
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anca Lucia Pop | + 2084 word(s) | 2084 | 2020-11-18 08:00:37 | | | |
| 2 | Vivi Li | -13 word(s) | 2071 | 2020-11-20 04:39:28 | | |
Video Upload Options
Clozapine (CLZ) use is precarious due to its neurological, cardiovascular, and hematological side effects; however, it is the gold standard in therapy-resistant schizophrenia (TRS) in adults and is underused. Objective: to examine the most recent CLZ data on (a) side effects concerning (b) recent pharmacological mechanisms, (c) therapy benefits, and (d) the particularities of the COVID-19 pandemic. Data sources: a search was performed in two databases (PubMed andWeb of Science) using the specific keywords “clozapine” and “schizophrenia”, “side effects”, “agranulocytosis”, “TRS”, or “bipolar affective disorder (BAF)” for the last ten years. Study eligibility criteria: clinical trials on adults with acute symptoms of schizophrenia or related disorders. Results: We selected 37 studies, randomized controlled trials (RCTs), and clinical case series (CCS), centered on six main topics in the search area: (a) CLZ in schizophrenia, (b) CLZ in bipolar disorder, (c) side effects during the clozapine therapy, (d) CLZ in pregnancy, (e) CLZ in early-onset schizophrenia, and (f) CLZ therapy and COVID-19 infection. Limitations: we considered RCTs and CCS from two databases, limited to the search topics. Conclusions and implications of key findings: (a) clozapine doses should be personalized for each patient based on pharmacogenetics testing when available; the genetic vulnerability postulates predictors of adverse reactions’ severity; patients with a lower genetic risk could have less frequent hematological monitoring; (b) a CLZ-associated risk of pulmonary embolism imposes prophylactic measures for venous thromboembolism; (c) convulsive episodes are not an indication for stopping treatment; the plasma concentration of clozapine is a better side effect predictor then the dosage; (d) COVID-19 infection may enhance clozapine toxicity, generating an increased risk of pneumonia. Therapy must be continued with the proper monitoring of the white blood count, and the clozapine dose decreased by half until three days after the fever breaks; psychiatrists and healthcare providers must act together.
1. Introduction
Controversies are frequent in psychiatric therapy, and consensus is hard to find [1]; however, there is widespread agreement regarding the exclusive role of clozapine in treating severe refractory schizophrenia [2]. Schizophrenia is a major mental illness with a lifelong impact on patients and their caregivers. The precise etiopathology of schizophrenia is unspecified and most probably multifactorial [3], implying neurodevelopmental (hypoxia, maternal infection, and stress), genetic (family history), and environmental factors (social and cannabis use) [4].
Second-generation antipsychotics (SGAP) are the first line of treatment for acute psychotic episodes and are currently prescribed for long-term management of schizophrenia, affective disorders, and some dementia-related symptoms; SGAP are considered atypical when comparing their clinical profile with first-generation antipsychotics and respond better to the negative symptoms of schizophrenia. Extrapyramidal side effects are less common than with typical ones [5][6]. Risperidone, ziprasidone, paliperidone, and aripiprazole are potent D2 dopamine receptor antagonists; quetiapine and clozapine are weak D2 antagonists and antagonists for 5-HT 2A, as well as agonists for 5-HT 1A receptors.
The most potent molecules that bind to the alpha-adrenergic receptors are clozapine, iloperidone, and risperidone. Clozapine, olanzapine, and quetiapine also bind to muscarinic cholinergic receptors [7][8]. The less common incidence of extrapyramidal side effects has made atypical antipsychotics very popular among psychiatrists [9]. However, they still carry a risk of side effects that must be monitored, including metabolic disorders (type 2 diabetes, weight gain, dyslipidemia) and cardiovascular disorders like the prolongation of the QT interval on Electrocardiogram [10], as well as neurological and hematological (agranulocytosis) complications.
The life expectancy of patients diagnosed with schizophrenia and affective bipolar disorder is between 11 and 20 years shorter, as patients are vulnerable and in continuous need of medical and social care to prolong their life expectancy [11]. One-third of patients respond to “typical” antipsychotics (e.g., chlorpromazine and haloperidol) [12]; the remaining two-thirds need a second strategy. Clozapine is established as the gold-standard treatment for treatment-resistant schizophrenia (TRS): 32% of short-term and almost 40% of long-term therapy TRS patients respond to clozapine [13][14][15]; the absolute reduction in overall positive and negative symptom scale (PANSS) scores is clinically significant.
