
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Himanshu Gupta | -- | 3216 | 2022-10-20 10:33:43 | | | |
| 2 | Conner Chen | -17 word(s) | 3199 | 2022-10-26 02:54:53 | | | | |
| 3 | Conner Chen | + 2 word(s) | 3201 | 2022-10-27 02:27:42 | | |
Video Upload Options
Rapid diagnostic tests (RDTs), the unsung hero in malaria diagnosis, work to eliminate the prevalence of Plasmodium falciparum malaria through their efficient, cost-effective, and user-friendly qualities in detecting the antigen HRP2 (histidine-rich protein 2), among other proteins. However, the testing mechanism and management of malaria with RDTs presents a variety of limitations. Such as the parasitic factors that limit the performance of HRP2-based RDTs. By understanding the factors that affect the performance of HRP2-based RDTs in the field, researchers can work toward creating and implementing more effective and accurate HRP2-based diagnostic tools. Further research is required to understand the extent of these factors, as the rapidly changing interplay between parasite and host directly hinders the effectiveness of the tool.
1. Introduction
2. Parasitic Factors
2.1. HRP2 Persistence
2.2. Variability of P. falciparum HRP2 and Homology with HRP3
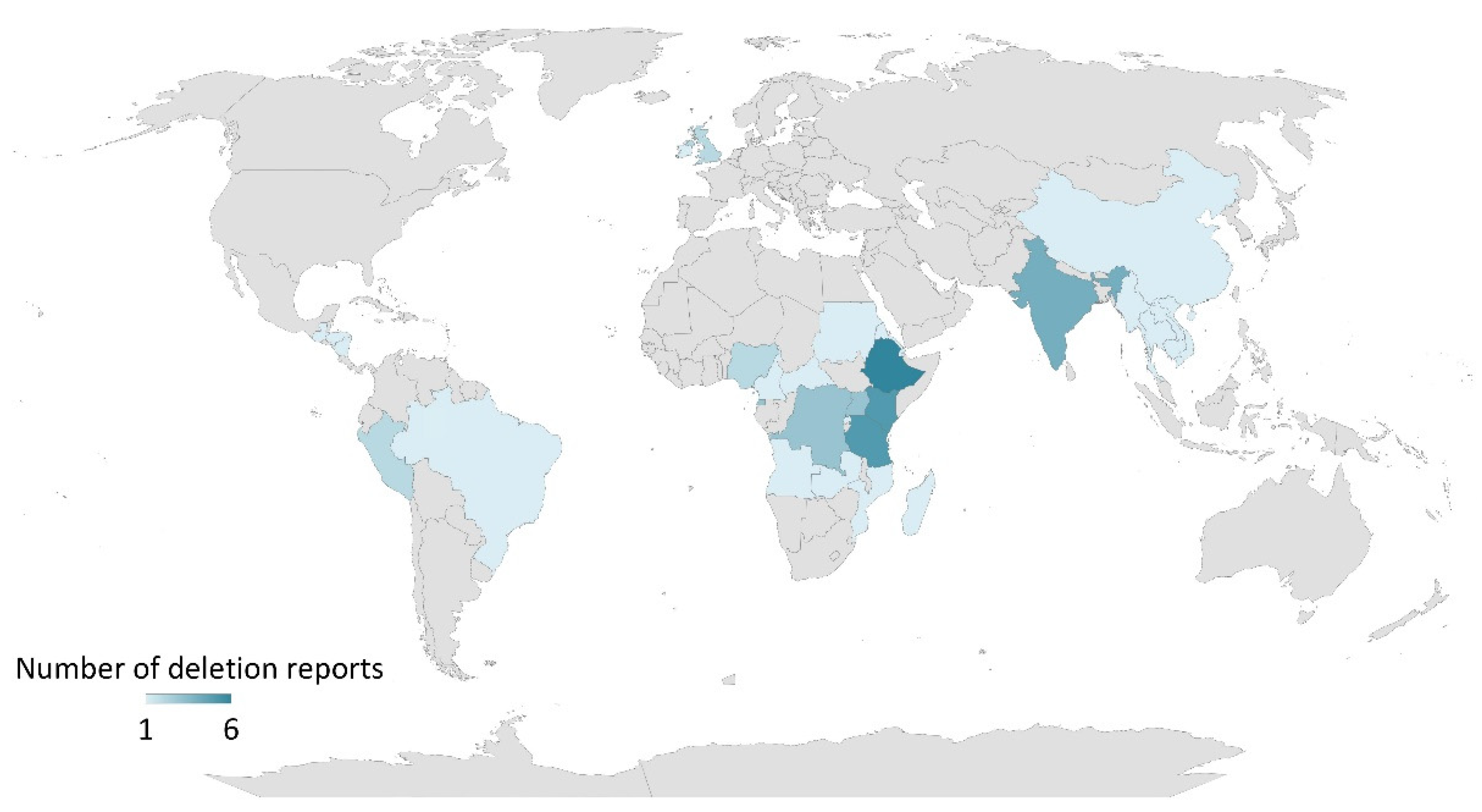
2.3. P. falciparum hrp2 and hrp3 Gene Deletions
References
- Feachem, R.G.A.; Chen, I.; Akbari, O.; Bertozzi-Villa, A.; Bhatt, S.; Binka, F.; Boni, M.F.; Buckee, C.; Dieleman, J.; Dondorp, A.; et al. Malaria eradication within a generation: Ambitious, achievable, and necessary. Lancet 2019, 394, 1056–1112.
