Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moshawih, S.; Dhaliwal, J.S.; Loy, M.J.; Hossain, M.S.; Kotra, V.; Kifli, N.; Goh, H.P.; Ming, L.C. Chemistry of Chalcone and Its Derivatives. Encyclopedia. Available online: https://encyclopedia.pub/entry/31096 (accessed on 03 March 2026).
Moshawih S, Dhaliwal JS, Loy MJ, Hossain MS, Kotra V, Kifli N, et al. Chemistry of Chalcone and Its Derivatives. Encyclopedia. Available at: https://encyclopedia.pub/entry/31096. Accessed March 03, 2026.
Moshawih, Said, Jagjit Singh Dhaliwal, Mei Jun Loy, Md. Sanower Hossain, Vijay Kotra, Nurolaini Kifli, Hui Poh Goh, Long Chiau Ming. "Chemistry of Chalcone and Its Derivatives" Encyclopedia, https://encyclopedia.pub/entry/31096 (accessed March 03, 2026).
Moshawih, S., Dhaliwal, J.S., Loy, M.J., Hossain, M.S., Kotra, V., Kifli, N., Goh, H.P., & Ming, L.C. (2022, October 25). Chemistry of Chalcone and Its Derivatives. In Encyclopedia. https://encyclopedia.pub/entry/31096
Moshawih, Said, et al. "Chemistry of Chalcone and Its Derivatives." Encyclopedia. Web. 25 October, 2022.
Copy Citation
Chalcone is a collective group of ketones (flavonoids) that has a three-carbon α,β-unsaturated carbonyl group attached to two aromatic rings. Other chemical names of chalcone include benzyl acetophenone or benzylideneacetophenone. They are produced by certain plant species such as Angelica, Glycyrrhiza, Humulus, and Scutellaria as precursors to the biosynthesis of flavonoids and isoflavonoids and intermediates to the synthesis of heterocyclic compounds with biologically interesting properties such as pyrazolines, isoxazoles, cyanopyridines and pyrimidines.
cancer
chalcone
heterogeneous acid catalyst
1. Introduction
Chalcone and its derivatives have been long used in various traditional medicine systems, including homeopathic and Chinese medicine. They are traditionally prepared by the reaction of benzaldehydes and active methylene ketones under homogeneous conditions using the Claisen-Schmidt condensation and a more recent invention known as the aldol condensation [1]. However, recent discoveries of methods for producing chalcones provide different advantages depending on the type of catalyst, solvent, base, and reaction conditions [2].
1.1 Claisen Schmidt Condensation
The Claisen Schmidt condensation reaction involves an aldehyde with the carbonyl group without hydrogen atoms in the α-position, and a ketone, using a heterogeneous acid catalyst to produce the desired α,β-unsaturated ketone. This is one of the methods of synthesizing chalcone in the laboratory (Figure 1) due to the equimolar quantities of acetophenone and benzaldehyde. Claisen Schmidt condensation uses an aqueous-alcoholic alkali with a (concentration of 10 to 60%) to catalyze the reaction between acetophenone and benzaldehyde by dehydration [3]. The reaction can take place either for 12–15 h at a temperature of 50 °C or for one week at room temperature (20–25 °C).