Considering the terrible burden that schizophrenia places on patients and their families, the discovery of clozapine, the first atypical antipsychotic, was a substantial pharmacological and clinical milestone. The significant therapeutic effect of clozapine compared with other classes of drugs and the reduced incidence of extrapyramidal side effects, which increased the stigma on psychiatric patients, and brought hope in the most severe cases of schizophrenia.
At the moment, clozapine is the most effective antipsychotic drug for therapy-resistant schizophrenia (TRS) [16][17][18], listed on the WHO Model List of Essential Medicines [19] and superior to other drugs in the class due to: (1) a lower risk of suicide, (2) lower risk for tardive dyskinesia, (3) the improvement of cognition and improved quality of life, and (4) the decreased risk of relapse. Clozapine is specific to psychiatric therapy due to its effectiveness but also due to a pharmacodynamic conundrum. Even though it had a rising prescription trend in 2005–2014 with a relative increase of 7.8%, up to 197.2%, clozapine (CLZ) remains underused due to its specific adverse reactions: hematological (agranulocytosis), cardiovascular, and neurological side effects. Despite the benefits, clozapine remains underutilized in up to two-thirds of TRS cases in most countries, as revealed in an Australian study in 2017 [20]. Less is known about clozapine use in Romania.
2. Pharmacogenetic Severity Markers as Potential Biomarkers in Clozapine Therapy
The current pharmacological doses are estimates for a standard patient; however, pharmacologists have highlighted two different genetic types: slow metabolizers and fast metabolizers. The type of metabolizer is generated by personal (genetic and metabolic) and environmental factors. Thus, when estimating the drug clearance (concentration-to-dose (C/D) ratio), a low C/D ratio indicates a fast metabolizer, while a high C/D ratio indicates a slow metabolizer. Clozapine C/D ratios range from 0.6 to 1.2 ng/mL per mg/day in the USA [21], with a double value in East Asians. Clozapine C/D ratios can be enhanced in interaction with inhibitors (including fluvoxamine and oral contraceptives) or an inflammatory state.
Two hundred and four studies were published on clozapine and pharmacogenetics topics in the PubMed® database starting in 1994, with 57 reviews, three systematic reviews or meta-analyses, and 12 clinical trials or RCTs (Figure 1).
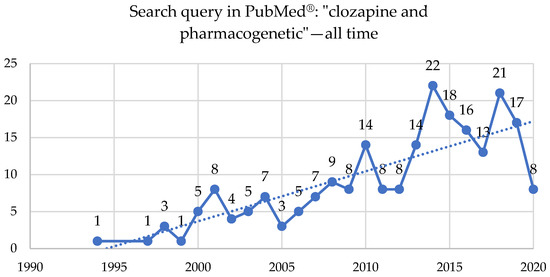
Figure 1. A search query in PubMed®: “clozapine” and “pharmacogenetic”—all time, up to 15 August 2020.
The genetic vulnerability is correlated with metabolic side effects with a higher prevalence of adverse metabolic reactions in clozapine-treated patients and postulates predictors of severity—pharmacogenetics markers such as receptors CYP2C19, LEP, LEPR, and HTR2C [22]. Clozapine’s metabolism, elimination, and response were evaluated by genotyping specific enzymes, such as CYP1A2 and CYO2C19, and measuring HTR2C serotonin receptors leptin receptor, taking into account concomitant medication if present. The results showed that metabolic syndrome was correlated with higher levels of clozapine and CYP2C19*2 and leptin receptor G alleles. Individuals who metabolize CLZ more slowly are at higher risk for this type of disorder.
Antipsychotic-related weight gain is linked with several variants from nine genes (Adrenoceptor (ADR) Alpha-2A, Beta-3 (ADRB3), Brain-Derived Neurotrophic Factor (BDNF), Dopamine Receptor D2 (DRD2), Guanine Nucleotide-Binding Protein (GNB3), 5-HT (Serotonin) Receptor 2C (HTR2C), Insulin-induced gene 2 (INSIG2), Melanocortin-4 Receptor (MC4R), and Synaptosomal-associated protein, 25kDa (SNAP25) [23].
Gressier et al. (2015) found three genetic variants linked to clozapine response in serotonin genes, rs6313 and rs6314 within the HTR2A gene, rs1062613 within the HT3A gene, suggesting a possible serotonergic modulation of clozapine clinical response but no link with weight gain [24].
DiGeorge syndrome (22q11.2 deletion syndrome) with a 1/4000 live births prevalence [25], is associated with high frequencies of attention-deficit/hyperactivity disorder (ADHD), psychotic disorders, and eating, mood, and anxiety disorders. The rates of schizophrenia spectrum disorders in adults with 22q11.2DS over 25 are up to 41% [26][27]. Much of the published literature on 22q11.2DS is on treatment-resistant schizophrenia; therapy with clozapine is the gold standard, but the side effects of CLZ seem to be more frequent.
