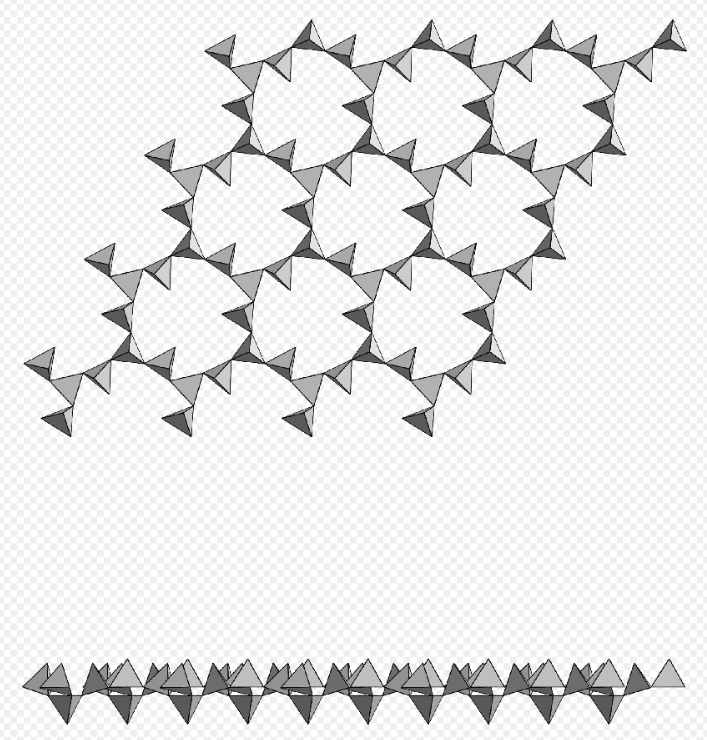
Phyllosilicate, single tetrahedral nets of 6-membered rings, zeophyllite. https://handwiki.org/wiki/index.php?curid=1289382
-
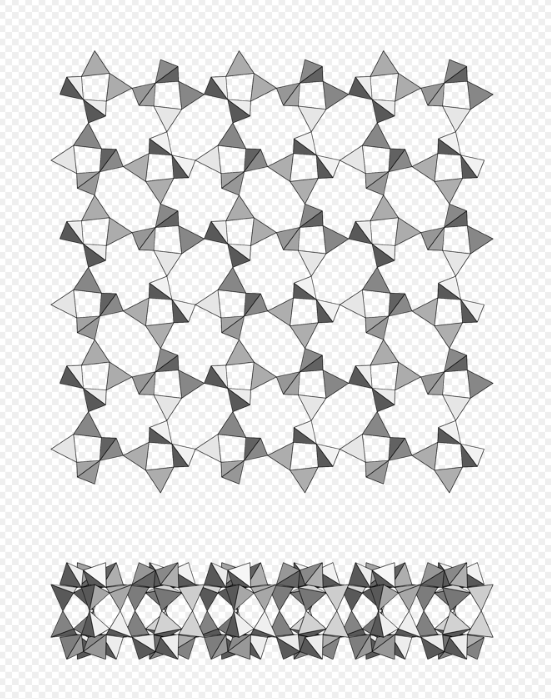
Phyllosilicate, double nets with 4- and 6-membered rings, carletonite. https://handwiki.org/wiki/index.php?curid=1890660
8. Tectosilicates
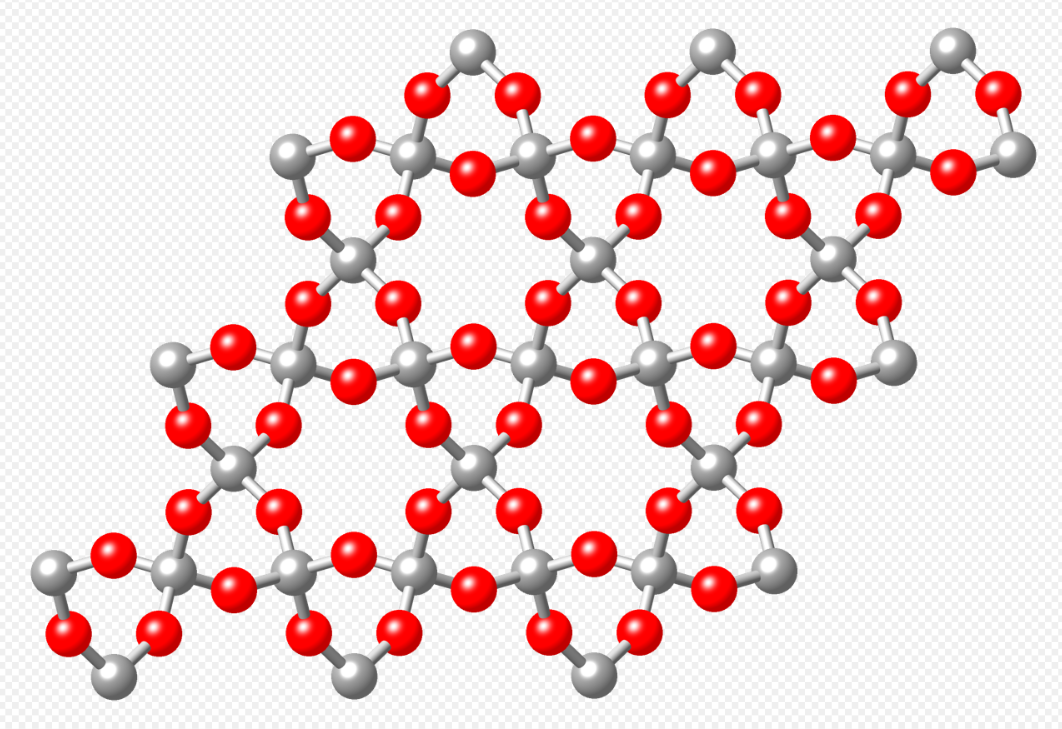
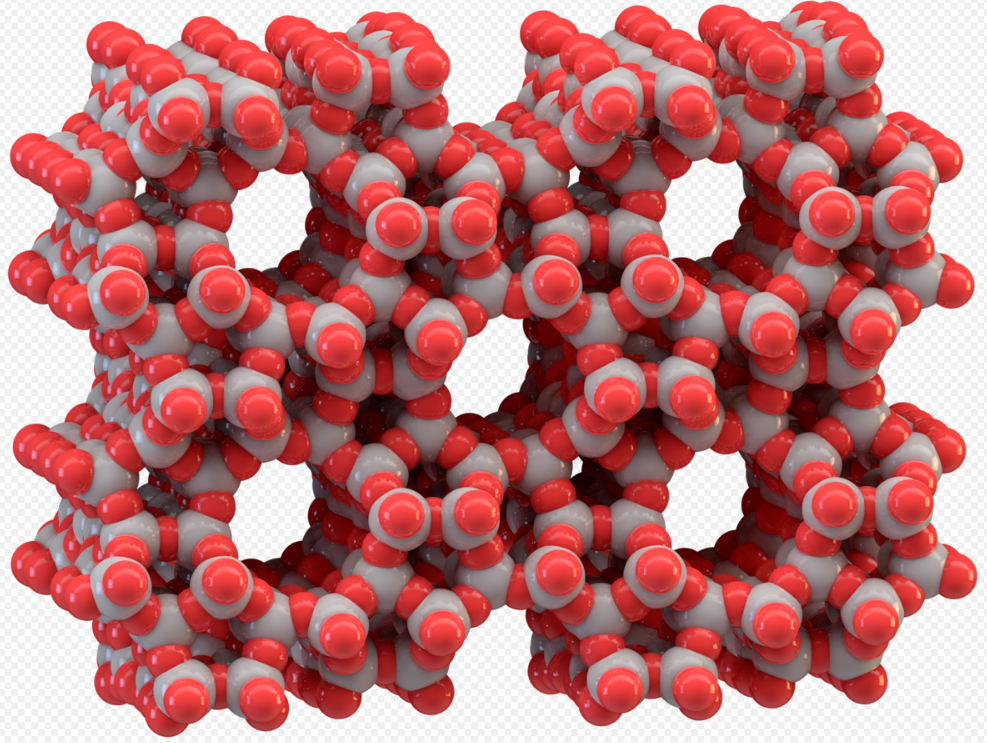


Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate tetrahedra with SiO2 or a 1:2 ratio. This group comprises nearly 75% of the crust of the Earth.[3] Tectosilicates, with the exception of the quartz group, are aluminosilicates. The Nickel–Strunz classifications are 09.F and 09.G, 04.DA (Quartz/ silica family). Examples include:
- 3D-Silicates, quartz family
- Quartz – SiO2
- Tridymite – SiO2
- Cristobalite – SiO2
- Coesite – SiO2