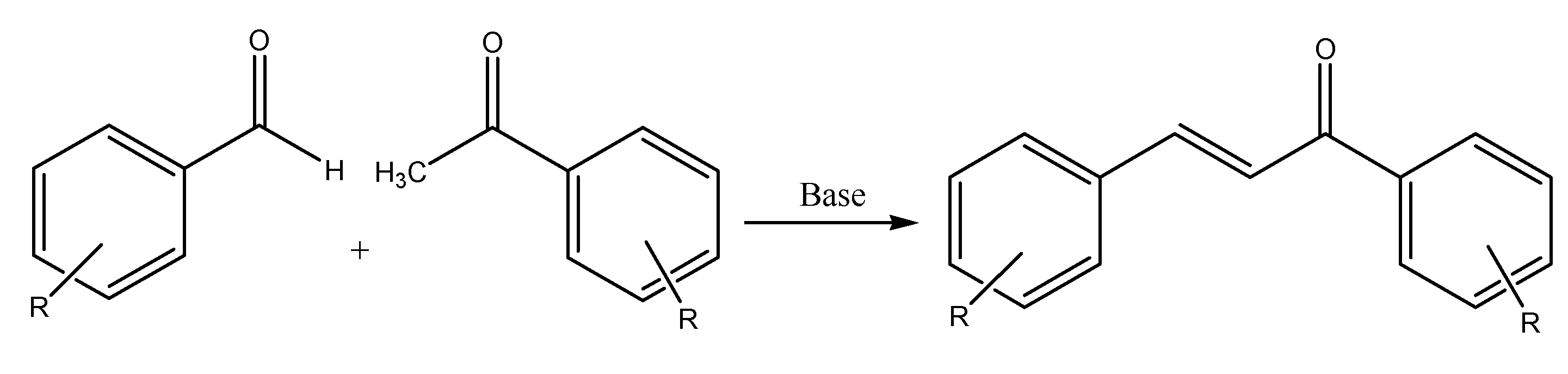
Figure 1. Claisen-Schmidt condensation reaction.
The heterogeneous acid catalyst is useful in producing chalcones because of the increased purity of end-products, decreased amounts of undesired products, reduced reaction time, and cost-effective procedure. Ionic liquids (ILs) are prepared using this condensation reaction but use a multi-sulfonic acid group ion liquid as the catalyst. ILs have gained interest due to their high catalytic activity, small catalyst usage, easy filtration, recyclable catalyst, and constant catalytic activity (Figure 1). However, the product yield will be decreased due to the reaction conditions, which promotes the Cannizzaro reaction. This redox reaction produces primary alcohol and carboxylic acid from two aldehyde molecules.
1.2. Aldol Condensation
Aldol condensation is another synthetic method commonly used after the Claisen-Schmidt condensation. The aldol condensation reaction (Figure 2), or the solid-state reaction, replaces aldehydes with benzylidene-diacetate and uses heat (200–350 °C) and a base such as potassium hydroxide as a catalyst for the reaction between the two compounds. It uses calcium, barium or strontium hydroxides or carbonates as catalysts in a liquid mixture containing water with a low boiling point that can perform distillation at a constant temperature. Other synthetic reactions can increase the reaction rate using microwave radiation without solvents; it also provides fluorescence emission profiles that can be used as biological markers.
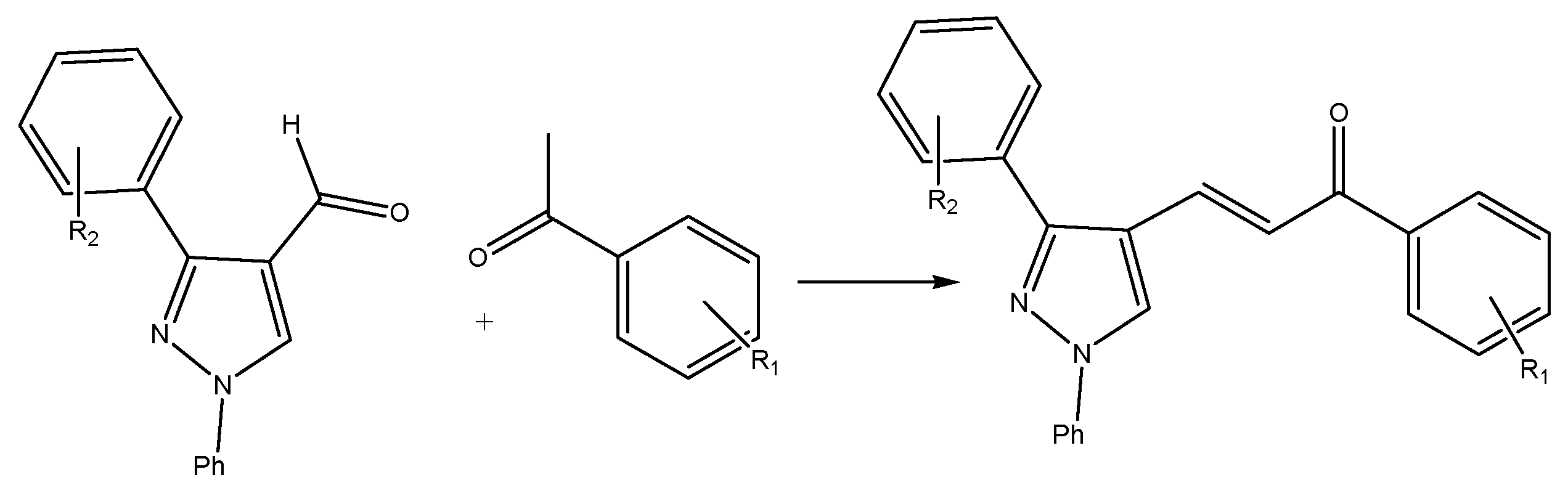
Figure 2. Heterocyclic ring-containing chalcone using a phase transfer catalyst.
Reacting a ketone with an aldehyde (with the carbonyl group-containing no hydrogen atoms in the α position with an acidic heterogeneous catalyst-activated carbon) will reduce the reaction time, cost, and impurities in the final products. Another technique involves the phase transfer method for synthesizing heterocyclic ring-containing chalcones, which incorporates a third ring into the chalcone’s skeleton chain. The starting constituents used are 1-phenyl-3-aryl-4-formyl pyrazole and acetophenone, with the catalyst being tetrabutylammonium bromide in the presence of an inorganic alkaline solution; the reaction is performed under microwave radiation (Figure 2).
