Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amin Gasmi | -- | 4256 | 2022-10-24 03:52:51 | | | |
| 2 | Conner Chen | -13 word(s) | 4243 | 2022-10-25 10:48:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/30760 (accessed on 07 February 2026).
Gasmi A, Mujawdiya PK, Noor S, Lysiuk R, Darmohray R, Piscopo S, et al. Polyphenols in Metabolic Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/30760. Accessed February 07, 2026.
Gasmi, Amin, Pavan Kumar Mujawdiya, Sadaf Noor, Roman Lysiuk, Roman Darmohray, Salva Piscopo, Larysa Lenchyk, Halyna Antonyak, Kateryna Dehtiarova, Mariia Shanaida, et al. "Polyphenols in Metabolic Diseases" Encyclopedia, https://encyclopedia.pub/entry/30760 (accessed February 07, 2026).
Gasmi, A., Mujawdiya, P.K., Noor, S., Lysiuk, R., Darmohray, R., Piscopo, S., Lenchyk, L., Antonyak, H., Dehtiarova, K., Shanaida, M., Polishchuk, A., Shanaida, V., Peana, M., & Bjørklund, G. (2022, October 24). Polyphenols in Metabolic Diseases. In Encyclopedia. https://encyclopedia.pub/entry/30760
Gasmi, Amin, et al. "Polyphenols in Metabolic Diseases." Encyclopedia. Web. 24 October, 2022.
Copy Citation
Polyphenols (PPs) are a large group of phytochemicals containing phenolic rings with two or more hydroxyl groups. They possess powerful antioxidant properties, multiple therapeutic effects, and possible health benefits in vivo and in vitro, as well as reported clinical studies. Considering their free-radical scavenging and anti-inflammatory properties, these substances can be used to treat different kinds of conditions associated with metabolic disorders. Many symptoms of metabolic syndrome (MtS), including obesity, dyslipidemia, atherosclerosis, elevated blood sugar, accelerating aging, liver intoxication, hypertension, as well as cancer and neurodegenerative disorders, are substantially relieved by dietary PPs.
phenolic compounds
natural sources
metabolic syndrome
bioprotective property
1. Introduction
Metabolic diseases such as hyperglycemia, obesity, dyslipidemia, and hypertension are now considered global problems of the world population [1][2]. Their occurrence increases yearly, and nowadays, they are considered a significant danger for human beings as the most prevalent disorders worldwide [3].
Metabolic syndrome (MtS) is a complex coexisting diagnosis including abdominal obesity, increased blood pressure, elevated fasting glucose, reduced high-density lipoprotein-cholesterol levels, and elevated triglyceride levels [4]. MtS increases the risk factors of cardiovascular diseases (CVD), which puts huge pressure on the healthcare economy of the whole society [4]. Therefore, it is an urgent challenge for researchers to determine active pharmaceutical ingredients to improve MtS and its complications.
Mts have a complex etiology, including several pathophysiological mechanisms and factors that may cause the development of MtS, such as genetics, lifestyle, diet, and gut microbiome state [5]. The molecular changes result from the interaction between environmental and genetic factors, and oxidative stress and systemic inflammation significantly contribute to MtS pathogenesis [1]. Thus, an effective method of combating MtS comprises not only appropriate diet and physical activity but also consuming drugs and/or food supplementation. Therefore, discovering new natural substances that could reduce the symptoms of MtS through antioxidant and anti-inflammatory effects and the ability to normalize lipid and carbohydrate metabolism are very important issues for researchers and clinicians [1].
Oxidative stress and inflammation are common pathophysiology keys involved in the development and progression of metabolic disorders [6]. Thus, finding appropriate natural compounds to mitigate metabolic disorder’s symptoms and prevent related diseases’ progression is necessary.
In recent years, polyphenols (PPs) have been considered the key plant-based bioactive compounds against various diseases, including metabolic diseases, cardiovascular and neurodegenerative disorders, and some varieties of cancer [7][8]. PPs are getting much attention in the medical industry to treat different metabolic diseases due to their intrinsic antioxidant and anti-inflammatory properties [9][10].
About eight thousand polyphenolic compounds have been recognized nowadays [11][12]. PPs are naturally occurring complexes in various plants, including herbs, tea, fruits, and vegetables. In plants, these compounds play a key role in pigmentation, growth, ultraviolet rays protection, and against pathogens. PPs are also an essential part of various chemical industries for producing commodity chemicals, food additives, cosmetics, and paints [13]. Generally, PPs are known as plant secondary metabolites and can be categorized by the presence of several phenolic groups [14]. These compounds are classified based on the chemical complexity of their respective phenolic structure (flavonoids and non-flavonoids, Figure 1) [15].
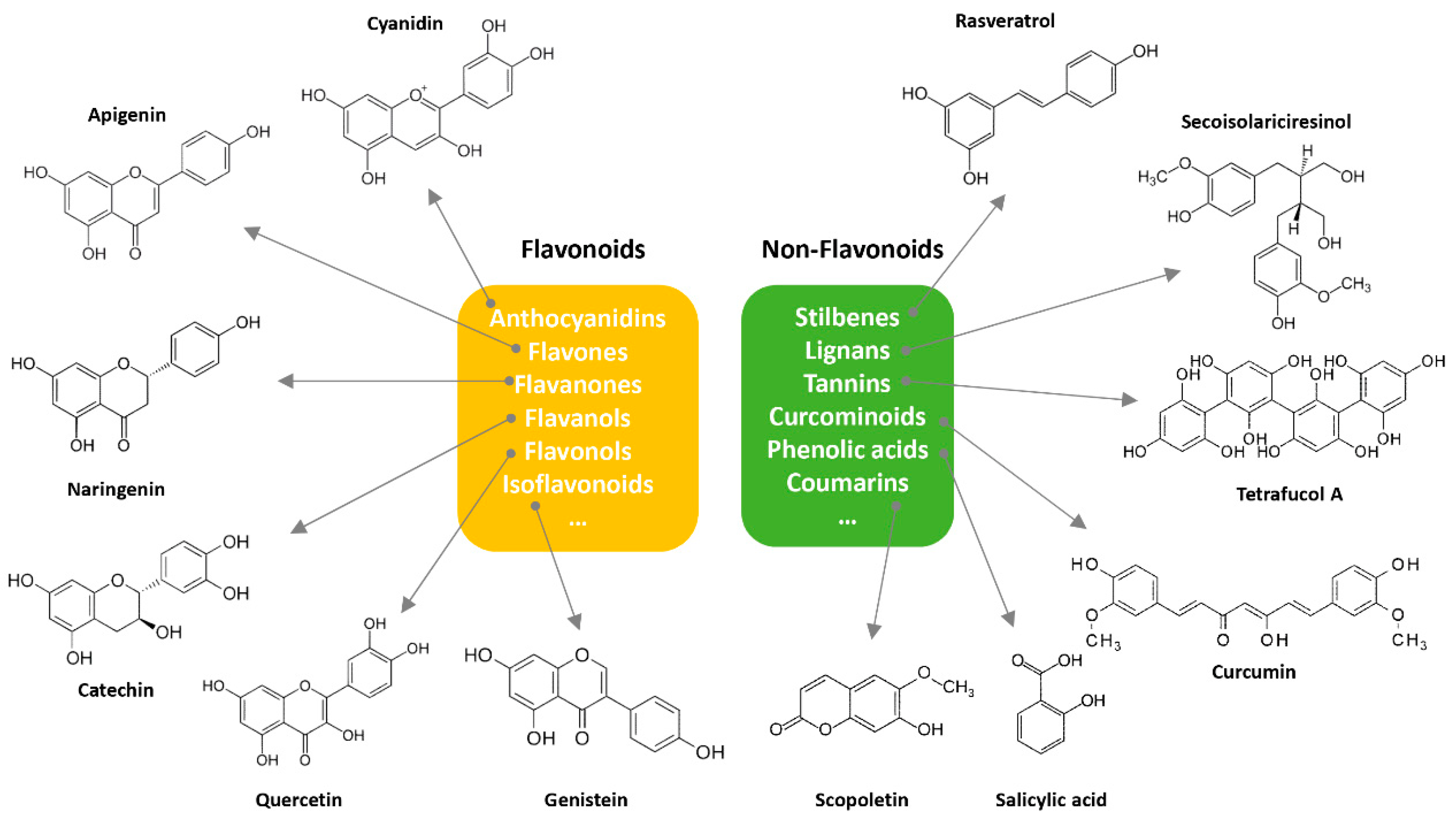
Figure 1. Classification of PPs with some representative examples.
