| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana da Silva | + 1658 word(s) | 1658 | 2020-11-13 05:12:05 | | | |
| 2 | Bruce Ren | Meta information modification | 1658 | 2020-11-19 02:33:58 | | |
Video Upload Options
The chicken immune system has provided an immense contribution to basic immunology knowledge by establishing major landmarks and discoveries that defined concepts widely used today. One of many special features on chickens is the presence of a compact and simple major histocompatibility complex (MHC). Despite its simplicity, the chicken MHC maintains the essential counterpart genes of the mammalian MHC, allowing for a strong association to be detected between the MHC and resistance or susceptibility to infectious diseases. This association has been widely studied for several poultry infectious diseases, including infectious bronchitis. In addition to the MHC and its linked genes, other non-MHC loci may play a role in the mechanisms underlying such resistance. It has been reported that innate immune responses, such as macrophage function and inflammation, might be some of the factors driving resistance or susceptibility, consequently influencing the disease outcome in an individual or a population. Information about innate immunity and genetic resistance can be helpful in developing effective preventative measures for diseases such as infectious bronchitis, to which a systemic antibody response is often not associated with disease protection.
1. Introduction
The avian immune system has been studied for decades and has provided immense knowledge on fundamental concepts in basic immunology that are widely accepted today. Despite numerous similarities to the mammalian immune system [1], avian species have unique immunological structures and mechanisms. For example, the serendipitous discovery of the bursa of Fabricius as the site for B cell antigen-specific repertoire development led to the differentiation between B and T cells, which are now identified as the two arms of adaptive immunity [2]. Unlike mammals, lymph nodes are absent in the chicken, although lymphoid tissue aggregates are distributed in several organs and systems. Some examples of these lymphoid tissues include lymphoid nodules in the walls of lymphatic vessels, the gut-, mucosa-, bronchial- and conjunctiva-associated lymphoid tissues (GALT, MALT, BALT, and CALT), cecal tonsils, Meckel’s diverticulum, Peyer’s patches, and Harderian glands [3]. These specific features of the chicken immune system and the importance of improving health in poultry production have turned the chicken into an important animal model for research, aiding the understanding of several aspects of basic immunology [4]. A special landmark of avian immunology was the unforeseen attenuation of a pathogen, later named Pasteurella multocida, by Louis Pasteur when studying fowl cholera in the late 1800s. His breakthrough led to the discovery of the first attenuated vaccine [5]. Some vaccine delivery systems that are inconvenient or inappropriate for mammalian livestock are widely used in poultry. These systems include mucosal vaccination via post-hatch spray and eye drop vaccines, which stimulate local innate and adaptive immune responses originating in the Harderian glands and local MALT, and in ovo vaccination, which enables easy automation and early stimulation of adaptive immune responses during embryonic development [3].
In addition to humoral responses elicited by natural infections and vaccination, cell-mediated adaptive immune responses in birds play an important role in minimizing disease outcome and preventing reinfections. For example, cytotoxic cluster of differentiation 8-positive (CD8+) T cells play a major role in eliminating infectious bronchitis virus (IBV) [6]. In addition, helper CD4+ T cells become activated by IBV antigens displayed on the surface of antigen presenting cells. After activation, CD4+ T cells interact with other T and B cells, amplifying cytotoxic and humoral responses to IBV [7]. Moreover, innate cellular responses also assist in fighting early stages of viral infections. The rates of macrophage differentiation and activation have been shown to be greater in relatively IBV-resistant cells than in IBV-susceptible cells, suggesting that disease resistance might be linked to a vigorous innate immune response [8][9][10]. Cytokine production and proinflammatory responses induced by IBV might also affect the severity of the disease and the development of appropriate adaptive immune responses [11][12][13].