- Cibulskis, R.E.; Alonso, P.; Aponte, J.; Aregawi, M.; Barrette, A.; Bergeron, L.; Fergus, C.A.; Knox, T.; Lynch, M.; Patouillard, E.; et al. Malaria: Global progress 2000–2015 and future challenges. Infect. Dis. Poverty 2016, 5, 61.
- World Health Organization. World Malaria Report 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 22 July 2022).
- World Health Organization. Parasitological Confirmation of Malaria Diagnosis. WHO Technical Consultation GENEVA, 6–8 October 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/44323/9789241599412_eng.pdf?sequence=1. (accessed on 1 May 2018).
- Wongsrichanalai, C.; Barcus, M.J.; Muth, S.; Sutamihardja, A.; Wernsdorfer, W.H. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT). Am. J. Trop. Med. Hyg. 2007, 77, 119–127.
- Rock, E.P.; Marsh, K.; Saul, A.J.; Wellems, T.E.; Taylor, D.W.; Maloy, W.L.; Howard, R.J. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 1987, 95 Pt 2, 209–227.
- Scherf, A.; Mattei, D. Cloning and characterization of chromosome breakpoints of Plasmodium falciparum: Breakage and new telomere formation occurs frequently and randomly in subtelomeric genes. Nucleic Acids Res. 1992, 20, 1491–1496.
- Wellems, T.E.; Howard, R.J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1986, 83, 6065–6069.
- Baker, J.; McCarthy, J.; Gatton, M.; Kyle, D.E.; Belizario, V.; Luchavez, J.; Bell, D.; Cheng, Q. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J. Infect. Dis. 2005, 192, 870–877.
- Cheng, Q.; Gatton, M.L.; Barnwell, J.; Chiodini, P.; McCarthy, J.; Bell, D.; Cunningham, J. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: A review and recommendations for accurate reporting. Malar. J. 2014, 13, 283.
- Gendrot, M.; Fawaz, R.; Dormoi, J.; Madamet, M.; Pradines, B. Genetic diversity and deletion of Plasmodium falciparum histidine-rich protein 2 and 3: A threat to diagnosis of P. falciparum malaria. Clin. Microbiol. Infect. 2019, 25, 580–585.
- Biswas, S.; Tomar, D.; Rao, D.N. Investigation of the kinetics of histidine-rich protein 2 and of the antibody responses to this antigen, in a group of malaria patients from India. Ann. Trop. Med. Parasitol. 2005, 99, 553–562.
- Desakorn, V.; Silamut, K.; Angus, B.; Sahassananda, D.; Chotivanich, K.; Suntharasamai, P.; Simpson, J.; White, N.J. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 479–483.
- Grandesso, F.; Nabasumba, C.; Nyehangane, D.; Page, A.L.; Bastard, M.; De Smet, M.; Boum, Y.; Etard, J.F. Performance and time to become negative after treatment of three malaria rapid diagnostic tests in low and high malaria transmission settings. Malar. J. 2016, 15, 496.