It is interesting to note that polyphenolic compounds share a similar chemical structure. The structure of naturally occurring PPs varies from simple molecules (phenolic acid) to complex molecules (tannins) depending on the length of the chain attached [16]. PPs occur primarily in conjugated form, with one or more sugar residues attached to hydroxyl groups, although direct linkages to an aromatic carbon atom can be found [17][18]. PPs can also be found in associations with other compounds, such as amines, carboxylic and organic acids, and lipids; also common are their linkages with other phenols [18].
As PPs are found abundantly in the plant kingdom, their consumption in the human diet is not much surprising. These compounds are found in almost every balanced diet, especially where fruits and vegetables are mostly consumed [19]. However, quantitative information on PPs for various foods is unavailable because of their diverse nature and other factors responsible for altering their concentration in the diet. PPs possess similar properties, but their complex linkage with other compounds makes their separation challenging. Ample research has been done on different samples from various sources (foods, beverages, and plants). Thus, a deep understanding of these naturally occurring compounds and their associated biosynthesis will open new avenues to design special dietary plans enriched with PPs that ultimately strengthen respective health benefits. Considering the antioxidant and anti-inflammatory features of PPs, these compounds can be employed to treat various diseases associated with metabolic disorders [1].
Treatments of MtS may involve the design of natural drugs and special food products enriched with high-quality PPs or by the controlled drug and/or supplement delivery system, which can lead to the health improvement of the human population. Besides this, Siroma et al. supposed that scientific studies regarding ingestion of PPs and other natural nutrients could improve global food education in different countries to help schools, families, and businesses to reduce obesity, hyperglycemia, and other metabolic diseases [20].
2. Polyphenols in the Prevention and Treatment of Different Metabolic Disorders
2.1. Oxidative Stress and Inflammation
Oxidative stress is one of the health conditions which occurs due to an imbalance of free radicals (oxygen or nitrogen species) and the defensive ability of the body to respond to reactive species to heal the respective disorder [21]. The production of significant reactive oxygen (ROS) and nitrogen species is one of the consequences of the normal functioning of alive intracellular structures [22]. The structural damage of various proteins, cell tissues, permeable membranes, and nucleic acids is due to exposure to highly reactive oxygen-based species such as hydrogen peroxide and superoxide anions [23]. To counter this oxidative stress, body cells continuously express several species, such as enzymes, to detoxify the resulting reactive species and ultimately heal the damage in the respective region. Species released by the body cells to encounter oxidative stress may come from enzymes, bacterial cells, mammalian cells, and PPs. In oxidative stress, the mechanism of cell damage occurs due to the different chemical actions of oxygen-based free radicals [24]. These reactive species may come from various sources by the abnormal metabolic system (aerobic metabolism) that may generate undesirable reactive species and cause cell death. It is found that the intake of antibiotics is also a source of generating these species [25][26].
2.2. Insulin Resistance/Hyperglycemia
Insulin resistance is the most common MtS in which body cells (fat, liver, and respective muscles) cannot provide an effective response to insulin and cannot consume glucose from the blood to produce energy [27]. Insulin resistance leads to the destruction of insulin-producing pancreatic B-cells.
This MtS can further complicate the health problems such as a rise in blood pressure, obesity, unbalanced cholesterol levels, and diabetes problems [28]. This type of syndrome can easily be identified by regular glucose checkups and other corresponding analyses to check the glucose tolerance level in the human body. The origin of this MtS may come from various sources such as the family history of the disease, dietary habits, smoking, age, and others [29]. In addition to a high blood sugar level, diabetes mellitus often has manifestations of metabolic disorders such as obesity, dyslipidemia, and hypertension, which increase the risk of cardiovascular and cerebrovascular diseases and increase mortality [30].
2.3. Obesity
Obesity, or a body having a higher weight than normal (as per BMI index), is one of the major problems in the new generation and society [31]. Over 1/3 of the world’s population is obese [1]. Obesity is closely linked with several health disorders, such as adipocyte hypertrophy, insulin resistance, diabetes mellitus, systematic inflammation, non-alcoholic fatty liver disorder, coronary heart diseases, cardiovascular diseases, and cancer [20][32][33].
2.4. Liver Intoxication
The liver is one of the vital internal organs of the body responsible for regulating more than 500 functions occurring in the human body. The liver’s most important function is to detoxify and neutralize the toxins coming into the body to avoid further health complications [34]. When it comes to liver cleansing, there are several home or market-wide methods available. However, many of these methods are not even tested on a clinical basis or are not regularized by the national drug authorities [35]. Liver detoxification is also associated with the metabolic system.
2.5. Aging
The idea of extension in life and a beautiful appearance is necessary for every human body to reverse the effects of aging. This effect can be achieved in several ways, either by using different medicines and supplements or by the dramatic physical changes in the body. Thus far, no such dramatic changes have been clinically observed that can prove effective for the subject. In this concern, various remedies are available over the counter, such as home remedies or allopathic medicines. In the medical industry, antiaging products and their corresponding cosmetic sector have promising contributions within the industry [36]. The consumption of these products is in demand worldwide. Consumers continually desire not only the long-term effects of these agents but also require a response after application to the target. Many cosmetic industries have introduced soft focus and lifting effect concepts considering consumer demand.