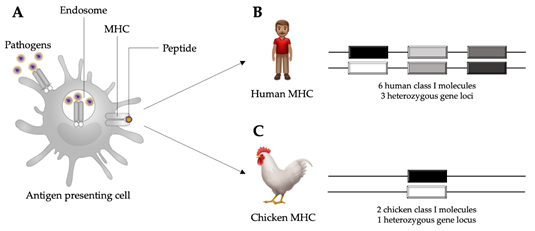
Another remarkable feature of the chicken immune system is the major histocompatibility complex (MHC). Unlike the MHC of mammals, the chicken MHC is compact and simple, while still maintaining the essential counterpart genes. The chicken MHC contains about 46 genes in a region of about 209 kb of the chicken genome [14], whereas the human MHC contains over 200 genes spanning a region of approximately 4000 kb [15]. Moreover, there is a single dominantly expressed chicken MHC class I antigen, which allows detection of a strong association between the chicken MHC and resistance or susceptibility to infectious diseases (Figure 1) [16][17]. The genetic makeup of the host determines how the immune system will respond to infectious challenges and, ultimately, if the response will be protective or not. Within a population, the genetic variability of the MHC and other related genes leads to a wide range of immune responses and disease outcomes, varying from mild clinical signs to mortality. This variability is partly due to the polymorphic nature of genes that regulate the expression of components of the immune system [18].
Figure 1. Schematic representation of an antigen presenting cell internalizing pathogens in the process of phagocytosis and subsequent antigen presentation through major histocompatibility complex (MHC) molecules (A). In humans, there are six MHC class I molecules occurring in three heterozygous loci (B). In chickens, there are two MHC class I molecules occurring in one heterozygous locus (C), which allows for stronger associations with disease resistance than the human MHC class I. Adapted from Kaufman, 2013 [19].
To study disease resistance in chickens, MHC congenic chicken lines that share the same genetic background with differences exclusively in their MHC B locus have been developed [20][21][22][23]. Using these chicken lines as animal models, associations between MHC haplotypes and disease resistance or susceptibility have been described for several infectious pathogens, including coccidia [24][25][26][27], pathogenic bacteria [28][29][30][31], oncogenic viruses [32][33][34][35][36][37], and other viruses including IBV [38][39][40][41][42][43][44][45][46][47]. The main focus of the present review is IBV.
Disease prevention and control are of major concern in commercial poultry production. Vaccination, in addition to management and biosecurity measures, is widely used to prevent disease and control pathogen loads when eradication is not possible. Vaccines, however, rarely provide 100% protection against infectious diseases [48]. With some diseases, such as infectious bronchitis, high serum antibody levels elicited by vaccination do not necessarily correspond to protection [49][50]. Studying the genetic resistance to IBV determined by the chicken MHC is essential to better understand the mechanisms underlying resistance and, consequently, to use this knowledge to improve IBV prevention [46][47].
This review summarizes the significance of the chicken MHC and its impact on poultry disease resistance, particularly in IBV infections, and the future of IBV genetic resistance research using MHC inbred and congenic chicken lines.
2. The Chicken MHC
The MHC is a well-studied genomic region of many animal species. It was first described in mice as the genetic locus responsible for rapid tissue allograft rejection and for encoding highly polymorphic alloantigens on cell surfaces [51]. In addition to its relation to graft rejection and autoimmunity, the MHC is also responsible for antigen presentation to T cells, representing an important bridge between the innate and the adaptive immune response. MHC class I molecules are present on nucleated cells and responsible for presenting peptides to CD8+ T cells. In contrast, the MHC class II molecules are only present on antigen presenting cells (dendritic cells, macrophages, and B cells) and are able to activate CD4+ T cells [52]. The mammalian MHC is polygenic, consisting of numerous genes, pseudogenes, and repetitive paralogous regions located on different chromosomes. The mammalian MHC is also polymorphic, with multiple alleles of each gene within a population [53]. Thus, the mammalian MHC is large and complex, with distantly related genes that provide extensive diversity to its antigen-presenting glycoproteins. In contrast, the chicken MHC is small and simple, yet contains the essential counterparts of genes present in the mammalian MHC. For this reason, the chicken MHC is considered a minimal essential set of genes, despite the differences from mammalian MHCs in organization and structure .
Upon discovery, the chicken MHC was classified as the B blood group or B locus, coding for agglutination factors present on the surface of chicken red blood cells [54]. Subsequently, these genes were associated with skin graft rejection and, therefore, histocompatibility [55]. Ultimately, it was discovered that the chicken MHC B locus is located on chicken microchromosome 16 and codes for molecules termed B-F (class I) and B-L (class II), which are closely linked and often referred to as the B-F/B-L region, and B-G (class IV), which is limited to Aves [56][57][58]. In addition to the B locus, a separate group of nonclassical MHC class I and II genes was identified and named Rfp-Y, which is also on microchromosome 16 but genetically distant and unlinked to the B locus (Figure 2) [59][60][61].