- Kiemde, F.; Bonko, M.D.A.; Tahita, M.C.; Lompo, P.; Rouamba, T.; Tinto, H.; van Hensbroek, M.B.; Mens, P.F.; Schallig, H. Accuracy of a Plasmodium falciparum specific histidine-rich protein 2 rapid diagnostic test in the context of the presence of non-malaria fevers, prior anti-malarial use and seasonal malaria transmission. Malar. J. 2017, 16, 294.
- World Health Organization. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 1–7 (2008–2016). Available online: https://apps.who.int/iris/bitstream/handle/10665/258597/9789241512916-eng.pdf?sequence=1 (accessed on 1 May 2018).
- World Health Organization. World Malaria Report 2019. Available online: https://www.who.int/publications/i/item/9789241565721 (accessed on 27 July 2022).
- Maltha, J.; Gillet, P.; Jacobs, J. Malaria rapid diagnostic tests in endemic settings. Clin. Microbiol. Infect. 2013, 19, 399–407.
- Zawawi, A.; Alghanmi, M.; Alsaady, I.; Gattan, H.; Zakai, H.; Couper, K. The impact of COVID-19 pandemic on malaria elimination. Parasite Epidemiol. Control. 2020, 11, e00187.
- Kusotera, T.; Nhengu, T.G. Coronavirus-19 and malaria: The great mimics. Afr. J. Prim. Health Care Fam. Med. 2020, 12, e1–e3.
- Mouatcho, J.C.; Goldring, J.P.D. Malaria rapid diagnostic tests: Challenges and prospects. J. Med. Microbiol. 2013, 62, 1491–1505.
- Dalrymple, U.; Arambepola, R.; Gething, P.W.; Cameron, E. How long do rapid diagnostic tests remain positive after anti-malarial treatment? Malar. J. 2018, 17, 228.
- Shiff, C.J.; Premji, Z.; Minjas, J.N. The rapid manual ParaSight-F test. A new diagnostic tool for Plasmodium falciparum infection. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 646–648.
- Abba, K.; Deeks, J.J.; Olliaro, P.; Naing, C.M.; Jackson, S.M.; Takwoingi, Y.; Donegan, S.; Garner, P. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst. Rev. 2011, 7, CD008122.
- Ochola, L.B.; Vounatsou, P.; Smith, T.; Mabaso, M.L.; Newton, C.R. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect. Dis. 2006, 6, 582–588.
- Desakorn, V.; Dondorp, A.M.; Silamut, K.; Pongtavornpinyo, W.; Sahassananda, D.; Chotivanich, K.; Pitisuttithum, P.; Smithyman, A.M.; Day, N.P.; White, N.J. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 517–524.
- Dondorp, A.M.; Desakorn, V.; Pongtavornpinyo, W.; Sahassananda, D.; Silamut, K.; Chotivanich, K.; Newton, P.N.; Pitisuttithum, P.; Smithyman, A.M.; White, N.J.; et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005, 2, e204.
- Marquart, L.; Butterworth, A.; McCarthy, J.S.; Gatton, M.L. Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance. Malar. J. 2012, 11, 74.
- Kyabayinze, D.J.; Tibenderana, J.K.; Odong, G.W.; Rwakimari, J.B.; Counihan, H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar. J. 2008, 7, 221.
- Plucinski, M.M.; Dimbu, P.R.; Fortes, F.; Abdulla, S.; Ahmed, S.; Gutman, J.; Kachur, S.P.; Badiane, A.; Ndiaye, D.; Talundzic, E.; et al. Posttreatment HRP2 Clearance in Patients with Uncomplicated Plasmodium falciparum Malaria. J. Infect. Dis. 2018, 217, 685–692.
- Das, S.; Jang, I.K.; Barney, B.; Peck, R.; Rek, J.C.; Arinaitwe, E.; Adrama, H.; Murphy, M.; Imwong, M.; Ling, C.L.; et al. Performance of a High-Sensitivity Rapid Diagnostic Test for Plasmodium falciparum Malaria in Asymptomatic Individuals from Uganda and Myanmar and Naive Human Challenge Infections. Am. J. Trop. Med. Hyg. 2017, 97, 1540–1550.