In the first one, results are observed in long-term applications, while in the lifting effect, an immediate effect of the proposed cosmetics is offered [37]. In various cosmetics, many naturally occurring compounds are used as skin mediators and healing agents. In these naturally occurring compounds, phenolic-based compounds have a very promising demand as antiaging and for other skin diseases such as skin cancer [38][39]. The antioxidative features of phenolic compounds enable extensive usage of these substances in the cosmetic industry as antiaging agents. Many phenolic compounds have been extracted from various sources, such as potato peels, apples, papaya, rosemary, Crataegus spp., and Ginkgo biloba, and their applications have been investigated for antiaging purposes [40].
Aging is associated with an increased risk of developing diabetes mellitus, neurodegeneration, cardiovascular diseases, osteoarthritis, or cancer [41]. Natural antioxidants might prevent aging-related pathologies via different signaling systems involving ROS and nitrogen species scavenging, with the Nrf2/Keap1-ARE system and the pathway mTOR/Foxo/SIRT1 [42]. However, to enhance the antiaging effect of PPs, various intermediates (ethanol and weak acids) are used to achieve maximum benefits [43]. In the cosmetic industry, the most used PPs are anthocyanins, phenolic acids, and flavonoids because of their excellent antioxidant activity. Anthocyanins are mostly found in colored vegetables and fruits, while flavonoids and phenolic acids are mostly found in various seed sources. Although the use of these compounds is much broader, their extraction and separation are key challenges in commercializing these compounds in medical research [44][45].
2.6. Carcinogenesis
Carcinogenesis or oncogenesis is the formation of various cancer types involving a multistep and complex process of normal cell division to malignant ones (cancer cells). Different biological alterations generally identify these processes at genetic, internal cellular, and epigenetic levels. These further cause cell division in dead cells and can occur in almost all body tissues under several circumstances [46]. The general theory of carcinogenesis suggests that DNA mutation is a major cause of developing cancer cells. However, only a few mutations can cause cancer cells while others cannot [47]. Carcinogenesis occurs due to human exposure to carcinogens, which can be chemical, biological, radiative, or viral substances. During the process of carcinogenesis, it is observed that the imbalance response of cytokines and their growth in the normal human body also plays a key role in the formation of cancer cells and their further progress by altering the cell cycle proteins [48]. The anticancer effects of natural phytochemicals, such as PPs, relevant to the modulation of cytokine signaling pathways in various cancer cells are evident from many reports.
2.7. Cardiovascular Diseases
The past few years have witnessed an extreme transformation in habits, pushing modern populations away from a natural diet and toward unhealthy foods and a sedentary lifestyle. The risk of developing CVDs has increased due to the integration of the modern lifestyle with a persistent intake of harmful intoxicants, including tobacco and misuse of drugs [49].
CVDs are a group of several heart-related complications, such as hypertension, heart failure, hyperlipidemia, acute coronary syndrome, peripheral artery disease, and stroke. Inflammation, atherosclerosis, immune responses, and any physical damage are the most common causes linked to the pathogenesis of heart stroke and failure. Furthermore, increased ROS generation results in altered molecular pathways and endothelial dysfunction; both play a significant role in the etiology of CVDs [50]. Several chemical-based drugs, such as antiplatelet drugs and cholesterol-lowering agents, are extensively utilized for CVDs treatment. However, these drugs pose several harmful effects.
Consequently, the use of herbal products is expanding rapidly in the 21st century [51]. Most plant species have remarkable safety records, making them a unique candidate for treating heart-related diseases [52]. PPs are the most promising plant compounds which showed significant cardioprotective properties due to their antioxidant potential. Recently, several research studies have evaluated their anti-atherosclerotic and immunomodulatory properties through pre-clinical and clinical trials. These studies highlight the importance of polyphenolic compounds as a natural way of reducing the risk of developing CVDs [53][54][55].
2.8. Other Health Problems
In the fourth century B.C., Hippocrates emphasized the importance of diet in health and disease, saying, “death sits in the bowels” [56]. Many in vivo studies and human trials suggest that gut microbiota dysbiosis is involved in gastrointestinal diseases and obesity, diabetes, and other MtS [57]. Recent data have revealed the ability of foods rich in polyphenols to modulate intestinal dysbiosis present in various diseases by reducing the number of potential pathobionts [57].
Table 1 summarizes the current state of the Mts treatments with PPs from clinical studies.
Table 1. The current state of the Mts treatments with polyphenols from clinical studies.