In clinical practice, pharmacogenetic testing is widely available, and psychiatrists should adjust and personalize clozapine doses for each patient, decreasing the risks of metabolic side effects to a minimum.
3. Clozapine and COVID-19 Infection
A “clozapine” and “COVID 19” search retrieved 22 results in a PubMed® database search and 26 results in Google Scholar®, from which two reviews considered topics of toxicity and side effects, management of clozapine monitoring and therapy during the SARS-COV-2 quarantine, COVID-19 patients, and clozapine and agranulocytosis—including the International Prospective Register of Systematic Reviews (PROSPERO)—CRD42020178819 trial investigating the influence of COVID-19 on mental health patients [28].
Patients with COVID-19 infection frequently experience lymphopenia but seldomly neutropenia [29]. COVID-19 infection may cause clozapine intoxication by dramatically increasing serum clozapine levels, affecting the cytokine release, and downregulating the metabolism of clozapine in the CYP450 system/CYP 1A2 [30], as revealed by recent studies and case reports [31][32]. SARS-CoV2 in CLZ therapy has increased pneumonia risk and clozapine toxicity risk, even the need for intervention in a critical care unit, and the disruption of CLZ therapy by COVID-19 induced lymphopenia [33][34][35][36].
Nevertheless, the therapy must be continued with the following recommendations [36][37]:
(1) The neutrophil count may be reduced to every three months, with a dispensation of up to a 90 day supply on receipt) for people fulfilling the following criteria: • continuous clozapine treatment for more than one year or • have never had a neutrophils count below 2000/µL or • safe and practical access to ANC testing.
(2) Patients on clozapine and with no COVID-19 symptoms (cough, fever, chills, sore throat, myalgia, fatigue, other flu-like symptoms) need an immediate in-person or distance medical evaluation, involving a complete blood count including neutrophils, according to the local protocols [38].
(3) If patients on clozapine become COVID-19 symptomatic, they may be required to decrease by half the dose of clozapine up to three days after the fever has passed, a point at which clozapine can be gradually increased to the pre-fever dose. Where available, clozapine levels could back up the clinical decision.
In a recent cohort study on 6309 participants, of whom 102 were positive for SARS-CoV-12, clozapine treatment was linked with an increased risk of COVID-19 infection, more than other antipsychotics, an association that needs further research to be confirmed [39].
Psychiatrists and healthcare providers involved in monitoring the absolute neutrophil count (ANC) and dispensing the prescription must be aware of the increased risks in clozapine-treated patients with COVID-19. They must communicate and strengthen the monitoring of side effects, including their duration, as the full impact of the COVID-19 pandemic is still unknown [40].
4. Conclusions
Clozapine has been known and used for an extended period, but in the past three or four decades, there has been a failure to generate effective novel psychopharmaceuticals. Due to the limited prospects for new, more effective antipsychotics in the short to medium term [41], there is a need to maximize access to clozapine therapy and investigate therapies that mitigate the side effects of CLZ in resistant cases.
The genetic vulnerability postulates predictors of adverse reactions’ severity, so clozapine doses should be personalized for each patient based on pharmacogenetic testing; patients with a lower genetic risk may have less frequent hematological monitoring.
The pulmonary embolism associated with clozapine has a mortality rate of 36.36%, so prophylactic measures for venous thromboembolism for six months after initiating therapy are mandatory. The convulsive episodes are not an indication for stopping treatment; side effect (s.e.) incidence increases with dose, so the plasma concentration of clozapine (1300 ng/mL) is a better s.e. predictor than the dosage.
Clozapine improves treatment-refractory early-onset schizophrenia by up to 69%, as assessed by the Brief Psychiatric Rating Scale (BPRS); more pharmacogenetic studies of Romanian schizophrenic patients are needed in relation with clozapine therapy in order to define more precise safety margins.
COVID-19 infection may enhance clozapine toxicity, generating an increased risk of pneumonia, so therapy must be continued with the proper monitoring of the white blood count and a decrease in the clozapine dose by half until three days after the end of the fever. Psychiatrists and healthcare providers must act together to choose the proper treatment and doses to achieve results in clozapine-treated patients with COVID-19.
Since in the past four decades research has failed to generate effective novel psychopharmaceuticals [42], there is an urgent need to enhance access to clozapine for people with TRS worldwide. Nowadays, progress in pharmacogenetic research, discoveries in the area of endocrinology, genetic testing, and other interdisciplinary approaches offer psychiatrists the chance to use this drug at its highest potential, in a personalized manner for every patient, minimizing the adverse side effects and perhaps decreasing the rate of clozapine resistance by correctly identifying the clinical situation and the neurobiology of the resistance.