- Niyukuri, D.; Sinzinkayo, D.; Troth, E.; Oduma, C.; Barengayabo, M.; Ndereyimana, M.; Holzschuh, A.; Vera-Arias, C.A.; Gebre, Y.; Badu, K.; et al. High sensitivity of a novel rapid test for the diagnosis of clinical and subclinical Plasmodium falciparum infections in a high transmission setting in Burundi. medRxiv 2022.
- Das, S.; Peck, R.B.; Barney, R.; Jang, I.K.; Kahn, M.; Zhu, M.; Domingo, G.J. Performance of an ultra-sensitive Plasmodium falciparum HRP2-based rapid diagnostic test with recombinant HRP2, culture parasites, and archived whole blood samples. Malar. J. 2018, 17, 118.
- Bashir, I.M.; Otsyula, N.; Awinda, G.; Spring, M.; Schneider, P.; Waitumbi, J.N. Comparison of PfHRP-2/pLDH ELISA, qPCR and microscopy for the detection of plasmodium events and prediction of sick visits during a malaria vaccine study. PLoS ONE 2013, 8, e56828.
- Rogier, E.; Plucinski, M.; Lucchi, N.; Mace, K.; Chang, M.; Lemoine, J.F.; Candrinho, B.; Colborn, J.; Dimbu, R.; Fortes, F.; et al. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS ONE 2017, 12, e0172139.
- Wu, L.; van den Hoogen, L.L.; Slater, H.; Walker, P.G.; Ghani, A.C.; Drakeley, C.J.; Okell, L.C. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015, 528, S86–S93.
- Atroosh, W.M.; Al-Mekhlafi, H.M.; Al-Jasari, A.; Sady, H.; Al-Delaimy, A.K.; Nasr, N.A.; Dawaki, S.; Abdulsalam, A.M.; Ithoi, I.; Lau, Y.L.; et al. Genetic variation of pfhrp2 in Plasmodium falciparum isolates from Yemen and the performance of HRP2-based malaria rapid diagnostic test. Parasit. Vectors 2015, 8, 388.
- Mussa, A.; Talib, M.; Mohamed, Z.; Hajissa, K. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. BMC Res. Notes 2019, 12, 334.
- Kumar, N.; Singh, J.P.; Pande, V.; Mishra, N.; Srivastava, B.; Kapoor, R.; Valecha, N.; Anvikar, A.R. Genetic variation in histidine rich proteins among Indian Plasmodium falciparum population: Possible cause of variable sensitivity of malaria rapid diagnostic tests. Malar. J. 2012, 11, 298.
- Baker, J.; Ho, M.F.; Pelecanos, A.; Gatton, M.; Chen, N.; Abdullah, S.; Albertini, A.; Ariey, F.; Barnwell, J.; Bell, D.; et al. Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: Implications for the performance of malaria rapid diagnostic tests. Malar. J. 2010, 9, 129.
- Mariette, N.; Barnadas, C.; Bouchier, C.; Tichit, M.; Menard, D. Country-wide assessment of the genetic polymorphism in Plasmodium falciparum and Plasmodium vivax antigens detected with rapid diagnostic tests for malaria. Malar. J. 2008, 7, 219.
- Bharti, P.K.; Chandel, H.S.; Ahmad, A.; Krishna, S.; Udhayakumar, V.; Singh, N. Prevalence of pfhrp2 and/or pfhrp3 Gene Deletion in Plasmodium falciparum Population in Eight Highly Endemic States in India. PLoS ONE 2016, 11, e0157949.
- Kumar Bharti, P.; Singh Chandel, H.; Krishna, S.; Nema, S.; Ahmad, A.; Udhayakumar, V.; Singh, N. Sequence variation in Plasmodium falciparum Histidine Rich Proteins 2 and 3 in Indian isolates: Implications for Malaria Rapid Diagnostic Test Performance. Sci. Rep. 2017, 7, 1308.
- Kumari, M.S.; Sharma, S.; Bhardwaj, N.; Kumar, S.; Ahmed, M.Z.; Pande, V.; Anvikar, A.R. Pfhrp2/3 gene deletion and genetic variation in PfHRP2-based RDTs with P. falciparum positive samples from India and its implication on malaria control. Infect. Genet. Evol. 2022, 99, 105232.