| PPs Type and Main Features of Treatment | Pathologies and Mechanism of Action | Refs. |
|---|---|---|
| Oxidative Stress and Inflammation | ||
| Oleuropein, hydroxytyrosol, curcumin, resveratrol, epigallocathechin | Cell protection (redox homeostasis) through the activation of vitagene signaling pathways | [58][59] |
| Grape products containing PPs (resveratrol, proanthocyanidin, quercetin, etc.) | Significant increase in the levels of total antioxidant capacity and oxygen radical absorbance capacity as well as improving various enzymatic systems such as superoxide dismutase or glutathione peroxidase (dependently on the dosage) | [60] |
| Genistein, silymarin caffeic acid, chlorogenic acid, ellagic acid | Healing chronic inflammation is the key pathomechanism of obesity-related metabolic disorders (insulin resistance, type 2 diabetes, and cardiovascular diseases) | [61] |
| PPs from cocoa, fruits, and vegetables | Alleviating the oxidative damage and inflammation parameters | [6][7][10][62] |
| Diabetes | ||
| Aloe Vera extract (enriched with PPs), PPs from grapes,and cinnamon | Control of insulin resistance | [63][64][65] |
| Quercetin, resveratrol and epigallocatechin-3-gallate | Enhancing glucose uptake in the adipocytes and muscles in type 2diabetes by the activation of the AMP-activated protein kinase pathway | [66] |
| Resveratrol | Reducing blood glucose levels | [30] |
| PPs from fruits and vegetables | Protecting pancreatic β-cells and activating glucose/lipid metabolism pathways, affecting glucose absorption and uptake | [62][66] |
| Obesity | ||
| Epigallocatechin gallate | Increasing energy consumption and weight loss due to a higher rate of fat oxidation | [67] |
| The total PP content (measured in urine samples using the Folin–Ciocalteu method) | Long-term intake of PPs led to significant loss of weight | [68] |
| Curcumin and resveratrol | Anti-obesity effect to avoid associated metabolic disorders | [69] |
| Brown seaweed PPs | Effective regulation of metabolic disorders via correction of fat function (transforming white adipose tissue into “brown” and enhancing energy consumption) | [70] |
| PPs from fruits and vegetables | Reducing lipid accumulation and regulating intestinal microflora | [62] |
| Liver Intoxication | ||
| Silymarin/Silybin | Hepatoprotection, preventing and treatment of chronic liver disease | [35] |
| Flavonoids (anthocyanins, flavonols, flavanones and isoflavones) |
Detoxifying and oxidative stress preventive abilities of flavonoids through regulation of the autophagy and apoptosis pathways as well as by impact on mitochondria-ER stress-proteasome | [71][72][73][74][75] |
| Foods’ PPs (whole-foods approach) | It affects the activity of detoxification pathways, including Nrf2 signaling, phase I cytochrome P450 enzymes, phase II conjugation enzymes, and metallothionein | [76] |
| Aging | ||
| Resveratrol | Vascular dysfunction in aging | [66] |
| Resveratrol, quercetin, curcumin and catechins | Modulation of some of the evolutionarily conserved hallmarks of aging, such as oxidative damage, cell senescence, and autophagy | [38] |
| Flavonoids, curcumin and resveratrol | Disruption of age-associated deterioration in physiological function | [44] |
| Isoflavones from soybean | Anti-arteriosclerotic effect | [77] |
| Flavonoids and tannins | Modulating genes associated with stress defense, drug-metabolizing enzymes, detoxification, and transporter proteins | [78] |
| Carcinogenesis | ||
| Epigallocatechin and other tea PPs | Chemopreventive effects on colorectal cancer | [79] |
| Pomegranate fruit extract, green tea PPs, grape seed proanthocyanidins, resveratrol, genistein, silymarin, and delphinidin | Inhibition of photocarcinogenesis (melanoma, squamous cell carcinoma, basal cell carcinoma) | [80][81] |
| Isoflavones from soybean | Prevention of prostate and breast cancer | [82][83] |
| Cardiovascular Diseases | ||
| Resveratrol | Increasing total plasminogen activator inhibitor and circulating vascular cell adhesion molecules | [84] |
| Green tea PPs | Prevention the coronary heart disease | [85] |
| Cocoa flavanols | Improving the levels of biomarkers for cardiometabolic disorders | [86][87] |
| Lignans, flavonoids, and hydroxybenzoic acids | Diminishing risk of major cardiovascular disorders (ischemia, myocardial infarction, stroke) | [9] |
| Rheumatoid Arthritis | ||
| Curcumin | Improving metabolic parameters and inflammatory factors in women with rheumatoid arthritis | [88] |
3. Proposed Panel of Polyphenols


It should be mentioned that PPs possess the ability to facilitate the MtS at different levels (Table 2). Several subclasses of flavonoids have proven free-radical scavenging activity and another valuable therapeutic potential: catechins (e.g., gallocatechin, epicatechin, epigallocatechin), flavones (e.g., luteolin, apigenin), flavanones (e.g., hesperidin, naringenin), anthocyanidins (e.g., cyanidin, delphinidin, pelargonidin), flavonols (e.g., quercetin, rutin, myricetin), and isoflavones (e.g., genistein) [89]. For instance, anthocyanins are water-soluble pigments that exist mainly in glycosylated forms. They are responsible for fruits and vegetables in red, purple, and blue colors [90]. The glycoside forms of cyanidin, delphinidin, pelargonidin, malvidin, peonidin, and petunidin are the major anthocyanins in plants [90][91].
Table 2. The main polyphenols and underlying mechanisms of their pharmacological activity in MtS treatment and prevention.
| The Common Name of Polyphenolic Compound | Structural Formula and IUPAC Name |
Class of Phenolic Compounds | Main Sources | Main Targets of Action (Metabolic Diseases and States) |
Refs. |
|---|---|---|---|---|---|
| Resveratrol |  3,5,4’-trihydroxystilbene |
Stilbenes | Grapes, raspberries, mulberries, blueberries, apples, plums, and peanuts |
|
[92][93][94] [30] [66] [69] [95] [96] [97] [98] [99] |
| Curcumin |  (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione |
Curcuminoids (diarylheptanoid) | Turmeric (Curcuma longa) rhizome |
|
[94] [66] [69] [100] [101] [102] [88] [103] [104] |
| Quercetin |  3,3′,4′,5,7-Pentahydroxyflavone |
Flavonoids (flavonols) |
Fruits and vegetables (mainly of yellow or orange color) |
|
[105] [106] [72] [73] [41] [107] [102] [99] [108] [109] [110] |
| Epigallo-catechin gallate |  (2R,3R)-3′,4′,5,5′,7-Pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate |
Flavonoids (catechins) |
Green tea |
|
[27] [111] [66] [41] [112] [113] [114] [102] |
| Anthocyanins | 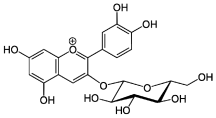 (2S,3R,4S,5S,6R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol chloride (Cyanidin-3-glucoside) |
Flavonoids (anthocyanins) |
Berries and flower corollas (in red, blue, or purple colors) |
|
[90] [115] [116] |
| Genistein |  4′,5,7-Trihydroxyisoflavone |
Flavonoids (isoflavone) |
Mainly Fabaceae plants (soy-beans in particular) |
|
[75] [77] [117] |
| Naringenin |  (2S)-4′,5,7-Trihydroxyflavan-4-one |
Flavonoids (flavanone) |
Citrus fruits (oranges, lemons, grapefruits, etc.) |
|
[118] [119] |
| Apigenin |  4′,5,7-Trihydroxyflavone |
Flavonoids (flavone) |
Celery, parsley, Lamiaceae plants |
|
[41] [120] [121] [122] |
| Luteolin | 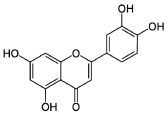 3′,4′,5,7-Tetrahydroxyflavone |
Flavonoids (flavone) |
Celery, carrot, parsley, broccoli, oranges, chamomile tea, and Lamiaceae plants (thyme, oregano, rosemary, etc.) |
|
[123] [89] [124] |
| Silybin |  Silybin A (2R,3R)-3,5,7-Trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydro-4H-chromen-4-one |
Flavonolignan (silymarin group) | Milk thistle (Silybum marianum) fruits. Silymarin is a flavonoid mixture in which silybin is the major one. |
|
[61] [125] [126] |
| Phlorotannins |  Tetrafucol A, [11,21:23,31:33,41-Quaterphenyl]-12,14,16,22,24,26,32,34,36,42,44,46-dodecol |
Oligomer of phloroglucinols (a fucol-type phlorotannin) |
Brown seaweeds |
|
[127] [70] [128] |
| Rosmarinic acid |  (2R)-3-(3,4-Dihydroxyphenyl)-2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}182propanoic acid |
Hydroxycinnamic acids | Mainly Lamiaceae plants (especially from the Nepetoideae subfamily) |
|
[129] [130] [131] |
| Hydroxytyrosol | 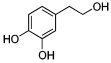 4-(2-Hydroxyethyl)benzene-1,2-diol |
Phenylethanoid (phenolic compound) |
Olive oil (in the form of oleuropein) |
|
[132] [133] |
| Chlorogenic acid |  (1S,3R,4R,5R)-3-{[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexane-1-carboxylic acid |
Hydroxycinnamic acids (phenolic compound) |
Coffee beans, peaches, eggplant, prunes |
|
[61] [134] |
| Caffecic acid |  3-(3,4-Dihydroxyphenyl)-2-propenoic acid3,4-Dihydroxycinnamic acid |
Hydroxycinnamic acids (phenolic compound) |
Coffee beans, Lamiaceae plants, etc. |
|
[61] [135] [136] |
| Ferulic acid |  (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid |
Hydroxycinnamic acids (phenolic compound) |
Mainly Apiaceae plants (Angelica sinensis, genus Ferula, etc.) |
|
[110] [136] [137] |
Berry-derived PPs are mainly obtained from the fleshy fruits of strawberry, blueberry, mulberry, currant, raspberry, blackberry, barberry, rosehip, and gooseberry [91]. Berries are rich sources of pigments, particularly anthocyanins (up to 5 g/kg), which are responsible for their red, blue, or purple colors. They also contain flavonols, phenolic acids, tannins, etc. Due to their ability to cross the blood-brain barrier, PPs with low molecular weight have beneficial antioxidant effects.