Figure 2. Schematic representation of the chicken microchromosome 16, indicating the MHC B and Rfp-Y loci. The B locus contains the B-F/B-L and the B-G regions corresponding to MHC class I, II, III, and IV (B-G) genes. The Rfp-Y locus contains nonclassical MHC class I and II genes. Adapted from Delany et al. (2009) and Kaufman (2013) [61].
In addition to the MHC, genetic resistance to infectious diseases has been associated with CD1 genes, which are located near the B locus on microchromosome 16. CD1 glycoproteins are present on the surface of antigen-presenting cells and are responsible for detecting lipids and glycolipids and presenting them to specific subsets of T cells [62].
As previously mentioned, the chicken MHC is small and simple while still preserving essential homologous genes present in the mammalian MHC. Furthermore, a single chicken MHC class I is predominantly expressed, showing a strong association with relative protection or vulnerability to various infectious diseases [62]. This genetic resistance to disease is demonstrated with specific MHC B haplotypes and, less frequently, with alleles in particular subregions of microchromosome 16 [[62]. For this reason, the chicken is an excellent animal model to study immunology, leading geneticists to develop genetically defined chicken lines for research purposes. By using inbreeding, congenic chicken lines that share the same genetic backbone and differ from one another with respect to only the MHC B locus have been generated. With these chicken lines, scientists have been able to mitigate background effects, isolate and characterize specific genes, and associate MHC genes with disease resistance.
References
- Sharma, J.M.; Tizard, I. Avian cellular immune effector mechanisms: A review. Avian Pathol. 1984, 13, 357–376, doi:10.1080/03079458408418541.
- Glick, B.; Chang, T.S.; Jaap, R.G. The bursa of Fabricius and antibody production. Poult. Sci. 1956, 35, 224–225, doi:10.3382/ps.0350224.
- Jaffredo, T.; Fellah, J.S.; Dunon, D. Immunology of Birds and Reptiles. In Encyclopedia of Life Sciences (eLS); Wiley online library: Hoboken, NJ, USA, 2006; doi:10.1038/npg.els.0000521.
- Davison, F. The importance of the avian immune system and its unique features. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1–9, doi:10.1016/B978-0-12-396965-1.00001-7.
- De Kruif, P. Microbe Hunters; Houghton Mifflin Harcourt: San Diego, CA, USA 1996.
- Collisson, E.W.; Pei, J.; Dzielawa, J.; Seo, S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000, 24, 187–200, doi:10.1016/S0145-305X(99)00072-5.
- Janse, E.M.; Roozelaar, D.V.; Koch, G. Leukocyte subpopulations in kidney and trachea of chickens infected with infectious bronchitis virus. Avian Pathol. 1994, 23, 513–523, doi:10.1080/03079459408419021.
- Dawes, M.E.; Griggs, L.M.; Collisson, E.W.; Briles, W.E.; Drechsler, Y. Dramatic differences in the response of macrophages from B2 and B19 MHC-defined haplotypes to interferon gamma and polyinosinic:polycytidylic acid stimulation. Poult. Sci. 2014, 93, 830–838, doi:10.3382/ps.2013-03511.
- Collisson, E.; Griggs, L.; Drechsler, Y. Macrophages from disease resistant B2 haplotype chickens activate T lymphocytes more effectively than macrophages from disease susceptible B19 birds. Dev. Comp. Immunol. 2017, 67, 249–256, doi:10.1016/j.dci.2016.09.013.
- Seo, S.H.; Collisson, E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997, 71, 5173–5177, doi:10.1128/jvi.71.7.5173-5177.1997.
- Amarasinghe, A.; Abdul-Cader, M.S.; Almatrouk, Z.; van der Meer, F.; Cork, S.C.; Gomis, S.; Abdul-Careem, M.F. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV). Vet. Microbiol. 2018, 215, 1–10, doi:10.1016/j.vetmic.2018.01.001.
- da Silva, A.P.; Schat, K.A.; Gallardo, R.A. Cytokine responses in tracheas from MHC congenic chicken lines with distinct susceptibilities to infectious bronchitis virus. Avian Dis. 2020, 64, 36–45, doi:10.1637/0005-2086-64.1.36.
- Asif, M.; Lowenthal, J.W.; Ford, M.E.; Schat, K.A.; Kimpton, W.G.; Bean, A.G. Interleukin-6 expression after infectious bronchitis virus infection in chickens. Viral. Immunol. 2007, 20, 479–486, doi:10.1089/vim.2006.0109.