1.3. Synthesisand Chemistry
Another method uses plants such as Sedum jinianum, S. plumbizincicola, S. alfredi and Potentilla griffithii with a high concentration of metals such as zinc, copper, cadmium, and magnesium, and at least one of the metal elements is used as a catalyst. They are first heated and treated with acid, then filtered and purified to be attached to a carrier, becoming a metal catalyst in synthesizing chalcones. Although this method promotes less pollution, it is unconventional to extract metal from these plants to be used as mere catalysts.
A different method uses a fluorine-containing biphasic catalyst as a result of reacting 4-dimethylamino pyridine with fluorinated alkyl iodide to react benzaldehyde with acetophenone at 50 to 100 °C for 1 to 3 h. The reacted compounds were cooled, filtered, distilled, and recrystallized with more than 99% purity. This invention requires an easy processing method, nature-friendly, low synthesis cost, and ease of re-obtaining the fluoric catalyst.
There are methods to synthesize hydroxyflavones that use soluble resin, specifically polyethylene glycol (PEG) that reacted with benzyloxy-2-hydroxy-acetophenone to be used in a reaction with benzaldehyde with a base as a catalyst. The advantages of this method are high accessibility to the reactant compounds and high purity percentage.
Another popular method is the one-pot synthesis. In this process, a direct reaction in one step is utilized to prepare inorganic components, while the organic component operates as a surface capping material or template. This method shortens the time to separate and purify the products and increases the product yield. Another technique of one-pot synthesis is slowly adding primary alcohol with chromium oxide (CrO3) to produce furochalcones, or the addition of cheap catalysts, which are copper salt, 2,2′-bipyridine, and 2,2,6,6-Tetramethylpiperidinyloxy (TEMPO) kept at a temperature of −10 to 100 °C for 10 to 96 h to produce a milder reaction.
