
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sirius Huang | -- | 1236 | 2022-10-25 01:36:32 |
Video Upload Options
A super-spreader is a host—an organism infected with a disease—that disproportionally infects more secondary contacts than other hosts who are also infected with the same disease. A sick human can be a super-spreader; they would be more likely to infect others than most people with the disease. Super-spreaders are thus of high concern in epidemiology (the study of the spread of diseases). Some cases of super-spreading conform to the 20/80 rule, where approximately 20% of infected individuals are responsible for 80% of transmissions, although super-spreading can still be said to occur when super-spreaders account for a higher or lower percentage of transmissions. In epidemics with super-spreading, the majority of individuals infect relatively few secondary contacts. Super-spreading events are shaped by multiple factors including a decline in herd immunity, nosocomial infections, virulence, viral load, misdiagnosis, airflow dynamics, immune suppression, and co-infection with another pathogen.
1. Defining a Super-Spreading Event
Although loose definitions of super-spreading exist, some effort has been made at defining what qualifies as a super-spreading event (SSE) more explicit. Lloyd-Smith et al. (2005) define a protocol to identify a super-spreading event as follows:[1]
- estimate the effective reproductive number, R, for the disease and population in question;
- construct a Poisson distribution with mean R, representing the expected range of Z due to stochasticity without individual variation;
- define an SSE as any infected person who infects more than Z(n) others, where Z(n) is the nth percentile of the Poisson(R) distribution.
This protocol defines a 99th-percentile SSE as a case which causes more infections than would occur in 99% of infectious histories in a homogeneous population.[1]
During the 2003 SARS outbreak in Beijing, China, epidemiologists defined a super-spreader as an individual with transmission of SARS to at least eight contacts.[2]
Super-spreaders may or may not show any symptoms of the disease.[3][4]
2. Factors in Transmission
Super-spreaders have been identified who excrete a higher than normal number of pathogens during the time they are infectious. This causes their contacts to be exposed to higher viral/bacterial loads than would be seen in the contacts of non-superspreaders with the same duration of exposure.[5]
2.1. Basic Reproductive Number
The basic reproduction number R0 is the average number of secondary infections caused by a typical infective person in a totally susceptible population.[6] The basic reproductive number is found by multiplying the average number of contacts by the average probability that a susceptible individual will become infected, which is called the shedding potential.
2.2. Individual Reproductive Number
The individual reproductive number represents the number of secondary infections caused by a specific individual during the time that individual is infectious. Some individuals have significantly higher than average individual reproductive numbers and are known as super-spreaders. Through contact tracing, epidemiologists have identified super-spreaders in measles, tuberculosis, rubella, monkeypox, smallpox, Ebola hemorrhagic fever and SARS.[1][7]
2.3. Co-Infections with Other Pathogens
Men with HIV who were co-infected with at least one other sexually transmitted disease, such as gonorrhea, hepatitis C, and herpes simplex 2 virus, were found to have an eight-fold higher HIV shedding rate than men without co-infection. This shedding rate was calculated in men with similar HIV viral loads. Once treatment for the co-infection had been completed, the HIV shedding rate returned to levels comparable to men without co-infection.[8][9]
2.4. Lack of Herd Immunity
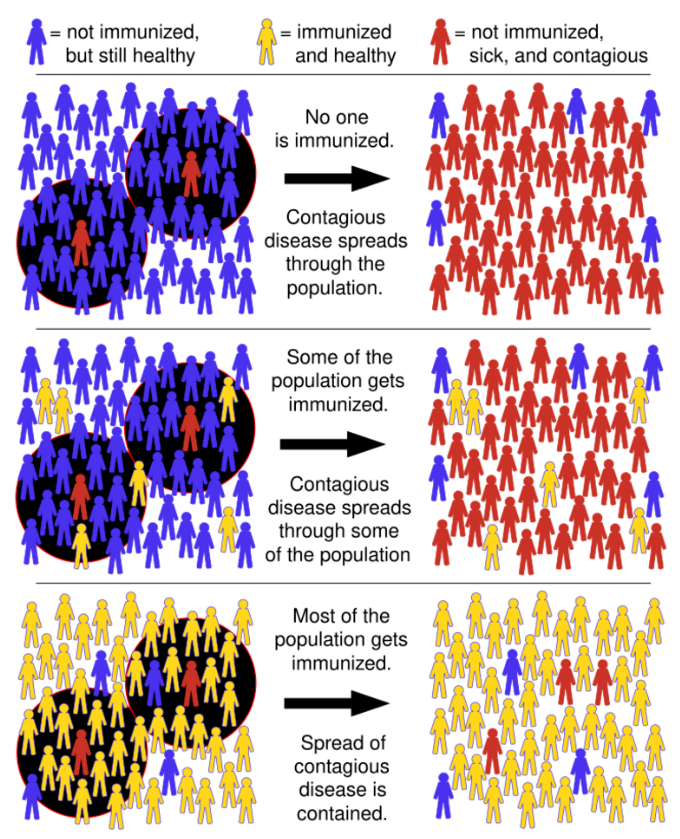
Herd immunity, or herd effect, refers to the indirect protection that immunized community members provide to non-immunized members in preventing the spread of contagious disease. The greater the number of immunized individuals, the less likely an outbreak can occur because there are fewer susceptible contacts. In epidemiology, herd immunity is known as a dependent happening because it influences transmission over time. As a pathogen that confers immunity to the survivors moves through a susceptible population, the number of susceptible contacts declines. Even if susceptible individuals remain, their contacts are likely to be immunized, preventing any further spread of the infection.[5][10] The proportion of immune individuals in a population above which a disease may no longer persist is the herd immunity threshold. Its value varies with the virulence of the disease, the efficacy of the vaccine, and the contact parameter for the population.[11] That is not to say that an outbreak can't occur, but it will be limited. [10][12][13]