The potential benefits of PPs on human health make them the best micronutrients obtained from plant foods. These active principles possess excellent antioxidant and anti-inflammatory properties that ultimately favor the metabolic system of the human body and can avoid various chronic diseases (Figure 2 and Figure 3). Epidemiologic and related case studies on mouse and human diets demonstrate that daily intake of PPs can easily prevent and provide efficient treatment for severe metabolic diseases that may cause complicated health conditions.

Figure 2. Influences of PPs on different manifestations of MtS.

Figure 3. Overall mechanisms of action of PPs against oxidative stress and inflammation leading to MtS.
It could be concluded that a single source of PPs, not always and not everywhere, can fulfill an effective role. Generally, the benefits of PPs involve the mechanism of bioactive scavenger theory in which free radicals are potentially absorbed by these healthy compounds and converted into a stabilized complex [138].
Recent studies suggested that various phenolic compounds can be made artificially or with the help of natural supplements that can influence and terminate the growth of pathogens in the gut. Thus, a careful investigation is required to provide mechanistic studies of various phenolic compounds.
4. Effective Delivery of Polyphenols to the Target
People are particularly interested in dietary PPs as there is a growing belief that their constant intake is healthy for the body. However, before reaching the target tissues, ingested PPs are substantially degraded in the digestive system or other sites, reducing their beneficial effects. Furthermore, some PPs are photosensitive and rapidly oxidized into undesirable forms. Although polyphenolic substances have been found to have several benefits, their pharmaceutical use in humans is currently restricted due to these limitations [139][140][141][142]. In recent years, several attempts have been made for intact distribution or delivery of PPs to target organs and tissues. Conversion into inactive forms by microbes, pH, enzymes, and the blood-brain barrier are the most common obstacles encountered by PPs before reaching the target organ. To overcome these challenges, several delivery systems have been introduced. Nanoparticles, liposomes, and microemulsions are the major delivery systems for their effective biodistribution (Figure 2) [143][144]. The most investigated biodegradable and biocompatible polymeric materials that enclose polyphenolic compounds are nanomaterials. Nanoshells, nanocarriers, solid lipid nanoparticles, cyclodextrins, liposomes, and micelles are the most commonly used nanoparticle-mediated delivery systems for PPs [145][146]. There are several methods for delivering these nanoparticles: orally, intravenously, intraperitoneally, and transdermally. Due to membrane adhesion and permeability properties, nanocarriers can transport relatively high concentrations of PPs to the intestine, effectively maintain their integrity, and increase their effectiveness [147].
In an in vitro study, Mathew and colleagues showed that curcumin nanoparticles enclosed in PLGA (poly lactic-co-glycolic acid) attached to Aβ clusters facilitated their dissociation. This study opened the door to minimize the amyloid-plaque development in Alzheimer’s by delivering curcumin-PLGA nanoparticles across the blood-brain barrier [148]. In a rat model of 3-nitropropionic acid-induced Huntington’s disease, it was observed that curcumin-encapsulated solid lipid nanoparticles effectively reduced mitochondrial dysfunction [149]. A remarkable research study demonstrated that solid-liquid nanoparticles functionalized with the anti-transferrin receptor (OX26) monoclonal antibody offered a reliable carrier to deliver the resveratrol and grape skin extract to target the brain and treat neurodegenerative disease [150]. Oral administration of resveratrol-loaded PLGA nanocarrier showed enhanced resveratrol bioavailability [151]. In another recent study, resveratrol oral bioavailability was enhanced in Sprague Dawley rats by binding the galactose ligand (N-oleoyl-d-galactosamine) on the surface of the resveratrol-loaded PLGA nanoparticles [152].
The literature notably documented that using polyphenol-loaded nanocarriers has increased their antioxidant and anti-inflammatory effects [153][154][155][156][157]. Therefore, although there are several types of delivery systems that have been assessed through in vitro and in vivo studies for improved bioavailability and target delivery of PPs in metabolic disease, very few clinical trials involving humans have been conducted so far to assess the effect of different delivery systems for PP delivery to target tissues and organs in metabolic diseases. Future clinical studies should be conducted.
References
- Niewiadomska, J.; Gajek-Marecka, A.; Gajek, J.; Noszczyk-Nowak, A. Biological Potential of Polyphenols in the Context of Metabolic Syndrome: An Analysis of Studies on Animal Models. Biology 2022, 11, 559.
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12.
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20.
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645.
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057.
- Martín, M.A.; Ramos, S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods 2016, 27, 570–588.
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243.
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672.
- Zern, T.L.; Fernandez, M.L. Cardioprotective Effects of Dietary Polyphenols. J. Nutr. 2005, 135, 2291–2294.
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 724–728.
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464.
- Durazzo, A.; Lucarini, M. Extractable and Non-Extractable Antioxidants. Molecules 2019, 24, 1933.
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural Polyphenols in Metabolic Syndrome: Protective Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6110.
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423.
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2010, 108, 4531–4538.
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333.
