
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luiza Sisdelli | + 5562 word(s) | 5562 | 2020-11-06 03:28:11 | | | |
| 2 | Luiza Sisdelli | -25 word(s) | 5537 | 2020-11-17 20:10:55 | | | | |
| 3 | Rita Xu | -3134 word(s) | 2428 | 2020-11-18 03:15:56 | | |
Video Upload Options
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer in both adult and pediatric populations, occurring more commonly in women at ages 50-59. PTC is characterized by the presence of cells arranged into papillae, presenting clear or ground-glass nuclei. It is further subdivided based on histological variants, such as the classic (CVPTC), follicular (FVPTC), solid (SVPTC), and diffuse sclerosing (DSVPTC) variants.
1. Introduction
Thyroid carcinoma is the most common malignancy of the endocrine system in adult and pediatric populations. In adults, this type of cancer is increasing dramatically in both men and women, with an average annual percentage change of 5.4% and 6.5%, respectively. It is projected to take the place of colon cancer and become the fourth leading cancer diagnosis in both sexes (second for women) by 2030 [1][2]. Thyroid cancer presents with relatively stable mortality, but it has been increasing globally since the 1970s [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18]. It is estimated that by the end of the year 2020, thyroid cancer will claim the lives of 2180 of the 52,890 new projected cases, corresponding to 0.4% of all cancer related deaths and 2.9% of new cancers throughout the world, respectively [19].
In the pediatric population (≤18 y.o. at diagnosis), thyroid cancer corresponds to 6% of all pediatric cancers (2012–2016 data) [20][21]. Even though there is no indication of ethnic or race susceptibility in pediatric thyroid cancer, there has been a prevalence related to increasing age range, i.e., ages 5–9, 10–14, and 15–19 showing a prevalence of 10,000, 80,000, and 310,000, respectively [19]. Considering gender, the prevalence is observed above age 10, and females are the most affected (more precisely between ages 13 and 19) [22][23][24]. Overall, among adolescents (ages 15–19), thyroid carcinoma is the eighth most diagnosed cancer [25][26].
Differentiated thyroid carcinoma (DTC) originates in the follicular cells of the thyroid and is the most common type (80–90%) of thyroid malignancy [27]. DTC is classified into follicular thyroid carcinoma (FTC) and papillary thyroid carcinoma (PTC). This classification relies on histological differences and the different metastatic dissemination routes between the two subtypes. FTC accounts for 10% of all DTC and is characterized by the presence of small follicles and the absence of ground-glass nuclei (characteristic of PTC). PTC encompasses the remaining 80–90% of all DTC and is characterized mainly by the presence of cells arranged into papillae, presenting clear or ground-glass nuclei. PTC is further subdivided based on histological variants, such as the classic (CVPTC), follicular (FVPTC), solid (SVPTC), and diffuse sclerosing (DSVPTC) variants. Among these variants, children under the age of 10 seem to be unaffected by the most common type, CVPTC, found in adults [26].
Oddly enough, regardless of studies suggesting that clinical presentation, pathophysiology, and long-term outcomes diverge between pediatric and adult populations, clinical assessment and treatment recommendations used in pediatric thyroid cancer are the same as those implemented for adults [21][28][29][30][31][32][33][34][35][36][37]. Looking closely, PTC differences in these populations could be explained by the distinct genetic alterations observed in the PTC of adults and children.
2. Epidemiology and Pathogenesis
According to the Surveillance, Epidemiology, and End Results (SEER) database, the incidence of PTC in adults increased between 2000 and 2017, from 7.9 to 16.9 per 100,000, compared to 0.6 to 1.0 per 100,000 in the pediatric group (Figure 1, bottom lines) [19]. Remarkably, as represented in Figure 1, PTC in adults occurs more commonly in women at ages 50–59 (37.3 × 100,000) and to a lower rate (17.3 × 100,000) in men, for whom the peak of incidence occurs at ages 65–69. Looking at the pediatric population, this difference in gender starts just above age 10, i.e., 0.3 per 100,000 for boys and 1.2 per 100,000 for girls (ages 10–14), with increasing distinction above age 15, where the incidence increases to 0.9 per 100,000 for boys vs. 5.3 per 100,000 for girls (ages 15–19) (Figure 1) [19].
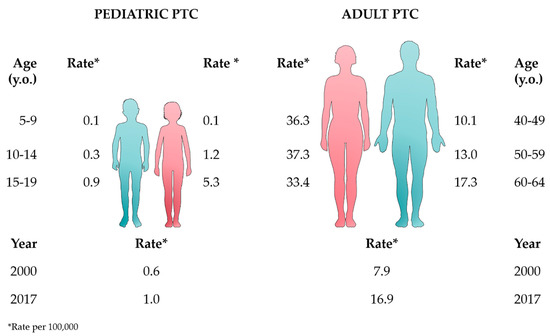
Figure 1. Epidemiologic data from the Surveillance, Epidemiology, and End Results (SEER) database (2000–2017) [19] comparing the rates of pediatric and adult papillary thyroid carcinoma (PTC) according to age, gender, and year. This figure was created using images from Servier Medical Art (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
The reasons associated with this progressive trend are controversial. Several authors propose that the increase in cases is due to better diagnosis, since this tendency coincides with the increased use of high resolution imaging techniques [3][8][38][39][40]. Others suggest that the reason is multifactorial and is related to environmental and lifestyle factors. Diet, obesity, smoking, drinking, sex hormones, iodine deficiency, and a history of benign nodules in the family may contribute to the increased PTC incidence [41][42][43][44].
