
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wim Ceelen | -- | 1820 | 2022-10-25 08:34:08 | | | |
| 2 | Sirius Huang | Meta information modification | 1820 | 2022-10-26 02:42:08 | | |
Video Upload Options
Local-regional administration of cytotoxic drugs is an important adjunct to systemic chemotherapy amongst cancer patients. It allows for targeted delivery of agents at high concentration to target sites while minimizing systemic side effects. Despite the pharmacokinetic advantages of the local–regional approach, drug transport into tumor nodules remains limited due to the biophysical properties of these tissues. Electromotive enhanced drug administration (EMDA) represents a potential solution to overcome challenges in local drug transport by applying electric currents. Through electrokinetic phenomena of electromigration, electroosmosis and electroporation, electric currents have been shown to improve drug penetration and distribution in a wide variety of clinical applications.
1. Introduction
2. Fundamental Principles in EMDA
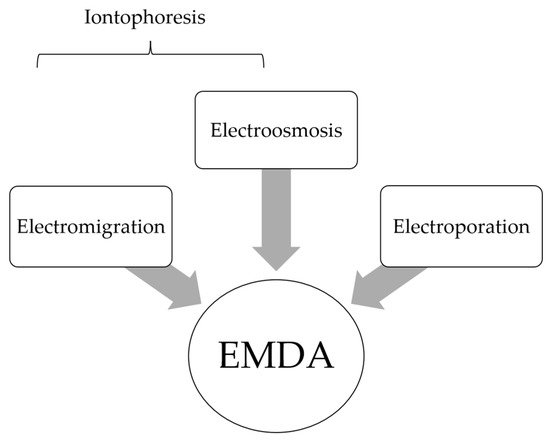
2.1. EMDA Devices
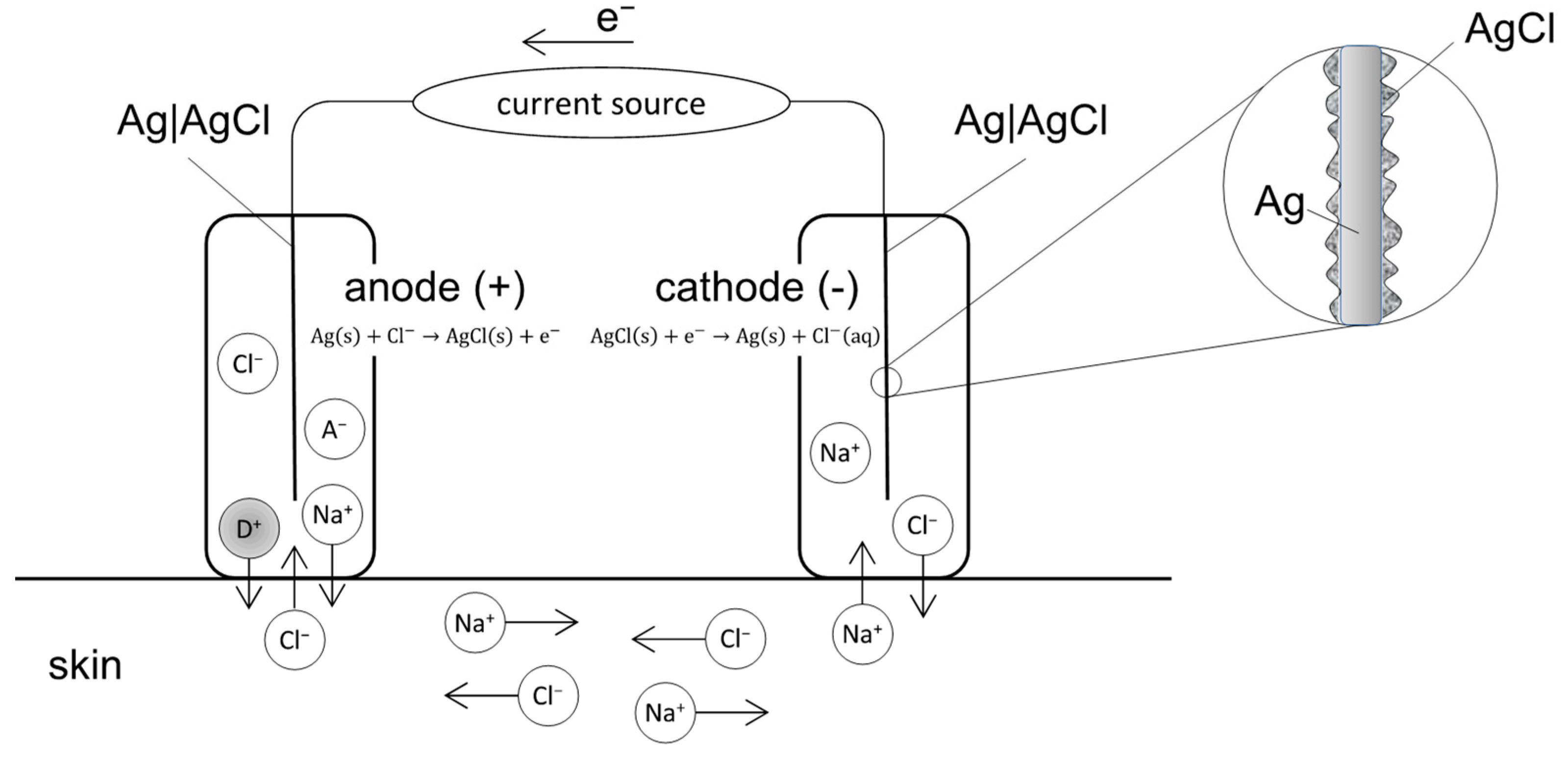
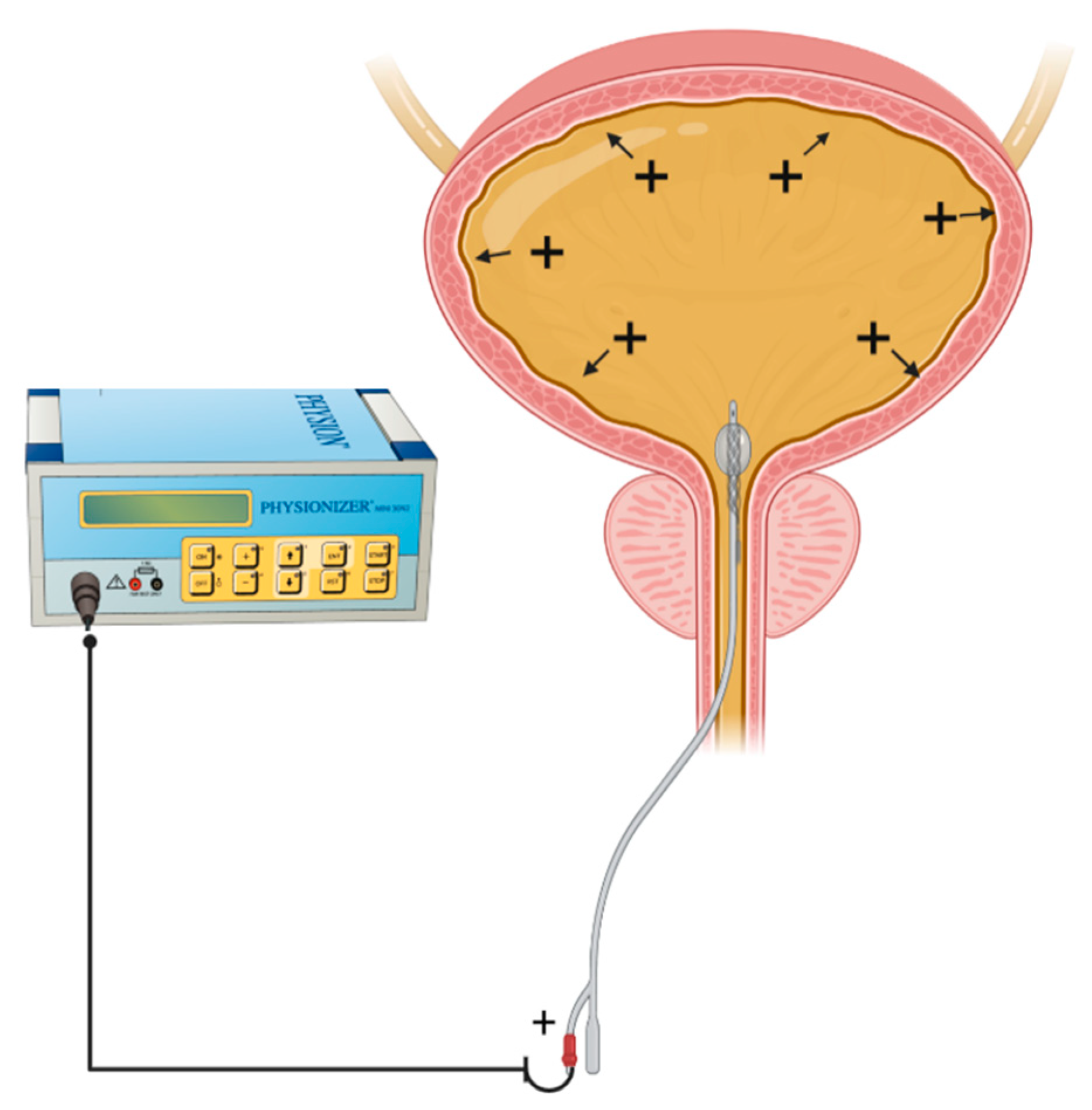