- Fulton, J.E.; McCarron, A.M.; Lund, A.R.; Pinegar, K.N.; Wolc, A.; Chazara, O.; Bed’Hom, B.; Berres, M.; Miller, M.M. A high-density SNP panel reveals extensive diversity, frequent recombination and multiple recombination hotspots within the chicken major histocompatibility complex B region between BG2 and CD1A1. Genet. Sel. Evol. 2016, 48, 1, doi:10.1186/s12711-015-0181-x.
- The MHC Sequencing Consortium. Complete sequence and gene map of a human major histocompatibility complex. Nature 1999, 401, 921–923, doi:10.1038/44853.
- Kaufman, J.; Milne, S.; Göbel, T.W.; Walker, B.A.; Jacob, J.P.; Auffray, C.; Zoorob, R.; Beck, S. The chicken B locus is a minimal essential major histocompatibility complex. Nature 1999, 401, 923–925, doi:10.1038/44856.
- Kaufman, J.; Jacob, J.; Shaw, J.; Walker, B.; Milne, S.; Beck, S.; Salomonsen, J. Gene organisation determines evolution of function in the chicken MHC. Immunol. Rev. 1999, 167, 101–117, doi:10.1111/j.1600-065X.1999.tb01385.x.
- Rautenschlein, S.; Cheng, H.H.; Lamont, S.J. Host factors for disease resistance. In Diseases of Poultry, 14th ed.; Swayne, D.E., Ed.; Wiley-Blackwell: Oxford, UK, 2020; pp. 79–108.
- Kaufman, J. The Avian MHC. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 149–167, doi:10.1016/B978-0-12-396965-1.00008-X.
- Waters, N.F.; Lambert, W.V. Inbreeding in the White Leghorn fowl. Res. Bull. (Iowa Agric. Home Econ. Exp. Stn. )1936, 18, 1.
- Delany, M.E.; Pisenti, J.M. Conservation of poultry genetic research resources: Consideration of the past, present and future. Poult. Avian Biol. Rev. 1998, 9, 25–42.
- Abplanalp, H. Inbred lines as genetic resources of chickens. Poult. Sci. Rev. 1992, 4, 29–39.
- Pisenti, J.M.; Delany, M.E.; Taylor Jr, R.L.; Abbott, U.K.; Abplanalp, H.; Arthur, J.A.; Bakst, M.R.; Baxter-Jones, C.; Bitgood, J.J.; Bradley, F.A.; et al. Avian Genetics Resources at Risk: An Assessment and Proposal for Conservation of Genetic Stocks in the USA and Canada; McGuire, P.E., Ed.; UC Davis Genetic Resources Conservation Program: Davis, CA, USA1999.
- Clare, R.A.; Strout, R.G.; Taylor, R.L., Jr.; Collins, W.M.; Briles, W.E. Major histocompatibility (B) complex effects on acquired immunity to cecal coccidiosis. Immunogenetics 1985, 22, 593–599.
- Brake, D.A.; Fedor, C.H.; Werner, B.W.; Miller, T.J.; Taylor, R.L.; Clare, R.A. Characterization of immune response to Eimeria tenella antigens in a natural immunity model with hosts which differ serologically at the B locus of the major histocompatibility complex. Infect. Immun. 1997, 65, 1204–1210.
- Collins, W.M.; Briles, W.E.; Zsigray, R.M.; Dunlop, W.R.; Corbett, A.C.; Clark, K.K.; Marks, J.L.; McGrail, T.P. The B locus (MHC) in the chicken: Association with the fate of RSV-induced tumors. Immunogenetics 1977, 5, 333–343, doi:10.1007/BF01570490.
- Schierman, L.W.; Watanabe, D.H.; McBride, R.A. Increased growth of Rous sarcomas in chickens pretreated with formalinized syngeneic tumor cells. Eur. J. Immunol. 1977, 7, 710–713, doi:10.1002/eji.1830071012.
- Lamont, S.J.; Bolin, C.; Cheville, N. Genetic resistance to fowl cholera is linked to the major histocompatibility complex. Immunogenetics 1987, 25, 284–289, doi:10.1007/BF00404420.
- Cotter, P.F.; Taylor, R.L.; Abplanalp, H. Differential Resistance to Staphylococcus aureus Challenge in Major Histocompatibility (B) Complex Congenic Lines. Poult. Sci. 1992, 71, 1873–1878, doi:10.3382/ps.0711873.