- Li, P.; Xing, H.; Zhao, Z.; Yang, Z.; Cao, Y.; Li, W.; Yan, G.; Sattabongkot, J.; Cui, L.; Fan, Q. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 in the China-Myanmar border area. Acta Trop. 2015, 152, 26–31.
- Le, H.G.; Kang, J.M.; Lee, J.; Yoo, W.G.; Myint, M.K.; Lin, K.; Kim, T.S.; Na, B.K. Genetic variations in histidine-rich protein 2 and histidine-rich protein 3 of Myanmar Plasmodium falciparum isolates. Malar. J. 2020, 19, 388.
- Deme, A.B.; Park, D.J.; Bei, A.K.; Sarr, O.; Badiane, A.S.; Gueye Pel, H.; Ahouidi, A.; Ndir, O.; Mboup, S.; Wirth, D.F.; et al. Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malar. J. 2014, 13, 34.
- Wurtz, N.; Briolant, S.; Lemarie, D.; de Pommier Santi, V.; Pascual, A.; Roodt, T.; Benoit, N.; Hupin, C.; Pradines, B. Delayed diagnosis of Plasmodium falciparum in a soldier in Uganda: False-positive rapid diagnostic test associated with reduced repeats in pfhrp2. Med. Sante Trop. 2013, 23, 181–184.
- Wurtz, N.; Fall, B.; Bui, K.; Pascual, A.; Fall, M.; Camara, C.; Diatta, B.; Fall, K.B.; Mbaye, P.S.; Dieme, Y.; et al. Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: Impact on rapid malaria diagnostic tests. Malar. J. 2013, 12, 34.
- Trouvay, M.; Palazon, G.; Berger, F.; Volney, B.; Blanchet, D.; Faway, E.; Donato, D.; Legrand, E.; Carme, B.; Musset, L. High performance of histidine-rich protein 2 based rapid diagnostic tests in French Guiana are explained by the absence of pfhrp2 gene deletion in P. falciparum. PLoS ONE 2013, 8, e74269.
- Nderu, D.; Kimani, F.; Thiong’o, K.; Akinyi, M.; Karanja, E.; Meyer, C.G.; Velavan, T.P. PfHRP2-PfHRP3 diversity among Kenyan isolates and comparative evaluation of PfHRP2/pLDH malaria RDT with microscopy and nested PCR methodologies. Parasitol. Int. 2018, 67, 793–799.
- Nderu, D.; Kimani, F.; Thiong’o, K.; Karanja, E.; Akinyi, M.; Too, E.; Chege, W.; Nambati, E.; Meyer, C.G.; Velavan, T.P. Plasmodium falciparum histidine-rich protein (PfHRP2 and 3) diversity in Western and Coastal Kenya. Sci. Rep. 2019, 9, 1709.
- Ramutton, T.; Hendriksen, I.C.; Mwanga-Amumpaire, J.; Mtove, G.; Olaosebikan, R.; Tshefu, A.K.; Onyamboko, M.A.; Karema, C.; Maitland, K.; Gomes, E.; et al. Sequence variation does not confound the measurement of plasma PfHRP2 concentration in African children presenting with severe malaria. Malar. J. 2012, 11, 276.
- Funwei, R.; Nderu, D.; Nguetse, C.N.; Thomas, B.N.; Falade, C.O.; Velavan, T.P.; Ojurongbe, O. Molecular surveillance of pfhrp2 and pfhrp3 genes deletion in Plasmodium falciparum isolates and the implications for rapid diagnostic tests in Nigeria. Acta Trop. 2019, 196, 121–125.
- Alemayehu, G.S.; Messele, A.; Blackburn, K.; Lopez, K.; Lo, E.; Janies, D.; Golassa, L. Genetic variation of Plasmodium falciparum histidine-rich protein 2 and 3 in Assosa zone, Ethiopia: Its impact on the performance of malaria rapid diagnostic tests. Malar. J. 2021, 20, 394.
- Dorado, E.J.; Okoth, S.A.; Montenegro, L.M.; Diaz, G.; Barnwell, J.W.; Udhayakumar, V.; Murillo Solano, C. Genetic Characterisation of Plasmodium falciparum Isolates with Deletion of the pfhrp2 and/or pfhrp3 Genes in Colombia: The Amazon Region, a Challenge for Malaria Diagnosis and Control. PLoS ONE 2016, 11, e0163137.