In the pediatric population, the only consolidated risk factor is the exposure to radiation in childhood, either environmental or as part of radiotherapy for a prior malignancy or treatment for another benign condition [45][46]. In fact, several studies have demonstrated a much greater sensitivity to radiation in children compared with adults. In the past 60 years, the incidence of pediatric cases peaked twice. The first peak occurred in the 1950s, due to the use of external irradiation of the head and neck to treat children with various benign non-thyroid disorders such as the enlargement of the thymus, tinea capitis, adenoids or neck lymph nodes, acne, eczema, otitis, and others [45][46][47]. The use of external radiation therapy on the neck essentially ended in the early 1960s, when a cause–effect relationship between radiation exposure and PTC was established [45][46][47]. However, radiation is still used in clinical practice to treat different types of cancers. Radiation-induced malignancies, such as thyroid cancer, are late complications of radiotherapy treatment, with increased frequency among survivors of both pediatric and adult cancers [48].
Although there was a sharp increase in the incidence of childhood thyroid cancer in the Minsk and Kiev centers 4–5 years after the explosion of the Chernobyl Nuclear Power Plant reactors in 1986, the second peak of incidence occurred just 10 years after the accident in some Eastern European countries. The high-risk group comprised children under the age of four at the time of exposure. Consequently, in this second peak, the majority of clinically evident tumors were present in children ~10–14 years old [22][24][26]. Regarding the Fukushima Daiichi nuclear disaster (March 2011), it is still unclear whether the radiation released after the nuclear accident could be considered the cause of a “third peak” of thyroid cancer incidence in the pediatric group, or if a potential peak is just an artefactual result of the intense screening of this population. The adverse effects of the Fukushima accident might have been partially mitigated by the measures taken, i.e., evacuation from most of the contaminated areas and the recommendation of a low iodine alimentary intake and food restrictions, which could have reduced the uptake of iodine-131. With an average radiation dose of < 1 mSv for the majority of Fukushima residents and a maximum of 30 mSv in few cases from evacuated sites near to the Fukushima Nuclear Power Plant, the first round of thyroid ultrasound screening, performed in all affected children under age 18, showed no clear evidence of a thyroid cancer increase due to radiation exposure [49]. Other studies have found a significant dose–response relationship between the rate of thyroid cancer detection and the external effective dose-rate in both the first and second rounds of the thyroid ultrasound screening [50][51]. The third and the fourth rounds of examinations are still in progress and further data may bring more light into this issue. Interestingly, as discussed in the next section, the pathological findings observed in the Fukushima PTC cases are similar to the pediatric cases found in non-exposed areas and to the mutational profile reported in adult PTC [52][53].
3. Clinical Features, Prognosis, and Treatment
The differences in clinical presentation and outcomes between pediatric and adult PTC are significant [54][55][56]. Compared to those of adults, pediatric thyroid cancers usually present with more advanced disease. Though the recurrence rates are higher than in adults, pediatric PTC has a better long-term outcome, with minimal or no mortality in most cases [54][57][58]. Pediatric PTC typically manifests as a palpable thyroid nodule/tumor, with or without cervical lymphadenopathy [59]. Although rare in children and adolescents, the presence of nodules in pediatric patients is clinically important. Thyroid nodules are associated with increased malignancy compared to adults (26% vs. 5%) [60][61]. Additionally, the mean tumor size is typically larger in pediatric patients. Hay et al. (2018) studied 190 children and 4242 adults consecutively treated during 1936–2015. They described a mean tumor size of 2.56 cm (median = 2.15 cm) in children vs. 1.94 cm (median = 1.5 cm) in adult patients [56]. Papillary thyroid microcarcinoma (≤1 cm) accounts for ~40% of tumors in adults [62] and represents < 10% of pediatric PTC [63]. This difference is probably due to the common practice of thyroid cancer screening in adults and the early detection of smaller tumors [64].
Furthermore, when compared to adults, childhood thyroid carcinomas are more frequently locally invasive. The metastatic involvement of regional lymph nodes at diagnosis was reported in ~50–75% of pediatric cases (Table 1) [55][56][65][66], compared to ~20–40% in adult PTC [56][66]. With respect to distant metastasis, data available from the literature also demonstrate a high frequency in pediatric vs. adult PTC patients [56]. The lungs are the most common site of distant metastases in all age groups, occurring in ~5–16% of pediatric PTC (Table 1) and in 2–4% of adults [54][55][56]. Liu et al. (2019) investigated the occurrence of factors influencing distant metastasis in pediatric thyroid cancer and identified the age at diagnosis as an important factor, with distant metastasis occurring in 1.73% of patients aged 15 and above, and in 6.73% of patients under the age of 15 [67].