2.2. Relationship between Current Intensity, Ion Valency and EMDA
2.3. Relationship between Drug Physicochemical Properties and EMDA
2.4. Membrane or Barrier Properties and EMDA
References
- Petrak, K. Essential properties of drug-targeting delivery systems. Drug Discov. Today 2005, 10, 1667–1673.
- Collins, J.M. Pharmacologic rationale for regional drug delivery. J. Clin. Oncol. 1984, 2, 498–504.
- Joice, G.A.; Bivalacqua, T.J.; Kates, M. Optimizing pharmacokinetics of intravesical chemotherapy for bladder cancer. Nat. Rev. Urol. 2019, 16, 599–612.
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114.
- Ceelen, W.P.; Flessner, M.F. Intraperitoneal therapy for peritoneal tumors: Biophysics and clinical evidence. Nat. Rev. Clin. Oncol. 2010, 7, 108–115.
- Mishina, T.; Watanabe, H.; Kobayashi, T.; Maegawa, M.; Nakao, M.; Nakagawa, S. Absorption of anticancer drugs through bladder epithelium. Urology 1986, 27, 148–157.
- Lasič, E.; Višnjar, T.; Kreft, M.E. Properties of the Urothelium that Establish the Blood-Urine Barrier and Their Implications for Drug Delivery. Rev. Physiol. Biochem. Pharmacol. 2015, 168, 1–29.
- Dedrick, R.L. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin. Oncol. 1985, 12 (Suppl. S4), 1–6.