References
- Blier, P. Do antidepressants really work? J. Psychiatry Neurosci. 2008, 33, 89–90.
- Joober, R.; Boksa, P. Clozapine: A distinct, poorly understood and under-used molecule. JPN 2010, 35, 147–149.
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645.
- PubChem. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 30 August 2020).
- Clissold, M.; Crowe, S.F. Comparing the effect of the subcategories of atypical antipsychotic medications on cognition in schizophrenia using a meta-analytic approach. J. Clin. Exp. Neuropsychol. 2019, 41, 26–42.
- Faay, M.D.M.; Czobor, P.; Sommer, I.E.C. Efficacy of typical and atypical antipsychotic medication on hostility in patients with psychosis-spectrum disorders: A review and meta-analysis. Neuropsychopharmacology 2018, 43, 2340–2349.
- Burry, L.; Mehta, S.; Perreault, M.M.; Luxenberg, J.S.; Siddiqi, N.; Hutton, B.; Fergusson, D.A.; Bell, C.; Rose, L. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 2018, 6, CD005594.
- Desai, N.; Patel, P.B.; Shah, S.; Patel, T.K.; Shah, S.N.; Vatsala, E. Prevalence and pattern of antipsychotic induced movement disorders in a tertiary care teaching hospital in India—A cross-sectional study. Int. J. Psychiatry Clin. Pract. 2018, 22, 101–108.
- Shulman, M.; Miller, A.; Misher, J.; Tentler, A. Managing cardiovascular disease risk in patients treated with antipsychotics: A multidisciplinary approach. J. Multidiscip. Healthc. 2014, 7, 489–501.
- Hasnain, M.; Vieweg, W.V.; Fredrickson, S.K.; Beatty-Brooks, M.; Fernandez, A.; Pandurangi, A.K. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim. Care Diabetes 2009, 3, 5–15.
- Laursen, T.M.; Wahlbeck, K.; Hällgren, J.; Westman, J.; Ösby, U.; Alinaghizadeh, H.; Gissler, M.; Nordentoft, M. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS ONE 2013, 8, e67133.
- Siskind, D.; Siskind, V.; Kisely, S. Clozapine response rates among people with treatment-resistant schizophrenia data from a systematic review and meta-analysis. Can. J. Psychiatry 2017, 62, 772–777.
- Taylor, D.M. Clozapine for treatment-resistant schizophrenia: Still the gold standard? CNS Drugs 2017, 31, 177–180.
- Siskind, D.; McCartney, L.; Goldschlager, R.; Kisely, S. Clozapine versus first and second generation antipsychotics in treatment refractory schizophrenia: A systematic review and meta-analysis. Br. J. Psychiatry 2016, 209, 385–392.
- Faden, J. Treatment resistant schizophrenia: A brief overview of treatment options. J. Clin. Psychiatry 2019, 80, 18ac12394.
- Yoshimura, B.; Yada, Y.; So, R.; Takaki, M.; Yamada, N. The critical treatment window of clozapine in treatment-resistant schizophrenia: Secondary analysis of an observational study. Psychiatry Res. 2017, 250, 65–70.
- Nucifora, F.C., Jr.; Woznica, E.; Lee, B.J.; Cascella, N.; Sawa, A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 2019, 131, 104257.
- Vanasse, A.; Blais, L.; Courteau, J.; Cohen, A.A.; Roberge, P.; Larouche, A.; Grignon, S.; Fleury, M.; Lesage, A.; Demers, M.; et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: A real-world observational study. Acta Psychiatr. Scand. 2016, 134, 374–384.
- Bachmann, C.J.; Aagaard, L.; Bernardo, M.; Brandt, L.; Cartabia, M.; Clavenna, A.; Hollingworth, S. International trends in clozapine use: A study in 17 countries. Acta Psychiatr. Scand. 2017, 136, 37–51.
- Forrester, T.; Siskind, D.J.; Winckel, K.; Wheeler, A.; Hollingworth, S. Increasing clozapine dispensing trends in Queensland, Australia 2004–2013. Pharmacopsychiatry 2015, 48, 164–169.
- De Leon, J. Personalizing dosing of risperidone, paliperidone and clozapine using therapeutic drug monitoring and pharmacogenetics. Neuropharmacology 2020, 168, 107656.