- Cotter, P.F.; Taylor, R.L., Jr.; Abplanalp, H. B-complex associated immunity to Salmonella enteritidis challenge in congenic chickens. Poult. Sci. 1998, 77, 1846–1851, doi:10.1093/ps/77.12.1846.
- Liu, W.; Miller, M.M.; Lamont, S.J. Association of MHC class I and class II gene polymorphisms with vaccine or challenge response to Salmonella enteritidis in young chicks. Immunogenetics 2002, 54, 582–590, doi:10.1007/s00251-002-0495-z.
- Schat, K.A.; Calnek, B.W.; Fabricant, J.; Abplanalp, H. Influence of oncogenicity of Marek’ disease virus on evaluation of genetic resistance. Poult. Sci. 1981, 60, 2559–2566.
- Briles, W.E.; Briles, R.W.; Pollock, D.L.; Pattison, M. Marek’s disease resistance of B (MHC) heterozygotes in a cross of purebred Leghorn lines. Poult. Sci. 1982, 61, 205–211.
- Bacon, L.D.; Crittenden, L.B.; Witter, R.L.; Fadly, A.; Motta, J. B5 and B15 associated with progressive Marek’s disease, Rous sarcoma, and avian leukosis virus-induced tumors in inbred 15I4 chickens. Poult. Sci. 1983, 62, 573–578, doi:10.3382/ps.0620573.
- Witter ,P.L. Calnek, B.W. Resistance to Marek’s disease of congenic lines differing in major histocompatibility haplotypes to 3 virus strains. In Proceedings of the International Symposium on Marek’s Disease, Abplanalp, H.; Schat, K.A. 2nd eds; Springer: Ithaca, NY, USA; 1984 pp. 347–358.
- Wakenell, P.S.; Miller, M.M.; Goto, R.M.; Gauderman, W.J.; Briles, W.E. Association between the Rfp-Y haplotype and the incidence of Marek’s disease in chickens. Immunogenetics 1996, 44, 242–245.
- Taylor, R.L. Major histocompatibility (B) complex control of responses against Rous sarcomas. Poult. Sci. 2004, 83, 638–649, doi:10.1093/ps/83.4.638.
- Bumstead, N.; Huggins, M.B.; Cook, J.K. Genetic differences in susceptibility to a mixture of avian infectious bronchitis virus and Escherichia coli. Br. Poult. Sci. 1989, 30, 39–48, doi:10.1080/00071668908417123.
- Cook, J.; Otsuki, K.; Huggins, M.; Bumstead, N. Investigations into resistance of chicken lines to infection with infectious bronchitis virus. Adv. Exp. Med. Biol. 1990, 276, 491–496.
- Otsuki, K.; Matsuo, K.; Maeda, N.; Sanekata, T.; Tsubokura, M. Selection of Variants of Avian Infectious Bronchitis Virus Showing Tropism for Different Organs; Springer, Boston, MA, USA; 1990 pp. 379–384.
- Ignjatovic, J.; Reece, R.; Ashton, F. Susceptibility of three genetic lines of chicks to infection with a nephropathogenic T strain of avian infectious bronchitis virus. J. Comp. Pathol. 2003, 128, 92–98.
- Bacon, L.D.; Hunter, D.B.; Zhang, H.M.; Brand, K.; Etches, R. Retrospective evidence that the MHC (B haplotype) of chickens influences genetic resistance to attenuated infectious bronchitis vaccine strains in chickens. Avian Pathol. 2004, 33, 605–609, doi:10.1080/03079450400013147.
- Joiner, K.S.; Hoerr, F.J.; Ewald, S.J.; van Santen, V.L.; Wright, J.C.; van Ginkel, F.W.; Toro, H. Pathogenesis of infectious bronchitis virus in vaccinated chickens of two different major histocompatibility B complex genotypes. Avian Dis. 2007, 51, 758–763, doi:10.1637/0005-2086(2007)51[758:poibvi]2.0.co;2.
- Banat, G.R.; Tkalcic, S.; Dzielawa, J.A.; Jackwood, M.W.; Saggese, M.D.; Yates, L.; Kopulos, R.; Briles, W.E.; Collisson, E.W. Association of the chicken MHC B haplotypes with resistance to avian coronavirus. Dev. Comp. Immunol. 2013, 39, 430–437, doi:10.1016/j.dci.2012.10.006.