3. Super-Spreaders During Outbreaks
3.1. SARS Outbreak 2003
The first cases of SARS occurred in mid-November 2002 in the Guangdong Province of China. This was followed by an outbreak in Hong Kong in February, 2003. A Guangdong Province doctor, Liu Jianlun, who had treated SARS cases there, had contracted the virus and was symptomatic. Despite his symptoms, he traveled to Hong Kong to attend a family wedding. He stayed on the ninth floor of the Metropole Hotel in Kowloon, infecting 16 other hotel guests also staying on that floor (pictured above). The guests then traveled to Canada, Singapore, Taiwan, and Vietnam, spreading SARS to those locations and transmitting what became a global epidemic.[14]
In another case during this same outbreak, a 54-year-old male was admitted to a hospital with coronary heart disease, chronic renal failure and type two diabetes. He had been in contact with a patient known to have SARS. Shortly after his admission he developed fever, cough, myalgia and sore throat. The admitting physician suspected SARS. The patient was transferred to another hospital for treatment of his coronary artery disease. While there, his SARS symptoms became more pronounced. Later, it was discovered he had transmitted SARS to 33 other patients in just two days. He was transferred back to the original hospital where he died of SARS.
The SARS pandemic was eventually contained, but not before it caused 8,273 cases and 775 deaths. Within two weeks of the original outbreak in Guangdong Province, SARS had spread to 37 countries.[15]
3.2. Measles Outbreak 1989
Measles is a highly contagious, air-borne virus that reappears even among vaccinated populations. In one Finnish town in 1989, an explosive school-based outbreak resulted in 51 cases, several of whom had been previously vaccinated. One child alone infected 22 others. It was noted during this outbreak that when vaccinated siblings shared a bedroom with an infected sibling, seven out of nine became infected as well.[16]
3.3. Typhoid Fever

Typhoid fever is a human-specific disease caused by the bacterium Salmonella typhi. It is highly contagious and becoming resistant to antibiotics.[17] S. typhi is susceptible to creating asymptomatic carriers. The most famous carriers are Mary Mallon, known as Typhoid Mary, from New York City, and Mr. N. the Milker, from Folkstone, England.[18] Both were active around the same time. Mallon infected 51 people from 1902 to 1909. Mr. N. infected more than 200 people over 14 years from 1901 to 1915. At the request of health officials, Mr. N. gave up working in food service. Mallon was at first also compliant, choosing other work - but eventually she returned to cooking and caused further outbreaks. She was involuntarily quarantined at Brothers Island in New York, where she stayed until she died in November 1938, aged 69.[19]
It has been found that Salmonella typhi persists in infected mice macrophages that have cycled from an inflammatory state to a non-inflammatory state. The bacteria remain and reproduce without causing further symptoms in the mice, and that this explains why[clarify] carriers are asymptomatic.[20][21][22][23]
References
- Lloyd-Smith, JO; Schreiber, SJ; Kopp, PE; Getz, WM (2005). "Superspreading and the effect of individual variation on disease emergence". Nature 438 (7066): 355–359. doi:10.1038/nature04153. PMID 16292310. Bibcode: 2005Natur.438..355L. https://dx.doi.org/10.1038%2Fnature04153
- Z. Shen, F. Ning, W. Zhou, L.He, C. Lin, D. Chin, Z. Zhus, A. Schuchat. Superspreading events, Beijing, 2003. Emerging Infectious Diseases. Vol. 10, No. 2. Feb. 2004.
- Stein, Richard A. (August 2011). "Super-spreaders in infectious diseases". International Journal of Infectious Diseases 15 (8): e510–e513. doi:10.1016/j.ijid.2010.06.020. PMID 21737332. "The minority of individuals who infect disproportionately more susceptible contacts, as compared to most individuals who infect few or no others, became known as super-spreaders, and their existence is deeply rooted in history: between 1900 and 1907, Typhoid Mary infected 51 individuals, three of whom died, even though she only had an asymptomatic infection.". https://dx.doi.org/10.1016%2Fj.ijid.2010.06.020
- Cory, David C. Wiley, Amy C. (2013). Encyclopedia of School Health. Los Angeles, Calif.: SAGE. ISBN 9781412996006. "Historically, one of the most famous examples of super-spreading was that of Mary Mallon, better known as Typhoid Mary, who infected many contacts, several of whom died, through food she prepared and consequently contaminated, even thought she did not show symptoms."