Table 1. Clinical pathological features of pediatric PTC.
| Reference | n | Distant Met. (%) | LN Met. (%) | Mean Age (y.o.) |
Gender F:M |
Mean size (cm) |
Mean Follow-up (years) | % NED | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Zimmerman et al. [29] | 58 | 6.9 | 89.7 | < 17 | 2.2: 1 | 3.1 | 26.7 | 52 | 14%* |
| Dottorini et al. [68] | 85 | 18.8 | 60 | 14.7 | 2.86: 1 | X | 9.25 | 63.5 | 0 |
| Kuo et al. [69] | 77 | 18 | 6.4 | 12.9 | 3.3: 1 | 6.93 | 8.2 | 89.6 | 0 |
| Vaisman et al. [70] | 65 | 29.2 | 61.5 | 14 | 3: 1 | 2.99 | 12.6 | 50.8 | 0 |
| Fridman et al. [71] | 94 | 20 | 66 | 15.1 | 3: 1 | 1.2 | 4.2 | 97 | 0 |
| Pires et al. [72] | 118 | 26.9 | 67.3 | 13.3 | 2.6: 1 | 2.5 | 8 | 63.5 | 0 |
| Cordioli et al. [73] | 38 | 26.3 | 73.7 | 11.8 | 3.2: 1 | 2.6 | 7.8 | 54.1 | 0 |
| Poyrazoğlu et al. [74] | 75 | 13.3 | 45.3 | 12.4 | 2.1: 1 | 2.2 | 4.3 | 65.3 | 1 patient |
| Hampson et al. [75] | 62 | 19.3 | 46.7 | 13.8 | 2.5: 1 | 2.3 | 3.6 | 59.6 | Not reported |
| Galuppini et al. [76] | 59 | 20.8 | 51 | 14.4 | 2.7: 1 | 2.0 | 5.9 | 66.7 | Not reported |
Despite the higher rate of disease recurrence when compared to adults, overall survival is higher in pediatric PTC [29][77]. Mazzaferri et al. (2001) [78], in a series of 16.6 years’ follow-up, found a disease recurrence rate of ~40% in patients under the age of 20 and ~20% in patients above the age of 20. Additionally, Demidchik et al. (2006) [79], with a cohort of 741 patients, found a survival rate of 99.3% at age 5 and 98.5% at age 10. Lazar et al. (2009) [80] demonstrated that patients under the age of 10, mainly pre-pubertal patients, presented a worse prognosis than older ones or those in more advanced puberty stages. It seems that large tumors (>2 cm), extra-thyroidal extension, and younger age are factors associated with worse prognosis. However, the ideal cut-off for age and pubertal status awaits future investigation. The same is true for gender, which two studies showed to be an important prognostic factor [70][72], whereas another study showed no significance [67].
PTC treatment is based on the combination of three therapeutic modalities: surgery, radioiodine therapy, and hormone replacement with levothyroxine. Surgery can range from lobectomy to total thyroidectomy, accompanied by cervical lymphadenectomy. The extent of thyroid surgery for adult PTC patients has shifted in a more conservative direction in most recent guidelines [30]. Since then, lobectomy has been an acceptable surgical treatment for low-risk tumors without extrathyroidal extension or clinical lymph node metastases. However, the American Thyroid Association (ATA) management guidelines for children with PTC recommend total thyroidectomy for the majority of children [21][30]. The rationale for this approach is based on an increased incidence of bilateral and multi-focal disease in pediatric patients. It consists of the dissection of the central cervical compartment, with the removal of lymph nodes and adjacent tissues suspected to present metastasis. Modified lateral cervical dissection is indicated in cases of metastasis to lateral lymph nodes. The main surgical complications include persistent hypoparathyroidism and injury to the recurrent laryngeal nerve, which can cause hoarseness to complete closure of the vocal cords, requiring a definitive tracheostomy [81][82]. Fridman et al. (2019) [83] have reported a number of complications of thyroid surgery in childhood PTC. However, they concluded that prophylactic neck dissections should be recommended in children and adolescents due to the high rates of node metastases. On the other hand, to avoid surgical morbidity, Francis et al. (2015) [21] proposed that surgery for pediatric patients should take into account the risk stratification variables, in which patients are divided into a low, intermediate, and high risk of recurrence.