A different synthesis method to produce the α,β-unsaturated carbonyl system that does not require condensation reaction offers more direct response with fewer vigor conditions, a cheaper cost of reactants, a more straightforward operating system, and a higher yield of products. The raw materials are the halogenated aromatic hydrocarbons with a ketone in the carbon skeleton and aromatic alkynes, using an alkali, a phosphine ligand, and palladium as catalysts, at a temperature of 60 to 150 °C [4][5].
1.4. Approach to Design of Chalcone Derivatives from the Natural Sources
Different approaches have been used to design chalcone derivatives, as outlined below:
1.4.1. Isoliquiritigenin
It can be extracted from the plant Radix Glycyrrhizae. Isoliquiritigenin is used in cosmetics due to its beneficial effects on the skin, including treatment of skin conditions such as acne, eczema, and irritation, as well as desirable effects, such as skin whitening, anti-aging, and eye drop preparations. There have been claims that isoliquiritigenin can activate the GABAA receptor, bind to the γ-subunit and act as a positive allosteric modulator that elicits similar effects as a benzodiazepine. It also prevents and treats cardio-cerebrovascular diseases [6] (Figure 3).
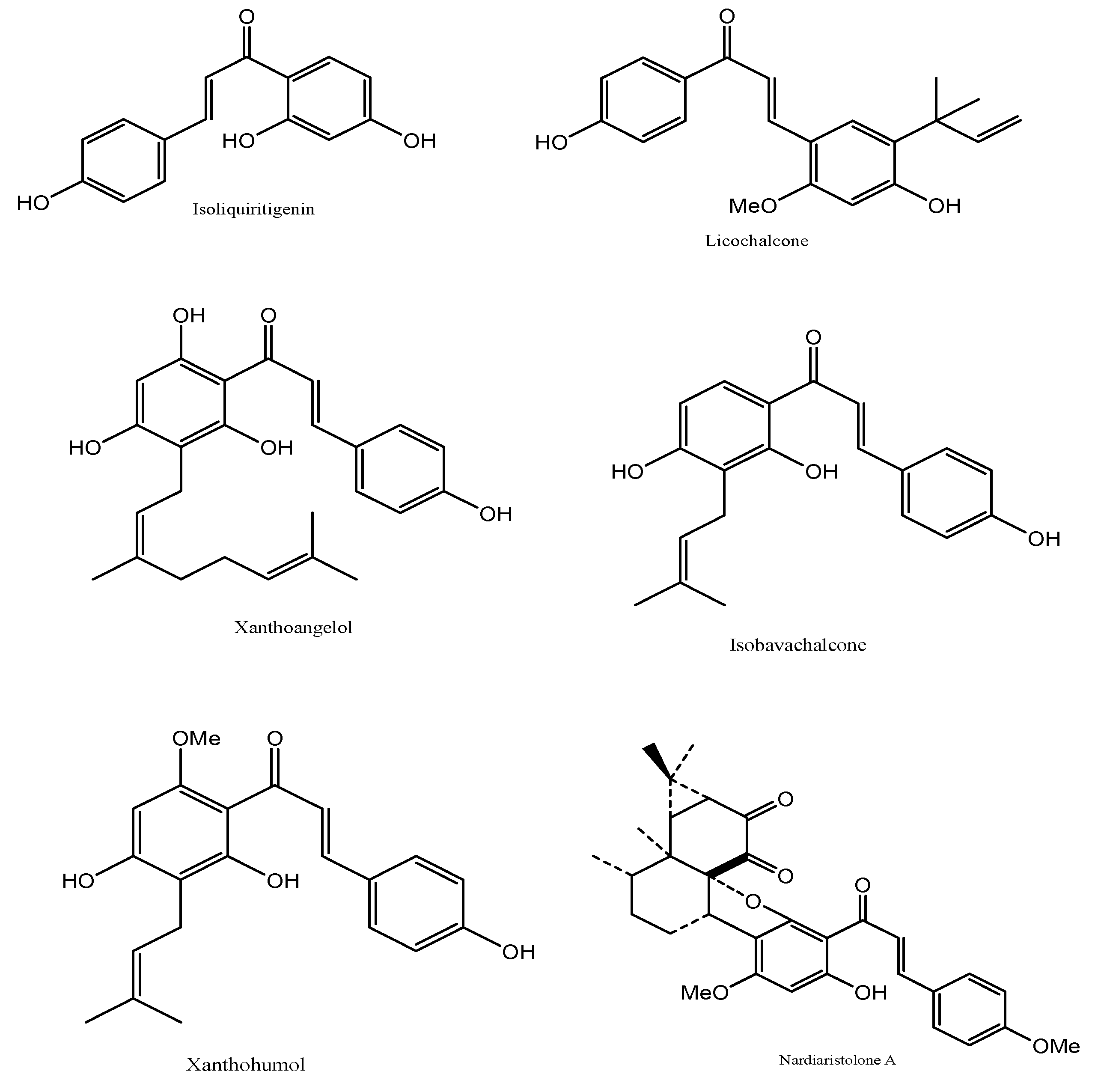
Figure 3. The derivatives of Chalcones from natural sources.
1.4.2. Licochalcone A
It is present in high concentrations in the plant Glycyrrhiza inflate [7]. Licochalcone A and isoliquiritigenin compounds are used in the cosmetic industry; they are both used in acne treatment and skin whitening. Licochalcone A is also used to prepare skin toner and hair cosmetics. The compound in essential oils produces bath salts to clean pores in the skin, control sebum production and retain skin moisture. Licochalcone A is also used for the treatment, improvement, and prevention of adenosine 5′ monophosphate-activated protein kinase (AMPK)-related diseases, an enzyme involved in metabolism, specifically for lipids [7]. Influenza virus infection-related diseases were treated for other conditions in which licochalcone A was used [8][9][10] (Figure 3).