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813.
- Hashemi, S.; Sahai, A.; Malde, S. Applications of electromotive drug administration in urology. Urol. Ann. 2020, 12, 301–308.
- Charoo, N.A.; Rahman, Z.; Repka, M.A.; Murthy, S.N. Electroporation: An avenue for transdermal drug delivery. Curr. Drug Deliv. 2010, 7, 125–136.
- Turnell, W.J. Therapeutic action of constant current. Proc. R. Soc. Med. 1921, 14, 41–52.
- Leduc, S. Introduction of medicinal substances into the depth of tissues by electric current. Ann. D’Eleetrobiol. 1900, 3, 545–560.
- Leduc, S. Electric Ions and Their Use in Medicine; Rebman Ltd.: London, UK, 1908.
- Banga, A.K.; Chien, Y.W. Iontophoretic delivery of drugs: Fundamentals, developments and biomedical application. J. Control. Release 1988, 7, 1–24.
- Phipps, J.B.; Padmanabhan, R.B.; Lattin, G.A. Iontophoretic delivery of model inorganic and drug ions. J. Pharm. Sci. 1989, 48, 365.
- Hughes, L.; Maurice, D.M. A fresh look at iontophoresis. Arch. Ophthalmol. 1984, 102, 1825–1829.
- Shelley, W.B.; Horwath, P.; Weidrnan, F.; Pillsbury, D.M. Experimental milaria in man. Production of sweat retention anhidrosis and vesicles by means of iontophoresis. J. Investig. Dermatol. 1948, 11, 275–291.