After total or almost total thyroidectomy, the volume of the remaining gland must be <2 mL at cervical ultrasound, performed up to 1 month after surgery [77][84]. Interestingly, even after total thyroid removal, with no thyroid detected by ultrasound, radioiodine (RAI) uptake in the thyroid bed occurs [85]. This phenomenon is usually attributed to remaining thyroid cells. However, since multifocality and metastasis are more common in the pediatric age group, the possibility that such foci still have malignant cells cannot be ruled out. Despite this, most societies recommend the ablation of reminiscent tissue in the majority of pediatric patients [21]. The pediatric recommendations regarding indications for RAI are still controversial. The National Comprehensive Cancer Network for adults suggests clinical features including tumor size >2–4 cm, gross extrathyroidal extension, and extensive regional nodal involvement as indicators for adjuvant RAI [86]. The guidelines for children recommend an individualized approach using post-operative thyroid-stimulating hormone (TSH)-stimulated thyroglobulin levels to determine who should receive adjuvant RAI [21]. There is no consensus in the calculation of the appropriate dose of iodine-131 (131I) for pediatric patients, since both body weight and body surface area methods are used. Whole body 131I dosimetry can also be used in patients with extensive metastases [87]. The success rate of ablation is significantly lower in patients who have undergone less extensive surgery, whether they are children or adults [22][78][84].
Successful ablation is usually defined as the absence of uptake or uptake of less than 0.1–1%, as detected by means of a total body scintigraphy performed 6–12 months after the procedure [85][88][89], accompanied by markedly decreased or undetectable serum thyroglobulin, and suboptimal TSH stimulus, all happening at the same time [77][78][88]. In most cases, one dose of radiodine therapy is able to achieve these goals [85], if not, the procedure may be repeated no earlier than 12 months after the first attempt [88][89]. The ablation should also be followed by a total body scintigraphy (post-therapeutic whole-body scan), performed ~5–7 days after the administration of the radioiodine, in order to detect or confirm the presence of functional metastases.
Lastly, thyroid hormone replacement, the third treatment modality, involves the oral use of levothyroxine. This modality is called suppressive therapy with thyroid hormone when a supraphysiological dose is used in order to keep serum TSH levels below the lower reference limit, reducing the risk of TSH-induced tumor growth or proliferation [90]. In children and adolescents, there are several studies guaranteeing the effectiveness and safety of this type of replacement, as long as it is carefully controlled, particularly regarding the patient’s final height [66][77][91]. The actual recommendation is to keep TSH suppressed as needed [21]. Possible side effects of long-term suppressive therapy, documented in adults, include osteoporosis [82] and cardiovascular diseases, especially left ventricular hypertrophy [92][93]. Regarding fertility, some studies suggest that radioiodine may affect testicular and ovarian function, at least temporarily [94][95][96].
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. J. Cancer 2019, 144, 1941–1953, doi:10.1002/ijc.31937.
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921.
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167, doi:10.1001/jama.295.18.2164.
- Lise, M.; Franceschi, S.; Buzzoni, C.; Zambon, P.; Falcini, F.; Crocetti, E.; Serraino, D.; Iachetta, F.; Zanetti, R.; Vercelli, M.; et al. Changes in the Incidence of Thyroid Cancer Between 1991 and 2005 in Italy: A Geographical Analysis. Thyroid 2012, 22, 27–34, doi:10.1089/thy.2011.0038.
- Keinan-Boker, L.; Silverman, B.G. Trends of Thyroid Cancer in Israel: 1980–2012. Rambam Maimonides Med. J. 2016, 7, e0001, doi:10.5041/rmmj.10228.
- Lubina, A.; Cohen, O.; Barchana, M.; Liphshiz, I.; Vered, I.; Sadetzki, S.; Karasik, A. Time trends of incidence rates of thyroid cancer in Israel: What might explain the sharp increase. Thyroid 2006, 16, 1033–1040, doi:10.1089/thy.2006.16.1033.
- Wang, Y.; Wang, W. Increasing incidence of thyroid cancer in Shanghai, China, 1983–2007. Asia-Pac. J. Public Health 2015, 27, NP223–NP229, doi:10.1177/1010539512436874.
- Ahn, H.S.; Kim, H.J.; Welch, G. Korea’s Thyroid-Cancer “Epidemic”—Screening and Overdiagnosis. Engl. J. Med. 2014, 371, 1765–1767.
- Veiga, L.H.S.; Neta, G.; Aschebrook-Kilfoy, B.; Ron, E.; Devesa, S.S. Thyroid cancer incidence patterns in Sao Paulo, Brazil, and the U.S. SEER program, 1997–2008. Thyroid 2013, 23, 748–757, doi:10.1089/thy.2012.0532.
- Sierra, M.S.; Soerjomataram, I.; Forman, D. Thyroid cancer burden in Central and South America. Cancer Epidemiol. 2016, 44, S150–S157, doi:10.1016/j.canep.2016.07.017.
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791, doi:10.1158/1055-9965.EPI-08-0960.
- Kent, W.D.T.; Hall, S.F.; Isotalo, P.A.; Houlden, R.L.; George, R.L.; Groome, P.A. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 2007, 177, 1357–1361, doi:10.1503/cmaj.061730.
- Liu, S.; Semenciw, R.; Ugnat, A.M.; Mao, Y. Increasing thyroid cancer incidence in Canada, 1970–1996: Time trends and age-period-cohort effects. J. Cancer 2001, 85, 1335–1339, doi:10.1054/bjoc.2001.2061.