- Smith, J.; Sadeyen, J.R.; Cavanagh, D.; Kaiser, P.; Burt, D.W. The early immune response to infection of chickens with Infectious Bronchitis Virus (IBV) in susceptible and resistant birds. BMC Vet. Res. 2015, 11, 256, doi:10.1186/s12917-015-0575-6.
- da Silva, A.P.; Hauck, R.; Zhou, H.; Gallardo, R.A. Understanding immune resistance to infectious bronchitis using major histocompatibility complex chicken lines. Avian Dis. 2017, 61, 358–365, doi:10.1637/11666-050117-RegR.
- da Silva, A.P.; Hauck, R.; Kern, C.; Wang, Y.; Zhou, H.; Gallardo, R.A. Effect of chicken MHC haplotype on resistance to distantly-related infectious bronchitis viruses. Avian Dis. 2019, 63, 310–317, doi:10.1637/11989-103118-Reg.1.
- Marangon, S.; Busani, L. The use of vaccination in poultry production. Rev. Sci. Tech. 2007, 26, 265–274.
- Raggi, L.G.; Lee, G.G. Lack of correlation between infectivity, serologic response and challenge results in immunization with an infectious bronchitis vaccine. J. Immunol. 1965, 94, 538–543.
- Toro, H.; Espinoza, C.; Ponce, V.; Rojas, V.; Morales, M.A.; Kaleta, E.F. Infectious bronchitis: Effect of viral doses and routes on specific lacrimal and serum antibody responses in chickens. Avian Dis. 1997, 41, 379–387.
- Browning, M.; McMichael, A. HLA and MHC: Genes, Molecules, and Function; Academic Press: Cambridge, MA, USA, 1999.
- Murphy, K.; Weaver, C. Antigen recognition by B-cell and T-cell receptors. In Janeway’s Immunobiology, 9th ed.; Garland Science: New york, NY, USA, 2016; pp. 139–172.
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017, 8, doi:10.3389/fimmu.2017.00292.
- Briles, W.E.; McGibbon, W.H.; Irwin, M.R. On multiple alleles effecting cellular antigens in the chicken. Genetics 1950, 35, 633–652.
- Schierman, L.W.; Nordskog, A.W. Relationship of blood type to histocompatibility in chickens. Science 1961, 134, 1008–1009, doi:10.1126/science.134.3484.1008.
- Pink, J.R.L.; Droege, W.; Hála, K.; Miggiano, V.C.; ZIegler, A. A three-locus model for the chicken major histocompatibility complex. Immunogenetics 1977, 5, 203–216, doi:10.1007/BF01570477.
- Wolf, H.; Hála, K.; Boyd, R.L.; Wick, G. MHC- and non-MHC-encoded surface antigens of chicken lymphoid cells and erythrocytes recognized by polyclonal xeno-, allo- and monoclonal antibodies. Eur. J. Immunol. 1984, 14, 831–839, doi:10.1002/eji.1830140912.
- Kline, K.; Briles, W.E.; Bacon, L.; Sanders, B.G. Characterization of two distinct disulfide-linked B-G molecules in the chicken. J. Hered. 1988, 79, 249–256, doi:10.1093/oxfordjournals.jhered.a110505.
- Briles, W.E.; Goto, R.M.; Auffray, C.; Miller, M.M. A polymorphic system related to but genetically independent of the chicken major histocompatibility complex. Immunogenetics 1993, 37, 408–414, doi:10.1007/BF00222464.
- Juul-Madsen, H.R.; Simonsen, M.; Hedemand, J.E.; Salomonsen, J. Restriction fragment length polymorphism analysis of the chicken B-F and B-L genes and their association with serologically defined B haplotypes. Anim. Genet. 1993, 24, 243–247, doi:10.1111/j.1365-2052.1993.tb00306.x.
- Delany, M.E.; Robinson, C.M.; Goto, R.M.; Miller, M.M. Architecture and organization of chicken microchromosome 16: Order of the NOR, MHC-Y, and MHC-B subregions. J. Hered. 2009, 100, 507–514, doi:10.1093/jhered/esp044.
- Miller, M.M.; Taylor, R.L., Jr. Brief review of the chicken major histocompatibility complex: The genes, their distribution on chromosome 16, and their contributions to disease resistance. Poult. Sci. 2016, 95, 375–392, doi:10.3382/ps/pev379.