- Harris, R. Treatment of post-therapeutic neuralgia. Lancet 1957, 269, 378–379.
- Stephen, R.; Miotti, D.; Bettaglio, R.; Rossi, C.; Bonezzi, C. Electromotive administration of a new morphine formulation: Morphine citrate. Artif. Organs 1994, 18, 461–465.
- Gangarosa, L.P.; Park, N.H.; Wiggins, C.A.; Hill, J.M. Increased penetration of nonelectrolytes into mouse skin during iontophoretic water transport (iontohydrokinesis). J. Pharmacol. Exp. Ther. 1980, 212, 377–381.
- Prausnitz, M.R.; Bose, V.G.; Langer, R.; Weaver, J.C. Electroporation of mammalian skin: A mechanism to enhance transdermal drug delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 10504–10508.
- Guy, R.H.; Kalia, Y.N.; Delgado-Charro, M.B.; Merino, V.; López, A.; Marro, D. Iontophoresis: Electrorepulsion and electroosmosis. J. Control. Release 2000, 64, 129–132.
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658.
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 1st ed.; Wiley: New York, NY, USA, 1980.
- Fick, A.V. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 63, 30–39.
- Li, S.K.; Hao, J.; Liddell, M.R. Electrotransport across membranes in biological media: Electrokinetic theories and applications in drug delivery. In Transport in Biological Media; Becker, S., Kuznetsoy, A., Eds.; Elsevier: Philadelphia, PA, USA, 2013; pp. 417–454.
- Banga, A.K.; Bose, S.; Ghosh, T.K. Iontophoresis and electroporation: Comparisons and contrasts. Int. J. Pharm. 1999, 179, 1–19.
- Available online: https://www.federalregister.gov/documents/2016/07/26/2016-17609/physical-medicine-devices-reclassification-of-iontophoresis-device-intended-for-any-other-purposes (accessed on 7 October 2022).
- Stillwell, G.K. Electrical stimulation and iontophoresis. In Handbook of Physical Medicine and Rehabilitation, 2nd ed.; Krussen, F.H., Ed.; W.B. Saunders Company: St. Louis, MO, USA, 1971; Chapter 14.
- Harding, J.W.; Felix, D. Quantification of angiotensin iontophoresis. J. Neurosci. Methods 1987, 19, 209–215.
- Sloan, J.B.; Soltani, K. Iontophoresis in dermatology. A review. J. Am. Acad. Dermatol. 1986, 15 Pt 1, 671–684.
- Sun, Y.; Siddiqui, O.; Liu, J.C.; Chien, Y.W. Transdermal modulated delivery of polypeptides: Effect of DC pulse wave-form on enhancement. In Proceedings of the 13th International Symposium on Controlled Release of Bioactive Materials, Controlled Release Society, Lincolnshire, IL, USA, 23–25 July 1986.
- Gangarosa, L.P.; Park, N.H.; Fong, B.C.; Scott, D.F.; Hill, J.M. Conductivity of drugs used for iontophoresis. J. Pharm. Sci. 1978, 67, 1439–1443.
- Narasimha Murthy, S.; Wiskirchen, D.E.; Bowers, C.P. Iontophoretic drug delivery across human nail. J. Pharm. Sci. 2007, 96, 305–311.
- Hadgraft, J. Skin, the final frontier. Int. J. Pharm. 2001, 224, 1–18.
- Chopra, P.; Hao, J.; Li, S.K. Iontophoretic transport of charged macromolecules across human sclera. Int. J. Pharm. 2010, 388, 107–113.
- Li, S.K.; Liddell, M.R.; Wen, H. Effective electrophoretic mobilities and charges of anti-VEGF proteins determined by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2011, 55, 603–607.
- Di Stasi, S.M.; Giannantoni, A.; Massoud, R.; Dolci, S.; Navarra, P.; Vespasiani, G.; Stephen, R.L. Electromotive versus passive diffusion of mitomycin C into human bladder wall: Concentration-depth profiles studies. Cancer Res. 1999, 59, 4912–4918.
- Lugnani, F.; Mazza, G.; Cerulli, N.; Rossi, C.; Stephen, R. Iontophoresis of drugs in the bladder wall: Equipment and preliminary studies. Artif. Organs 1993, 17, 8–17.