- Uhry, Z.; Colonna, M.; Remontet, L.; Grosclaude, P.; Carré, N.; Couris, C.M.; Velten, M. Estimating infra-national and national thyroid cancer incidence in France from cancer registries data and national hospital discharge database. J. Epidemiol. 2007, 22, 607–614, doi:10.1007/s10654-007-9158-6.
- Colonna, M.; Uhry, Z.; Guizard, A.V.; Delafosse, P.; Schvartz, C.; Belot, A.; Grosclaude, P. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015, 39, 511–518, doi:10.1016/j.canep.2015.04.015.
- Reynolds, R.M.; Weir, J.; Stockton, D.L.; Brewster, D.H.; Sandeep, T.C.; Strachan, M.W.J. Changing trends in incidence and mortality of thyroid cancer in Scotland. Endocrinol. (Oxf.) 2005, 62, 156–162, doi:10.1111/j.1365-2265.2004.02187.x.
- Smailyte, G.; Miseikyte-Kaubriene, E.; Kurtinaitis, J. Increasing thyroid cancer incidence in Lithuania in 1978–2003. BMC Cancer 2006, 6, doi:10.1186/1471-2407-6-284.
- Pandeya, N.; McLeod, D.S.; Balasubramaniam, K.; Baade, P.D.; Youl, P.H.; Bain, C.J.; Allison, R.; Jordan, S.J. Increasing thyroid cancer incidence in Queensland, Australia 1982–2008—True increase or overdiagnosis. Endocrinol. (Oxf.) 2016, 84, 257–264, doi:10.1111/cen.12724.
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. Cancer Statistics Review, 1975–2017—SEER Statistics. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 13 July 2020).
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. SEER Cancer Statistics Review, 1975–2016. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 3 April 2020).
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 25, 716–759, doi:10.1089/thy.2014.0460.
- Jarza̧b, B.; Handkiewicz-Junak, D.; Włoch, J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: A qualitative review. Relat. Cancer 2005, 12, 773–803, doi:10.1677/erc.1.00880.
- Hogan, A.; Zhuge, Y.; Perez, E.; Koniaris, L.; Lew, J.; Sola, J. The incidence of pediatric thyroid cancer is increasing and is higher in girls than in boys and may have an adverse outcome. Thyroidol. 2009, 21, 10–12.
- Vaisman, F.; Corbo, R.; Vaisman, M. Thyroid Carcinoma in Children and Adolescents—Systematic Review of the Literature. Thyroid Res. 2011, 2011, 845362, doi:10.4061/2011/845362.
- Wu, X.-C.; Chen, V.W.; Steele, B.; Roffers, S.; Klotz, J.B.; Correa, C.N.; Carozza, S.E. Cancer incidence in adolescents and young adults in the United States, 1992–1997. Adolesc. Health 2003, 32, 405–415, doi:10.1016/S1054-139X(03)00057-0.
- Cordioli, M.I.; Moraes, L.; Cury, A.N.; Cerutti, J.M. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Relat. Cancer 2015, 22, R311–R324, doi:10.1530/ERC-15-0381.
- Tuttle, R.M.; Ball, D.W.; Byrd, D.; Dilawari, R.A.; Gerard, M.; Duh, Q.; Ehya, H.; Farrar, W.B.; Haddad, R.I.; Kandeel, F.; et al. Thyroid Carcinoma. Natl. Compr. Cancer Netw. 2010, 8, 1228–1274.
- Chan, C.M.; Young, J.; Prager, J.; Travers, S. Pediatric Thyroid Cancer. Pediatr. 2017, 64, 171–190.
- Zimmerman, D.; Hay, I.D.; Gough, I.R.; Goellner, J.R.; Ryan, J.J.; Grant, C.S.; McConahey, W.M. Papillary thyroid carcinoma in children and adults: Long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 1988, 104, 1157–1166.
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133, doi:10.1089/thy.2015.0020.
- Karapanou, O.; Tzanela, M.; Vlassopoulou, B.; Kanaka-Gantenbein, C. Differentiated thyroid cancer in childhood: A literature update. Hormones 2017, 16, 381–387, doi:10.14310/horm.2002.1758.
- Creo, A.; Alahdab, F.; Al Nofal, A.; Thomas, K.; Kolbe, A.; Pittock, S.T. Ultrasonography and the American Thyroid Association Ultrasound-Based Risk Stratification Tool: Utility in Pediatric and Adolescent Thyroid Nodules. Res. Paediatr. 2018, 90, 93–101, doi:10.1159/000490468.
- Hay, I.D.; Gonzalez-Losada, T.; Reinalda, M.S.; Honetschlager, J.A.; Richards, M.L.; Thompson, G.B. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J. Surg. 2010, 34, 1192–1202, doi:10.1007/s00268-009-0364-0.
- Zaydfudim, V.; Feurer, I.D.; Griffin, M.R.; Phay, J.E. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008, 144, 1070–1078, doi:10.1016/j.surg.2008.08.034.