1.4.3. Xanthoangelol
It is a major component of the plant Angelica keiskei. Xanthoangelol is an isoprenyl-chalcone compound with antioxidant properties that are used to prevent diseases involving lipid metabolism or inflammation [11] (Figure 3).
1.4.4. Isobavachalcone
It can be isolated from Psoralea corylifolia or Piper longum fruits. It belongs to the same chalcone family as xanthoangelol but has different properties [12]. It inhibits melanin formation causing skin whitening [13]. Isobavachalcone is also used to reduce nerve inflammation and inhibit cholesterol absorption [14]. Other uses of isobavachalcone prevent and control diseases [15] (Figure 3).
1.4.5. Xanthohumol
It is present in the Humulus lupulus and belongs to the prenylated chalcone family. Its antioxidant property is a ‘broad spectrum’ cancer chemo-preventive agent. Along with hydroxytyrosol, it is used as a nasal spray for viral infection, allergic reactions, or vasomotor rhinitis of the nasal mucosa [16]. Xanthohumol has demonstrated the inhibitory activity of the enzyme α-glucosidase, an enzyme responsible for carbohydrate metabolism. Therefore, it is used in metabolic diseases such as diabetes and other diseases such as AIDS, osteoporosis, and malignant tumors [17][18] (Figure 3).
1.4.6. Nardoaristolone A
It can be extracted from Nardostachys chinensis and classified as terpenoid chalcones [19]. Like any other chalcones, it has been used to treat different skin cancers. It has systemic effects such as increasing the red blood cell count and aids in small bowel movements. Nardoaristolone A has been used in medications for tuberculosis and endometrium cancers [18][20] (Figure 3).