- Kenneth J. Rothman, Sander Greenland, and Timothy L. Lash. Modern Epidemiology, 3rd Edition. 2008. page 561. Lippincott, Williams & Wilkins. Philadelphia.
- Galvani, Alison P.; Robert M. (17 November 2005). "Epidemiology: Dimensions of super-spreading". Nature 438 (7066): 239–295. doi:10.1038/438293a. PMID 16292292. Bibcode: 2005Natur.438..293G. https://dx.doi.org/10.1038%2F438293a
- De Serres, GExpression error: Unrecognized word "etal". (2013). "Largest measles epidemic in North America in a decade–Quebec, Canada, 2011: contribution of susceptibility, serendipity, and superspreading events". J Infect Dis 207 (6): 990–998. doi:10.1093/infdis/jis923. PMID 23264672. https://dx.doi.org/10.1093%2Finfdis%2Fjis923
- Cohen, M.S. HoffmanExpression error: Unrecognized word "etal". (1997). "Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group". Lancet 349 (9069): 1868–73. doi:10.1016/s0140-6736(97)02190-9. PMID 9217758. https://dx.doi.org/10.1016%2Fs0140-6736%2897%2902190-9
- Winter, AJ; Taylor, S. Workman J.; White, D.; Ross, JD.; Swan, AV; Pillay, D. (1999). "Asymptomatic urethritis and detection of HIV-1 RNA in seminal plasma". Sex Transm Infect 75 (261): 261–3. doi:10.1136/sti.75.4.261. PMID 10615314. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1758225
- Fine, P (1993). "Herd immunity: history, theory, practice". Epidemiol Rev 15 (2): 265–302. doi:10.1093/oxfordjournals.epirev.a036121. PMID 8174658. https://dx.doi.org/10.1093%2Foxfordjournals.epirev.a036121
- "Chapter 4: Cost-Effective Strategies for the Excess Burden of Disease in Developing CountriesSection: Vaccine-preventable Diseases". Priorities in Health: Disease Control Priorities Companion Volume. World Bank Publications. 2006. ISBN 0-8213-6260-7. https://www.ncbi.nlm.nih.gov/books/NBK10258/#A151.
- Yeung LF1, Lurie P, Dayan G, Eduardo E, Britz PH, Redd SB, Papania MJ, Seward JF.A limited measles outbreak in a highly vaccinated US boarding school. Pediatrics. 2005 Dec;116(6):1287-91.
- Paul Fine. Ken Eames. David L. Heymann. Herd Immunity:A Rough Guide. Clinical Infectious Dis. (2011) 52 (7). 911-916.
- "How SARS changed the world in less than six months". Archived from the original on April 5, 2012. https://web.archive.org/web/20120405125444/http://www.scielosp.org/pdf/bwho/v81n8/v81n8a14.pdf. Retrieved February 4, 2016.
- Shen, Zhuang; Fang Ning (February 2004). "Superspreading SARS Events, Beijing 2003". Emerging Infectious Diseases 10 (2): 256–260. doi:10.3201/eid1002.030732. PMID 15030693. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3322930
- Paunio, Mikko; Peltola, Heikki; Davidkin, Irja; Virtanen, Martti; Heinonen, Olli P.; Valle, Martti (1998). "Explosive School-based Measles Outbreak Intense Exposure May Have Resulted in High Risk, Even among Revaccinees". Am J Epidemiol 148 (11): 1103–10. doi:10.1093/oxfordjournals.aje.a009588. PMID 9850133. https://dx.doi.org/10.1093%2Foxfordjournals.aje.a009588
- Roger Highfield (28 November 2006). "Typhoid is with us to stay". The Telegraph. https://www.telegraph.co.uk/health/healthnews/3345526/Typhoid-is-with-us-to-stay.html. Retrieved 2015-03-03.
- Mortimer, PP. Mr. N. the Milker and Dr. Koch's concept of the healthy carrier. The Lancet. 1999; 353:1354-1356.
- Marr, J. Typhoid Mary. The Lancet. 1999; 353:1714
- TM, Ng, DM Monack. Revisiting Caspase-II Function in Host Defense. Cell Host & Microbe. 17 July 2013. 14(1). pp. 9-14.
- JS Cox, L.Lam, L. Mukundan, A. Chawla, DM Monack. Salmonella Require the Fatty Acid Regulator PPAR. Cell Host & Microbe. 14 August 2013. 14(2) pp. 171-182.
- Geoffrey Mohan (August 14, 2013). "Typhoid Mary case may be cracked, a century later". Los Angeles Times. http://www.latimes.com/science/sciencenow/la-sci-sn-typhoid-mary-20130814-story.html. Retrieved 2015-03-03.
- Donald G. McNeil Jr. (August 26, 2013). "Bacteria study offers clues to Typhoid Mary mystery". The New York Times. https://www.nytimes.com/2013/08/27/health/bacteria-study-offers-clues-to-typhoid-mary-mystery.html?_r=0. Retrieved 2015-03-03.