- Ahn, B.H.; Kim, J.R.; Jeong, H.C.; Lee, J.S.; Chang, E.S.; Kim, Y.H. Predictive factors of central lymph node metastasis in papillary thyroid carcinoma. Surg. Treat. Res. 2015, 88, 63–68, doi:10.4174/astr.2015.88.2.63.
- Pawelczak, M.; David, R.; Franklin, B.; Kessler, M.; Lam, L.; Shah, B. Outcomes of children and adolescents with well-differentiated thyroid carcinoma and pulmonary metastases following 131I treatment: A systematic review. Thyroid 2010, 20, 1095–1101, doi:10.1089/thy.2009.0446.
- Handkiewicz-Junak, D.; Wloch, J.; Roskosz, J.; Krajewska, J.; Kropinska, A.; Pomorski, L.; Kukulska, A.; Prokurat, A.; Wygoda, Z.; Jarzab, B. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. Nucl. Med. 2007, 48, 879–888, doi:10.2967/jnumed.106.035535.
- Brito, J.P.; Davies, L. Is there really an increased incidence of thyroid cancer? Opin. Endocrinol. Diabetes Obes. 2014, 21, 405–408, doi:10.1097/MED.0000000000000094.
- Franceschi, S.; Vaccarella, S.; La Vecchia, C.; Bosetti, C.; Malvezzi, M.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer: An epidemic of disease or an epidemic of diagnosis? J. Cancer 2015, 136, 2738–2739, doi:10.1002/ijc.29311.
- Morris, L.G.; Tuttle, R.M.; Davies, L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol. Head Neck Surg. 2016, doi:10.1001/jamaoto.2016.0230.
- Sholl, L.M.; Barletta, J.A.; Hornick, J.L. Radiation-associated neoplasia: Clinical, pathological and genomic correlates. Histopathology 2017, 70, 70–80, doi:10.1111/his.13069.
- Cléro, É.; Doyon, F.; Chungue, V.; Rachédi, F.; Boissin, J.-L.; Sebbag, J.; Shan, L.; Bost-Bezeaud, F.; Petitdidier, P.; Dewailly, É.; et al. Dietary Iodine and Thyroid Cancer Risk in French Polynesia: A Case—Control Study. Thyroid 2012, 22, 422–429, doi:10.1089/thy.2011.0173.
- Engeland, A.; Tretli, S.; Akslen, L.A.; Bjørge, T. Body size and thyroid cancer in two million Norwegian men and women. J. Cancer 2006, 95, 366–370, doi:10.1038/sj.bjc.6603249.
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Cell. Endocrinol. 2016, doi:10.1016/j.mce.2016.10.027.
- Ron, E.; Lubin, J.H.; Shore, R.E.; Mabuchi, K.; Modan, B.; Pottern, L.M.; Schneider, A.B.; Tucker, M.A.; Boice, J.D. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Res. 1995, 141, 259–277, doi:10.2307/3579003.
- Sadetzki, S.; Chetrit, A.; Lubina, A.; Stovall, M.; Novikov, I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. Clin. Endocrinol. Metab. 2006, 91, 4798–4804, doi:10.1210/jc.2006-0743.
- Goldschmidt, H. Dermatologic Radiotherapy and Thyroid Cancer. Dermatol. 1977, 113, 362–364, doi:10.1001/archderm.1977.01640030108019.
- Wijnen, M.; van den Heuvel-Eibrink, M.M.; Medici, M.; Peeters, R.P.; van der Lely, A.J.; Neggers, S.J. Risk factors for subsequent endocrine-related cancer in childhood cancer survivors. Relat. Cancer 2016, 23, R299–R321, doi:10.1530/ERC-16-0113.
- Ishikawa, T. Radiation Doses and Associated Risk from the Fukushima Nuclear Accident. Asia Pac. J. Public Health 2017, 29, 18S–28S, doi:10.1177/1010539516675703.
- Yamamoto, H.; Hayashi, K.; Scherb, H.; Efird, J.T. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine 2019, 98, doi:10.1097/MD.0000000000017165.
- Toki, H.; Wada, T.; Manabe, Y.; Hirota, S.; Higuchi, T.; Tanihata, I.; Satoh, K.; Bando, M. Relationship between environmental radiation and radioactivity and childhood thyroid cancer found in Fukushima health management survey. Rep. 2020, 10, 1–12, doi:10.1038/s41598-020-60999-z.
- Mitsutake, N.; Fukushima, T.; Matsuse, M.; Rogounovitch, T.; Saenko, V.; Uchino, S.; Ito, M.; Suzuki, K.; Suzuki, S.; Yamashita, S. BRAFV600E mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: A different oncogenic profile from Chernobyl. Rep. 2015, 5, 16976, doi:10.1038/srep16976.
- Yamashita, S.; Suzuki, S.; Suzuki, S.; Shimura, H.; Saenko, V. Lessons from Fukushima: Latest Findings of Thyroid Cancer after the Fukushima Nuclear Power Plant Accident. Thyroid 2018, 28, 11–22, doi:10.1089/thy.2017.0283.