- Gamboa, D.; Ho, M.F.; Bendezu, J.; Torres, K.; Chiodini, P.L.; Barnwell, J.W.; Incardona, S.; Perkins, M.; Bell, D.; McCarthy, J.; et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: Implications for malaria rapid diagnostic tests. PLoS ONE 2010, 5, e8091.
- Akinyi, S.; Hayden, T.; Gamboa, D.; Torres, K.; Bendezu, J.; Abdallah, J.F.; Griffing, S.M.; Quezada, W.M.; Arrospide, N.; De Oliveira, A.M.; et al. Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci. Rep. 2013, 3, 2797.
- Rachid Viana, G.M.; Akinyi Okoth, S.; Silva-Flannery, L.; Lima Barbosa, D.R.; Macedo de Oliveira, A.; Goldman, I.F.; Morton, L.C.; Huber, C.; Anez, A.; Dantas Machado, R.L.; et al. Histidine-rich protein 2 (pfhrp2) and pfhrp3 gene deletions in Plasmodium falciparum isolates from select sites in Brazil and Bolivia. PLoS ONE 2017, 12, e0171150.
- Mayor, A.; da Silva, C.; Rovira-Vallbona, E.; Roca-Feltrer, A.; Bonnington, C.; Wharton-Smith, A.; Greenhouse, B.; Bever, C.; Chidimatembue, A.; Guinovart, C.; et al. Prospective surveillance study to detect antimalarial drug resistance, gene deletions of diagnostic relevance and genetic diversity of Plasmodium falciparum in Mozambique: Protocol. BMJ Open 2022, 12, e063456.
- Taylor, S.M.; Parobek, C.M.; Aragam, N.; Ngasala, B.E.; Martensson, A.; Meshnick, S.R.; Juliano, J.J. Pooled deep sequencing of Plasmodium falciparum isolates: An efficient and scalable tool to quantify prevailing malaria drug-resistance genotypes. J. Infect. Dis. 2013, 208, 1998–2006.
- Sepulveda, N.; Phelan, J.; Diez-Benavente, E.; Campino, S.; Clark, T.G.; Hopkins, H.; Sutherland, C.; Drakeley, C.J.; Beshir, K.B. Global analysis of Plasmodium falciparum histidine-rich protein-2 (pfhrp2) and pfhrp3 gene deletions using whole-genome sequencing data and meta-analysis. Infect. Genet. Evol. 2018, 62, 211–219.
- Beshir, K.B.; Sepulveda, N.; Bharmal, J.; Robinson, A.; Mwanguzi, J.; Busula, A.O.; de Boer, J.G.; Sutherland, C.; Cunningham, J.; Hopkins, H. Plasmodium falciparum parasites with histidine-rich protein 2 (pfhrp2) and pfhrp3 gene deletions in two endemic regions of Kenya. Sci. Rep. 2017, 7, 14718.
- Jang, I.K.; Tyler, A.; Lyman, C.; Kahn, M.; Kalnoky, M.; Rek, J.C.; Arinaitwe, E.; Adrama, H.; Murphy, M.; Imwong, M.; et al. Simultaneous Quantification of Plasmodium Antigens and Host Factor C-Reactive Protein in Asymptomatic Individuals with Confirmed Malaria by Use of a Novel Multiplex Immunoassay. J. Clin. Microbiol. 2019, 57, e00948-18.
- Martianez-Vendrell, X.; Jimenez, A.; Vasquez, A.; Campillo, A.; Incardona, S.; Gonzalez, R.; Gamboa, D.; Torres, K.; Oyibo, W.; Faye, B.; et al. Quantification of malaria antigens PfHRP2 and pLDH by quantitative suspension array technology in whole blood, dried blood spot and plasma. Malar. J. 2020, 19, 12.
- Plucinski, M.M.; Herman, C.; Jones, S.; Dimbu, R.; Fortes, F.; Ljolje, D.; Lucchi, N.; Murphy, S.C.; Smith, N.T.; Cruz, K.R.; et al. Screening for Pfhrp2/3-Deleted Plasmodium falciparum, Non-falciparum, and Low-Density Malaria Infections by a Multiplex Antigen Assay. J. Infect. Dis. 2019, 219, 437–447.