- Dodevska, M.; Sobajic, S.; Djordjevic, B. Fibre and polyphenols of selected fruits, nuts and green leafy vegetables used in Serbian diet. J. Serb. Chem. Soc. 2015, 80, 21–33.
- Siroma, T.K.; Machate, D.J.; Zorgetto-Pinheiro, V.A.; Figueiredo, P.S.; Marcelino, G.; Hiane, P.A.; Bogo, D.; Pott, A.; Cury, E.R.J.; Guimarães, R.D.C.A.; et al. Polyphenols and ω-3 PUFAs: Beneficial Outcomes to Obesity and Its Related Metabolic Diseases. Front. Nutr. 2022, 8, 781622.
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 1865–1879.
- Tian, C.; Hao, L.; Yi, W.; Ding, S.; Xu, F. Polyphenols, Oxidative Stress, and Metabolic Syndrome. Oxidative Med. Cell. Longev. 2020, 2020, 7398453.
- Niess, A.M.; Dickhuth, H.H.; Northoff, H.; Fehrenbach, E. Free radicals and oxidative stress in exercise—Immunological aspects. Exerc. Immunol. Rev. 1999, 5, 22–56.
- Storz, G.; Imlayt, J.A. Oxidative stress. Curr. Opin. Microbiol. 1999, 2, 188–194.
- Kuczyńska-Wiśnik, D.; Matuszewska, E.; Furmanek-Blaszk, B.; Leszczyńska, D.; Grudowska, A.; Szczepaniak, P.; Laskowska, E. Antibiotics promoting oxidative stress inhibit formation of Escherichia coli biofilm via indole signalling. Res. Microbiol. 2010, 161, 847–853.
- Wright, G.D. On the Road to Bacterial Cell Death. Cell 2007, 130, 781–783.
- Martín, M.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850.
- Singhal, A. Early Nutrition and Long-Term Cardiovascular Health. Nutr. Rev. 2006, 64, 44–49.
- Leroux, C.; Brazeau, A.-S.; Gingras, V.; Desjardins, K.; Strychar, I.; Rabasa-Lhoret, R. Lifestyle and Cardiometabolic Risk in Adults with Type 1 Diabetes: A Review. Can. J. Diabetes 2014, 38, 62–69.
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pr. 2022, 2022, 9734738.
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts Through 2030. Am. J. Prev. Med. 2012, 42, 563–570.
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and their anti-obesity role mediated by the gut microbiota: A comprehensive review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388.
- Lyons, C.L.; Kennedy, E.B.; Roche, H.M. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients 2016, 8, 247.
- Grant, D.M. Detoxification pathways in the liver. J. Inherit. Metab. Dis. 1991, 14, 421–430.
- Sánchez, B.; Casalots-Casado, J.; Quintana, S.; Arroyo, A.; Martín-Fumadó, C.; Galtés, I. Fatal manganese intoxication due to an error in the elaboration of Epsom salts for a liver cleansing diet. Forensic Sci. Int. 2012, 223, e1–e4.
- opaciuk, A.; Łoboda, M. Global beauty industry trends in the 21st century. In Proceedings of the Management, Knowledge and Learning International Conference, Zadar, Croatia, 19–21 June 2013; pp. 19–21.
- Vivó-Sesé, I.; Pla, M. Bioactive Ingredients in Cosmetics. Anal. Cosmet. Prod. 2007, 380–389.
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2019, 173, 113719.
- Cattuzzato, L.; Dumont, S.; Le Gelebart, E.; Loeuil, J. Obtaining an Extract from Brown Algae Gametophytes, and Use of Said Extract as a Cosmetic Anti-Aging Active Principle. U.S. Patent 10206869, 2019.
- Abdelmoez, W.; Abdelfatah, R. Therapeutic Compounds From Plants Using Subcritical Water Technology. Water Extr. Bioact. Compd. 2017, 51–68.
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.-R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344.
- Bjørklund, G.; Dadar, M.; Martins, N.; Chirumbolo, S.; Goh, B.H.; Smetanina, K.; Lysiuk, R. Brief Challenges on Medicinal Plants: An Eye-Opening Look at Ageing-Related Disorders. Basic Clin. Pharmacol. Toxicol. 2018, 122, 539–558.
- Do, Y.-K.; Kim, J.-M.; Chang, S.-M.; Hwang, J.-H.; Kim, W.-S. Enhancement of polyphenol bio-activities by enzyme reaction. J. Mol. Catal. B: Enzym. 2009, 56, 173–178.
- Obrenovich, M.E.; Nair, N.G.; Beyaz, A.; Aliev, G.; Reddy, V.P. The Role of Polyphenolic Antioxidants in Health, Disease, and Aging. Rejuvenation Res. 2010, 13, 631–643.
- Ratz-Łyko, A.; Arct, J.; Majewski, S.; Pytkowska, K. Influence of Polyphenols on the Physiological Processes in the Skin. Phytother. Res. 2015, 29, 509–517.
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2009, 38, 96–109.
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68.
- Catalgol, B. Proteasome and Cancer. Prog. Mol. Biol. Transl. Sci. 2012, 109, 277–293.
- Biglu, M.-H.; Ghavami, M.; Biglu, S. Cardiovascular diseases in the mirror of science. J. Cardiovasc. Thorac. Res. 2016, 8, 158–163.
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56.
- Li, L.; Zhou, X.; Li, N.; Sun, M.; Lv, J.; Xu, Z. Herbal drugs against cardiovascular disease: Traditional medicine and modern development. Drug Discov. Today 2015, 20, 1074–1086.
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315.
- Mehmood, A.; Usman, M.; Patil, P.; Zhao, L.; Wang, C. A review on management of cardiovascular diseases by olive polyphenols. Food Sci. Nutr. 2020, 8, 4639–4655.
- Kishimoto, Y.; Tani, M.; Kondo, K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013, 67, 532–535.
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904.
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. BioFactors 2021, 48, 255–273.
- Osawa, T. Protective role of dietary polyphenols in oxidative stress. Mech. Ageing Dev. 1999, 111, 133–139.
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250.
- Sarkhosh-Khorasani, S.; Sangsefidi, Z.S.; Hosseinzadeh, M. The effect of grape products containing polyphenols on oxidative stress: A systematic review and meta-analysis of randomized clinical trials. Nutr. J. 2021, 20, 25.
- Zamani-Garmsiri, F.; Emamgholipour, S.; Fard, S.R.; Ghasempour, G.; Ahvazi, R.J.; Meshkani, R. Polyphenols: Potential anti-inflammatory agents for treatment of metabolic disorders. Phytother. Res. 2021, 36, 415–432.
- Chen, L.; Pu, Y.; Xu, Y.; He, X.; Cao, J.; Ma, Y.; Jiang, W. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res. Int. 2022, 157, 111202.