- Alzahrani, A.S.; Alkhafaji, D.; Tuli, M.; Al-Hindi, H.; Sadiq, B. Bin Comparison of differentiated thyroid cancer in children and adolescents (≤20 years) with young adults. Endocrinol. (Oxf.) 2015, 84, 571–577, doi:10.1111/cen.12845.
- Lee, Y.A.; Jung, H.W.; Kim, H.Y.; Choi, H.; Kim, H.-Y.; Hah, J.H.; Park, D.J.; Chung, J.-K.; Yang, S.W.; Shin, C.H.; et al. Pediatric Patients with Multifocal Papillary Thyroid Cancer Have Higher Recurrence Rates than Adult Patients: A Retrospective Analysis of a Large Pediatric Thyroid Cancer Cohort over 33 Years. Clin. Endocrinol. Metab. 2015, 100, 1619–1629, doi:10.1210/jc.2014-3647.
- Hay, I.D.; Johnson, T.R.; Kaggal, S.; Reinalda, M.S.; Iniguez-Ariza, N.M.; Grant, C.S.; Pittock, S.T.; Thompson, G.B. Papillary Thyroid Carcinoma (PTC) in Children and Adults: Comparison of Initial Presentation and Long-Term Postoperative Outcome in 4432 Patients Consecutively Treated at the Mayo Clinic during Eight Decades (1936–2015). World J. Surg. 2018, 42, 329–342, doi:10.1007/s00268-017-4279-x.
- Hogan, A.R.; Zhuge, Y.; Perez, E.A.; Koniaris, L.G.; Lew, J.I.; Sola, J.E. Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. Surg. Res. 2009, 156, 167–172, doi:10.1016/j.jss.2009.03.098.
- de Jong, M.C.; Gaze, M.N.; Szychot, E.; Rozalén García, V.; Brain, C.; Dattani, M.; Spoudeas, H.; Hindmarsh, P.; Abdel-Aziz, T.E.; Bomanji, J.; et al. Treating papillary and follicular thyroid cancer in children and young people: Single UK-center experience between 2003 and 2018. Pediatr. Surg. 2020, doi:10.1016/j.jpedsurg.2020.07.034.
- Rah, C.S.; Kim, W.W.; Lee, Y.M.; Kim, W.G.; Song, D.E.; Chung, K.W.; Kim, S.C.; Hong, S.J.; Sung, T.Y. Recent Trends in the Clinicopathological Features of Thyroid Nodules in Pediatric Patients: A Single Tertiary Center Experience over 25 Years. J. Endocrinol. 2019, 2019, doi:10.1155/2019/1829043.
- Niedziela, M. Pathogenesis, diagnosis and management of thyroid nodules in children. Relat. Cancer 2006, 13, 427–453, doi:10.1677/erc.1.00882.
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The diagnosis and management of thyroid nodules a review. JAMA J. Am. Med. Assoc. 2018, 319, 919–924.
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322, doi:10.1001/jamaoto.2014.1.
- Lerner, J.; Goldfarb, M. Pediatric Thyroid Microcarcinoma. Surg. Oncol. 2015, 22, 4187–4192, doi:10.1245/s10434-015-4546-8.
- Bernier, M.O.; Withrow, D.R.; Berrington de Gonzalez, A.; Lam, C.J.K.; Linet, M.S.; Kitahara, C.M.; Shiels, M.S. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 2019, 125, 2497–2505, doi:10.1002/cncr.32125.
- Golpanian, S.; Perez, E.A.; Tashiro, J.; Lew, J.I.; Sola, J.E.; Hogan, A.R. Pediatric papillary thyroid carcinoma: Outcomes and survival predictors in 2504 surgical patients. Surg. Int. 2016, 32, 201–208, doi:10.1007/s00383-015-3855-0.
- Chow, S.M.; Law, S.C.; Mendenhall, W.M.; Au, S.K.; Yau, S.; Mang, O.; Lau, W.H. Differentiated thyroid carcinoma in childhood and adolescence-clinical course and role of radioiodine. Blood Cancer 2004, 42, 176–183, doi:10.1002/pbc.10410.
- Liu, Z.; Hu, D.; Huang, Y.; Chen, S.; Zeng, W.; Zhou, L.; Zhou, W.; Wang, M.; Feng, H.; Wei, W.; et al. Factors associated with distant metastasis in pediatric thyroid cancer: Evaluation of the seer database. Connect. 2019, 8, 78–85, doi:10.1530/EC-18-0441.
- Dottorini, M.E.; Vignati, A.; Mazzucchelli, L.; Lomuscio, G.; Colombo, L. Differentiated thyroid carcinoma in children and adolescents: A 37-year experience in 85 patients. Nucl. Med. 1997, 38, 669–675.