2. Role of Chalcone Moiety in Synthesis of Derivatives
The chalcone moiety can be used to produce other chalcone derivatives such as cyanopyridines, pyrazolines, isoxazoles, and pyrimidines with different heterocyclic ring systems. These derivatives containing hydroxyl, ether, acid, or amino groups have diverse functionality and can be used to produce more complex chalcones. Aminochalcones, a chalcone moiety, can produce benzothiazole chalcones and other derivatives through alkylation, hydrolysis, and esterification or amide formation. Chalcone derivatives can be produced through the Phase Transfer Catalysis through alkylation, using oxygen or sulfur.
Chalcone derivatives can also be produced using the α,β-unsaturated system by substitution of the functional group at the two positions, and dihydrochalcones can be produced by reducing the double bond in the saturated system; these derivatives will be used in the synthesis of pyrazoles and flavonoids as well as other heterocyclic compounds.
A chalcone derivative which is 3-phenyl-1-(4-methyl) phenyl-2-bromo-propylene-1-one, can be produced by reacting ρ-toualdehyde with acetophenone using an inorganic base as a catalyst in an aldol condensation reaction, and the products react with a halogen in an addition reaction followed by an elimination reaction with an inorganic base. Another method also used a heterogeneous catalyst (hexagonal boron nitride h-BN) that is hydrogenated and has a Frustrated Lewis Pair (FLP)-type electronic structure that produces 100% yield.
Another method to obtain dihydrochalcones, specifically phloretin, used bacterial or plant chalcone isomerases and enolate reductase as a catalyst. An electrolytic hydrogenation method using hydrogen, which becomes active by electrolysis of water, is needed, followed by an addition reaction with a ketone without a catalyst to obtain neohesperidin dihydrochalcones. Dihydrochalcones and quinazolinyl derivatives can be produced by substituting carboxylic or nitro groups at the β carbon of the carbon skeleton of chalcones, respectively [21] (Figure 4).