- Vasudev, K.; Choi, Y.H.; Norman, R.; Kim, R.B.; Schwarz, U.I. Genetic Determinants of Clozapine-Induced Metabolic Side Effects. Can. J. Psychiatry 2017, 62, 138–149.
- Kohlrausch, F.B. Pharmacogenetics in schizophrenia: A review of clozapine studies. Braz. J. Psychiatry 2013, 35, 305–317.
- Gressier, F.; Porcelli, S.; Calati, R.; Serretti, A. Pharmacogenetics of clozapine response and induced weight gain: A comprehensive review and meta-analysis. Eur. Neuropsychopharmacol. 2016, 26, 163–185.
- Chromosome 22q11.2 Deletion Syndrome—NORD (National Organization for Rare Disorders); NORD (National Organization for Rare Disorders): Danbury, CT, USA, 2017.
- Mosheva, M.; Korotkin, L.; Gur, R.E.; Weizman, A.; Gothelf, D. Effectiveness and side effects of psychopharmacotherapy in individuals with 22q11.2 deletion syndrome with comorbid psychiatric disorders: A systematic review. Eur. Child Adolesc. Psychiatry 2019, 29, 1035–1048.
- Kohlrausch, F.B.; Salatino-Oliveira, A.; Gama, C.S.; Lobato, M.I.; Belmonte-de-Abreu, P.; Hutz, M.H. G-protein gene 825C>T polymorphism is associated with response to clozapine in Brazilian schizophrenics. Pharmacogenomics 2008, 9, 1429–1436.
- Remington, G.; Powell, V. Clozapine and COVID-19. J. Psychiatry Neurosci. 2020, 45, E1.
- Levine, M.; Burns, M.J. Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, Antipsychotic Agents; Burns, M.J., Shannon, M.W., Borron, S.W., Eds.; Saunders: Yonkers, NY, USA, 2007; pp. 703–720.
- Smith, K.; Ostinelli, E.; Macdonald, O.; Cipriani, A. COVID-19 and Telepsychiatry: Development of evidence-based guidance for clinicians. JMIR Ment. Health 2020, 7, e21108.
- Gee, S.; Taylor, D. The effect of COVID-19 on absolute neutrophil counts in patients taking clozapine. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320940935.
- Leon de, J.; Ruan, C.-J.; Verdoux, H.; Wang, C. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen. Psychiatry 2020, 33, e100183.
- Leung, J.G.; Wittenberger, T.S.; Schak, K.M. Clozapine treated patients and COVID-19: Ensuring continued care through collaboration. Schizophr. Res. 2020.
- Cranshaw, T.; Harikumar, T. COVID-19 Infection May Cause Clozapine Intoxication: Case Report and Discussion. Schizophr. Bull. 2020, 46, 751.
- Kuo, C.-J.; Yang, S.-Y.; Liao, Y.-T.; Chen, W.J.; Lee, W.-C.; Shau, W.-Y.; Chang, Y.-T.; Tsai, S.-Y.; Chen, C.-C. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr. Bull. 2013, 39, 648–657.
- Gee, S.; Taylor, D. Clozapine and Blood Dyscrasias in Patients with Coronavirus (COVID-19). RCPsych 2020. Available online: https://www.ncl-mon.nhs.uk/wp-content/uploads/Guidelines/0_covid19_clozapine.pdf (accessed on 30 September 2020).
- Siskind, D.; Honer, W.G.; Clark, S.; Correll, C.U.; Hasan, A.; Howes, O.; Kane, J.M.; Kelly, D.L.; Laitman, R.; Lee, J.; et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J. Psychiatry Neurosci. JPN 2020, 45, 222–223.
- Honer, W.G.; Correll, C.U.; Hasan, A.; Kane, J.M.; Laitman, R.; Myles, N.; Freudenreich, O. Clozapine and COVID-19/respond. JPN 2020, 45, E1–E2.
- Boland, X.; Dratcu, L. Clozapine in the time of COVID-19. Clin. Psychopharmacol. Neurosci. 2020, 18, 450–453.
- Govind, R.; Fonseca de Freitas, D.; Pritchard, M.; Hayes, R.D.; MacCabe, J.H. Clozapine treatment and risk of COVID-19 infection: Retrospective cohort study. Br. J. Psychiatry J. Ment. Sci. 2020, 1–7.
- Ifteni, P.; Teodorescu, A. Switching bipolar disorder patients treated with clozapine to another antipsychotic medication: A mirror image study. Eur. Psychiatry 2017, 41, S118.
- Fibiger, H.C. Psychiatry, the Pharmaceutical Industry, and the Road to Better Therapeutics. Schizophr. Bull. 2012, 38, 649–650.