- Stishovite – SiO2
- Moganite – SiO2
- Chalcedony – SiO2
- Tectosilicates, feldspar group
- Alkali feldspars (potassium feldspars)
- Microcline – KAlSi3O8
- Orthoclase – KAlSi3O8
- Anorthoclase – (Na,K)AlSi3O8
- Sanidine – KAlSi3O8
- Plagioclase feldspars
- Albite – NaAlSi3O8
- Oligoclase – (Na,Ca)(Si,Al)4O8 (Na:Ca 4:1)
- Andesine – (Na,Ca)(Si,Al)4O8 (Na:Ca 3:2)
- Labradorite – (Ca,Na)(Si,Al)4O8 (Na:Ca 2:3)
- Bytownite – (Ca,Na)(Si,Al)4O8 (Na:Ca 1:4)
- Anorthite – CaAl2Si2O8
- Alkali feldspars (potassium feldspars)
- Tectosilicates, feldspathoid family
- Nosean – Na8Al6Si6O24(SO4)
- Cancrinite – Na6Ca2(CO3,Al6Si6O24) • 2H2O
- Leucite – KAlSi2O6
- Nepheline – (Na,K)AlSiO4
- Sodalite – Na8(AlSiO4)6Cl2
- Hauyne – (Na,Ca)4–8Al6Si6(O,S)24(SO4,Cl)1–2
- Lazurite – (Na,Ca)8(AlSiO4)6(SO4,S,Cl)2
- Tectosilicates, scapolite group
- Marialite – Na4(AlSi3O8)3(Cl2,CO3,SO4)
- Meionite – Ca4(Al2Si2O8)3(Cl2CO3,SO4)
- Tectosilicates, zeolite family
- Natrolite – Na2Al2Si3O10·2H2O
- Erionite – (Na2,K2,Ca)2Al4Si14O36·15H2O
- Chabazite – CaAl2Si4O12·6H2O
- Heulandite – CaAl2Si7O18·6H2O
- Stilbite – NaCa2Al5Si13O36·17H2O
- Scolecite – CaAl2Si3O10·3H2O
- Mordenite – (Ca,Na2,K2)Al2Si10O24·7H2O
- Analcime – NaAlSi2O6·H2O
References
- Deer, W.A.; Howie, R.A., & Zussman, J. (1992). An introduction to the rock forming minerals (2nd edition ed.). London: Longman ISBN:0-582-30094-0
- Hurlbut, Cornelius S.; Klein, Cornelis ||1985). Manual of Mineralogy, Wiley, (20th edition ed.). ISBN:0-471-80580-7
- Deer, W.A.; Howie, R.A.; Wise, W.S.; Zussman, J. (2004). Rock-forming minerals. Volume 4B. Framework silicates: silica minerals. Feldspathoids and the zeolites (2nd ed.). London: Geological Society of London. p. 982 pp.