- Pérez, Y.Y.; Jiménez-Ferrer, E.; Zamilpa, A.; Hernández-Valencia, M.; Alarcón-Aguilar, F.J.; Tortoriello, J.; Román-Ramos, R. Effect of a Polyphenol-Rich Extract from Aloe vera Gel on Experimentally Induced Insulin Resistance in Mice. Am. J. Chin. Med. 2007, 35, 1037–1046.
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape Polyphenols Prevent Fructose-Induced Oxidative Stress and Insulin Resistance in First-Degree Relatives of Type 2 Diabetic Patients. Diabetes Care 2013, 36, 1454–1461.
- Anderson, R.A. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc. Nutr. Soc. 2008, 67, 48–53.
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.; Hasan, P.M.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579.
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045.
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Portillo, M.P.; Moreno, J.J.; et al. Polyphenol Levels Are Inversely Correlated with Body Weight and Obesity in an Elderly Population after 5 Years of Follow Up (The Randomised PREDIMED Study). Nutrients 2017, 9, 452.
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340.
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589.
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Lysiuk, R. Flavonoids as detoxifying and pro-survival agents: What’s new? Food Chem. Toxicol. 2017, 110, 240–250.
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234.
- Hollman, P.C.; De Vries, J.H.; Van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282.
- Bugianesi, R.; Catasta, G.; Spigno, P.; D’Uva, A.; Maiani, G. Naringenin from Cooked Tomato Paste Is Bioavailable in Men. J. Nutr. 2002, 132, 3349–3352.
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy Isoflavone Aglycones Are Absorbed Faster and in Higher Amounts than Their Glucosides in Humans. J. Nutr. 2000, 130, 1695–1699.
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689.
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863.
- Soory, M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: Potential for therapeutic targeting. Infect. Disord. Drug Targets 2009, 9, 400–414.
- Wang, S.-T.; Cui, W.-Q.; Pan, D.; Jiang, M.; Chang, B.; Sang, L.-X. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J. Gastroenterol. 2020, 26, 562–597.
- Afaq, F. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini-Rev. Med. Chem. 2011, 11, 1200–1215.
- De Oca, M.K.M.; Pearlman, R.L.; McClees, S.F.; Strickland, R.; Afaq, F. Phytochemicals for the Prevention of Photocarcinogenesis. Photochem. Photobiol. 2017, 93, 956–974.
- De Souza, P.L.; Russell, P.J.; Kearsley, J.H.; Howes, L.G. Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr. Rev. 2010, 68, 542–555.
- Nagata, C. Factors to Consider in the Association Between Soy Isoflavone Intake and Breast Cancer Risk. J. Epidemiol. 2010, 20, 83–89.
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821.
- Reis, J.P.; Loria, C.M.; Steffen, L.M.; Zhou, X.; van Horn, L.; Siscovick, D.S.; JacobsJr, D.R.; Carr, J.J. Coffee, Decaffeinated Coffee, Caffeine, and Tea Consumption in Young Adulthood and Atherosclerosis Later in Life. Arter. Thromb. Vasc. Biol. 2010, 30, 2059–2066.
- Lin, X.; Zhang, I.; Li, A.; Manson, J.E.; Sesso, H.D.; Wang, L.; Liu, S. Cocoa Flavanol Intake and Biomarkers for Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2016, 146, 2325–2333.
- Larsson, S.C.; Åkesson, A.; Gigante, B.; Wolk, A. Chocolate consumption and risk of myocardial infarction: A prospective study and meta-analysis. Heart 2016, 102, 1017–1022.
- Pourhabibi-Zarandi, F.; Rafraf, M.; Zayeni, H.; Asghari-Jafarabadi, M.; Ebrahimi, A. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2022, 36, 1797–1806.
- Ross, J.A.; Kasum, C.M. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Bouyahya, A.; El Omari, N.; EL Hachlafi, N.; El Jemly, M.; Hakkour, M.; Balahbib, A.; El Menyiy, N.; Bakrim, S.; Mrabti, H.N.; Khouchlaa, A.; et al. Chemical Compounds of Berry-Derived Polyphenols and Their Effects on Gut Microbiota, Inflammation, and Cancer. Molecules 2022, 27, 3286.
- Labinskyy, N.; Csiszar, A.; Veress, G.; Stef, G.; Pacher, P.; Oroszi, G.; Wu, J.; Ungvari, Z. Vascular Dysfunction in Aging: Potential Effects of Resveratrol, an Anti- Inflammatory Phytoestrogen. Curr. Med. Chem. 2006, 13, 989–996.
- Das, S.D.A.D.K.; Das, D.K. Anti-Inflammatory Responses of Resveratrol. Inflamm. Allergy-Drug Targets 2007, 6, 168–173.
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–125.
- El-Din, S.H.S.; El-Lakkany, N.; Salem, M.B.; Hammam, O.; Saleh, S.; Botros, S.S. Resveratrol mitigates hepatic injury in rats by regulating oxidative stress, nuclear factor-kappa B, and apoptosis. J. Adv. Pharm. Technol. Res. 2016, 7, 99–104.
- Matsuno, Y.; Atsumi, Y.; Alauddin, M.; Rana, M.M.; Fujimori, H.; Hyodo, M.; Shimizu, A.; Ikuta, T.; Tani, H.; Torigoe, H.; et al. Resveratrol and its Related Polyphenols Contribute to the Maintenance of Genome Stability. Sci. Rep. 2020, 10, 5388.
- Rytsyk, O.; Soroka, Y.; Shepet, I.; Vivchar, Z.; Andriichuk, I.; Lykhatskyi, P.; Fira, L.; Nebesna, Z.; Kramar, S.; Lisnychuk, N. Experimental Evaluation of the Effectiveness of Resveratrol as an Antioxidant in Colon Cancer Prevention. Nat. Prod. Commun. 2020, 15, 1934578X2093274.
- Hou, C.-Y.; Tain, Y.-L.; Yu, H.-R.; Huang, L.-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535.
- Chan, M.M.-Y.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem. Pharmacol. 2000, 60, 1539–1548.
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3149223.
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in Cancer Chemoprevention: Molecular Targets, Pharmacokinetics, Bioavailability, and Clinical Trials. Arch. Pharm. 2010, 343, 489–499.
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206.
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440.
- Osali, A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 2020, 12, 26.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618.
- Kannappan, S.; Anuradha, C.V. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J. Med. Res. 2009, 129, 401–408.
- Lin, R.; Piao, M.; Song, Y.; Liu, C. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. 2020, 2020, 9242601.
- Stechyshyn, I.; Pavliuk, B. The Quercetine Containing Drugs in Pharmacological Correction of Experimental Diabetes with Myocardial Injury. Romanian J. Diabetes Nutr. Metab. Dis. 2019, 26, 393–399.