Figure 4. Chemical reactions from Chalcones derivatives.
2.1. Semi Synthetic Derivatives of Chalcones
Chalcone isocordoin and its semisynthetic derivatives were tested for Anti-inflammatory and anti-hypersensitive effects in mice (Figure 5).
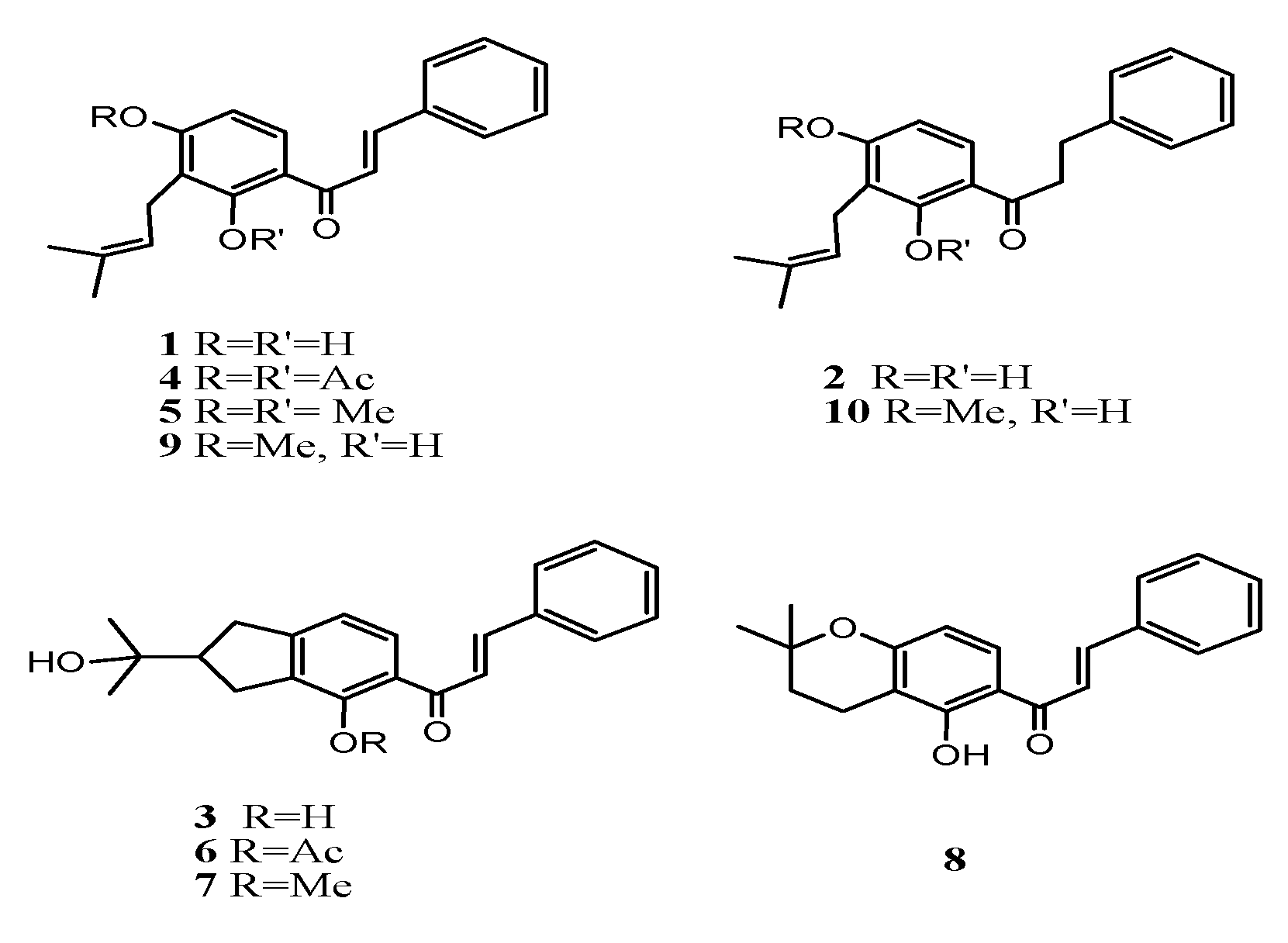
Figure 5. Semisynthetic derivatives of chalcones.
2.2. Characterization of Chalcones
The structure of the synthesized chalcones can be characterized by IR, NMR and mass spectroscopy.
2.3. UV Spectrum
The UV spectrum of chalcones consists of two essential absorption band: band I and relatively a minor band, band II. In chalcones, band I usually appears in 340–390 nm, although a minor inflection or peak often occurs at 300–320 nm. Band II appears in 220–270 nm.
2.4. IR Spectrum
In the IR spectra of chalcones asymmetric and symmetric stretching vibrations of the aromatic C–H bonds are seen at 3120–3080 cm−1 and 3060–3040 cm−1 ranges with two low intensity bands. C–H stretching band of the =C–H group is observed at 3030–3010 cm−1. The bands at 1610–1570 cm−1 are assigned to the vibrations of the aromatic ring. The inplane deformation of the =C–H bond is appeared as broad weak band at 1460–1430 cm−1. The carbonyl stretching vibrations for the enones (=C–C=O) can be found between 1650 and 1685 cm−1.
2.5. NMR Spectrum
The 1H-NMR spectrum of double bonds of chalcones were seen at 5.4 and 6.1 ppm. The aromatic regions were observed at 6.9–8.1 ppm.
In 13C-NMR spectrum of chalcones, the carbonyl carbon usually appears between δ 186.6 and 196.8. The α- and β- carbon atoms with respect to the carbonyl group give characteristic signals between δ 116.1–128.1 and δ 136.9–145.4 respectively.
2.6. Mass Spectrum
Basic fragmentation pathways of chalcones are obtained by loss of the phenyl group from the A or B ring, and loss of CO.
References
- Sun, Y.-F.; Cui, Y.-P. The synthesis, characterization and properties of coumarin-based chromophores containing a chalcone moiety. Dyes Pigments 2008, 78, 65–76.
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210.