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796.
- Little, R.; Houghton, M.; Carr, I.M.; Wabitsch, M.; Kerimi, A.; Williamson, G. The Ability of Quercetin and Ferulic Acid to Lower Stored Fat is Dependent on the Metabolic Background of Human Adipocytes. Mol. Nutr. Food Res. 2020, 64, e2000034.
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2008, 101, 886–894.
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135.
- Demeule, M.; Michaud-Levesque, J.; Annabi, B.; Gingras, D.; Boivin, D.; Jodoin, J.; Lamy, S.; Bertrand, Y.; Beliveau, R. Green Tea Catechins as Novel Antitumor and Antiangiogenic Compounds. Curr. Med. Chem. Agents 2002, 2, 441–463.
- Zhang, M.; Huang, J.; Xie, X.; Holman, C.D.J. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int. J. Cancer 2008, 124, 1404–1408.
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89.
- Naseri, R.; Farzaei, F.; Haratipour, P.; Nabavi, S.F.; Habtemariam, S.; Farzaei, M.H.; Khodarahmi, R.; Tewari, D.; Momtaz, S. Anthocyanins in the Management of Metabolic Syndrome: A Pharmacological and Biopharmaceutical Review. Front. Pharmacol. 2018, 9, 1310.
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22.
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417.
- Kumar, S.R.; Ramli, E.S.M.; Nasir, N.A.A.; Ismail, N.H.M.; Fahami, N.A.M. Preventive Effect of Naringin on Metabolic Syndrome and Its Mechanism of Action: A Systematic Review. Evidence-Based Complement. Altern. Med. 2019, 2019, 9752826.
- Xu, Y.; Li, X.; Wang, H. Protective Roles of Apigenin Against Cardiometabolic Diseases: A Systematic Review. Front. Nutr. 2022, 9, 875826.
- Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Dec, K.; Styburski, D.; Nowakowska, A.; Gutowska, I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere 2019, 240, 124901.
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305.
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Augustin, O.M.; de Medina, F.S. Effects of Flavonoids and other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362.
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454.
- Zaulet, M.; Kevorkian, S.E.M.; Dinescu, S.; Cotoraci, C.; Suciu, M.; Herman, H.; Buburuzan, L.; Badulescu, L.; Ardelean, A.; Hermenean, A. Protective effects of silymarin against bisphenol A-induced hepatotoxicity in mouse liver. Exp. Ther. Med. 2017, 13, 821–828.
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191.
- Chen, L.; Liu, R.; He, X.; Pei, S.; Li, D. Effects of brown seaweed polyphenols, a class of phlorotannins, on metabolic disorders via regulation of fat function. Food Funct. 2021, 12, 2378–2388.
- Barbosa, M.; Valentão, P.; Andrade, P.B. Polyphenols from Brown Seaweeds (Ochrophyta, Phaeophyceae): Phlorotannins in the Pursuit of Natural Alternatives to Tackle Neurodegeneration. Mar. Drugs 2020, 18, 654.
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and Pharmacological Screening of a Monarda fistulosa L. dry Extract Based on a Hydrodistilled Residue By-Product. Front. Pharmacol. 2021, 12, 563436.
- Nyandwi, J.B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic Acid Exhibits a Lipid-Lowering Effect by Modulating the Expression of Reverse Cholesterol Transporters and Lipid Metabolism in High-Fat Diet-Fed Mice. Biomolecules 2021, 11, 1470.
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid–Human Pharmacokinetics and Health Benefits. Planta Med. 2020, 87, 273–282.
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306.
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128.
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508.
- Kadar, N.N.M.A.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490.
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2017, 124, 410–417.
- Senaphan, K.; Kukongviriyapan, U.; Sangartit, W.; Pakdeechote, P.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E.; Kukongviriyapan, V. Ferulic Acid Alleviates Changes in a Rat Model of Metabolic Syndrome Induced by High-Carbohydrate, High-Fat Diet. Nutrients 2015, 7, 6446–6464.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Reis, F.; Madureira, A.R.; Nunes, S.; Campos, D.A.; Fernandes, J.C.; Marques, C.; Zuzarte, M.; Gullón, B.; Rodríguez-Alcalá, L.M.; Calhau, C.; et al. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, ume 11, 3621–3640.
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem. Pharmacol. 2018, 154, 303–317.
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of Polyphenols and Flavonoids in the Era of Precision Medicine. Mol. Pharm. 2017, 14, 2861–2863.
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051.
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397.
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2019, 60, 2083–2097.
- Ghurghure, S.M.; Pathan, M.S.A.; Surwase, P.R. Nanosponges: A novel approach for targeted drug delivery system. Int. J. Chem. Studies 2018, 2, 2.
- Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; Di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols nanoencapsulation for therapeutic applications. J. Biomol. Res. Ther. 2016, 5, 2.
- Tie, S.; Tan, M. Current Advances in Multifunctional Nanocarriers Based on Marine Polysaccharides for Colon Delivery of Food Polyphenols. J. Agric. Food Chem. 2022, 70, 903–915.
- Mathew, A.; Aravind, A.; Fukuda, T.; Hasumura, T.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin nanoparticles-a gateway for multifaceted approach to tackle Alzheimer’s disease. In Proceedings of the 2011 11th IEEE International Conference on Nanotechnology, Portland, OR, USA, 15–18 August 2011; pp. 833–836.
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin Nanoparticles Attenuate Neurochemical and Neurobehavioral Deficits in Experimental Model of Huntington’s Disease. NeuroMolecular Med. 2013, 16, 106–118.
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277.
- Singh, G.; Pai, R.S. Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin. Drug Deliv. 2014, 11, 647–659.
- Siu, F.Y.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 2018, ume 13, 4133–4144.
- Lee, C.-W.; Yen, F.-L.; Huang, H.-W.; Wu, T.-H.; Ko, H.-H.; Tzeng, W.-S.; Lin, C.-C. Resveratrol Nanoparticle System Improves Dissolution Properties and Enhances the Hepatoprotective Effect of Resveratrol through Antioxidant and Anti-Inflammatory Pathways. J. Agric. Food Chem. 2012, 60, 4662–4671.
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 2014, 25, 485102.
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629.
- Zhang, W.; Liu, Y.; Zhang, X.; Wu, Z.; Weng, P. Tea polyphenols-loaded nanocarriers: Preparation technology and biological function. Biotechnol. Lett. 2022, 44, 387–398.
- Beconcini, D.; Felice, F.; Fabiano, A.; Sarmento, B.; Zambito, Y.; Di Stefano, R. Antioxidant and Anti-Inflammatory Properties of Cherry Extract: Nanosystems-Based Strategies to Improve Endothelial Function and Intestinal Absorption. Foods 2020, 9, 207.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
25 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