- Dong, F.; Jian, C.; Zhenghao, F.; Kai, G.; Zuliang, L. Synthesis of chalcones via Claisen–Schmidt condensation reaction catalyzed by acyclic acidic ionic liquids. Catal. Commun. 2008, 9, 1924–1927.
- Wadey, B.L. Plasticizers. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 441–456.
- Larsen, R.D. 3.08-1,3-Selenazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; pp. 493–510.
- Zhan, C.; Yang, J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol. Res. 2006, 53, 303–309.
- Furusawa, J.; Funakoshi-Tago, M.; Mashino, T.; Tago, K.; Inoue, H.; Sonoda, Y.; Kasahara, T. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway. Int. Immunopharmacol. 2009, 9, 499–507.
- Chen, M.; Christensen, S.B.; Blom, J.; Lemmich, E.; Nadelmann, L.; Fich, K.; Theander, T.G.; Kharazmi, A. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother. 1993, 37, 2550–2556.
- Chen, M.; Christensen, S.B.; Theander, T.G.; Kharazmi, A. Antileishmanial activity of licochalcone A in mice infected with Leishmania major and in hamsters infected with Leishmania donovani. Antimicrob. Agents Chemother. 1994, 38, 1339–1344.
- Zhai, L.; Blom, J.; Chen, M.; Christensen, S.B.; Kharazmi, A. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 1995, 39, 2742–2748.
- Ogawa, H.; Okada, Y.; Kamisako, T.; Baba, K. Beneficial effect of xanthoangelol, a chalcone compound from Angelica keiskei, on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 238–243.
- Li, Y.; Qin, X.; Li, P.; Zhang, H.; Lin, T.; Miao, Z.; Ma, S. Isobavachalcone isolated from Psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3β/β-catenin pathway in colorectal cancer cells. Drug Des. Dev. Ther. 2019, 13, 1449–1460.
- Martinson, K.; Stueven, N.; Monte, A.; Huang, C. A Novel Stilbene-Like Compound That Reduces Melanin through Inhibiting Melanocyte Differentiation and Proliferation without Inhibiting Tyrosinase. Cosmetics 2018, 5, 45.
- Lee, H.; Li, H.; Kweon, M.; Choi, Y.; Kim, M.J.; Ryu, J.H. Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation. Int. J. Mol. Sci. 2018, 19, 1693.
- Liu, X.; Li, W.; Hu, B.; Wang, M.; Wang, J.; Guan, L. Identification of isobavachalcone as a potential drug for rice blast disease caused by the fungus Magnaporthe grisea. J. Biomol. Struct. Dyn. 2019, 37, 3399–3409.
- Liu, M.; Yin, H.; Liu, G.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Beer Hops, Acts as an α-Glucosidase Inhibitor in Vitro. J. Agric. Food Chem. 2014, 62, 5548–5554.
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J Enzym. Inhib Med Chem 2017, 32, 1216–1228.
- Rahman, M. Chalcone: A Valuable Insight into the Recent Advances and Potential Pharmacological Activities. Chem. Sci. 2011, 2011.
- Liu, M.L.; Duan, Y.H.; Hou, Y.L.; Li, C.; Gao, H.; Dai, Y.; Yao, X.S. Nardoaristolones A and B, two terpenoids with unusual skeletons from Nardostachys chinensis Batal. Org. Lett. 2013, 15, 1000–1003.
- Matos, M.J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L. Potential pharmacological uses of chalcones: A patent review (from June 2011–2014). Expert Opin. Ther. Pat. 2015, 25, 351–366.
- El-Meligie, S.; Taher, A.T.; Kamal, A.M.; Youssef, A. Design, synthesis and cytotoxic activity of certain novel chalcone analogous compounds. Eur. J. Med. Chem. 2017, 126, 52–60.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.4K
Revisions:
2 times
(View History)
Update Date:
25 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