- Kuo, S.-F.; Chao, T.-C.; Hsueh, C.; Chuang, W.-Y.; Yang, C.-H.; Lin, J.-D. Prognosis and Risk Stratification in Young Papillary Thyroid Carcinoma Patients. J. 2008, 55, 269–275, doi:10.1507/endocrj.K07E-127.
- Vaisman, F.; Bulzico, D.A.; Pessoa, C.H.; Bordallo, M.A.; de Mendonça, U.B.; Dias, F.L.; Coeli, C.M.; Corbo, R.; Vaisman, M. Prognostic factors of a good response to initial therapy in children and adolescents with differentiated thyroid cancer. Clinics 2011, 66, 281–286, doi:10.1590/S1807-59322011000200017.
- Fridman, M.V.; Savva, N.N.; Krasko, O.V.; Zborovskaya, A.A.; Mankovskaya, S.V.; Schmid, K.W.; Demidchik, Y.E. Clinical and Pathologic Features of “Sporadic” Papillary Thyroid Carcinoma Registered in the Years 2005 to 2008 in Children and Adolescents of Belarus. Thyroid 2012, 22, 1016–1024, doi:10.1089/thy.2011.0005.
- Pires, B.P.; Alves, P.A.G.; Bordallo, M.A.; Bulzico, D.A.; Lopes, F.P.; Farias, T.; Dias, F.; Lima, R.A.; Santos Gisler, I.C.; Coeli, C.M.; et al. Prognostic Factors for Early and Long-Term Remission in Pediatric Differentiated Thyroid Carcinoma: The Role of Sex, Age, Clinical Presentation, and the Newly Proposed American Thyroid Association Risk Stratification System. Thyroid 2016, 26, 1480–1487, doi:10.1089/thy.2016.0302.
- Cordioli, M.I.; Moraes, L.; Alves, M.T.; Delcelo, R.; Monte, O.; Longui, C.A.; Cury, A.N.; Cerutti, J.M. Thyroid-specific genes expression uncovered age-related differences in pediatric thyroid carcinomas. J. Endocrinol. 2016, 2016, doi:10.1155/2016/1956740.
- Poyrazoğlu, Ş.; Bundak, R.; Baş, F.; Yeğen, G.; Şanlı, Y.; Darendeliler, F. Clinicopathological characteristics of papillary thyroid cancer in children with emphasis on pubertal status and association with BRAFV600E mutation. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 185–193, doi:10.4274/jcrpe.3873.
- Hampson, S.; Stephens, D.; Wasserman, J.D. Young age is associated with increased rates of residual and recurrent paediatric differentiated thyroid carcinoma. Endocrinol. (Oxf.) 2018, 89, 212–218, doi:10.1111/cen.13720.
- Galuppini, F.; Vianello, F.; Censi, S.; Barollo, S.; Bertazza, L.; Carducci, S.; Colato, C.; Manso, J.; Rugge, M.; Iacobone, M.; et al. Differentiated Thyroid Carcinoma in Pediatric Age: Genetic and Clinical Scenario. Endocrinol. (Lausanne) 2019, 10, 1–11, doi:10.3389/fendo.2019.00552.
- Mazzaferri, E.L.; Massoll, N. Management of papillary and follicular (differentiated) thyroid cancer: New paradigms using recombinant human thyrotropin. Relat. Cancer 2002, 9, 227–247.
- Mazzaferri, E.L.; Kloos, R.T. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. Clin. Endocrinol. Metab. 2001, 86, 1447–1463, doi:10.1210/jcem.86.4.7407.
- Demidchik, Y.E.; Demidchik, E.P.; Reiners, C.; Biko, J.; Mine, M.; Saenko, V.A.; Yamashita, S. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Surg. 2006, 243, 525–532, doi:10.1097/01.sla.0000205977.74806.0b.
- Lazar, L.; Lebenthal, Y.; Steinmetz, A.; Yackobovitch-Gavan, M.; Phillip, M. Differentiated Thyroid Carcinoma in Pediatric Patients: Comparison of Presentation and Course between Pre-Pubertal Children and Adolescents. Pediatr. 2009, 154, 708–714, doi:10.1016/j.jpeds.2008.11.059.
- Van Santen, H.M.; Aronson, D.C.; Vulsma, T.; Tummers, R.F.; Geenen, M.M.; De Vijlder, J.J.; Van Den Bos, C. Frequent adverse events after treatment for childhood-onset differentiated thyroid carcinoma: A single institute experience. J. Cancer 2004, 40, 1743–1751, doi:10.1016/j.ejca.2004.03.006.
- Schneider, R.; Reiners, C. The Effect of Levothyroxine Therapy on Bone Mineral Density: A Systematic Review of the Literature. Clin. Endocrinol. Diabetes 2003, 111, 455–470.
- Fridman, M.; Krasko, O.; Branovan, D.I.; Dabryian, S.; Pisarenko, A.; Lo, C.Y.; Lam, A.K. yin Factors affecting the approaches and complications of surgery in childhood papillary thyroid carcinomas. J. Surg. Oncol. 2019, 45, 2078–2085, doi:10.1016/j.ejso.2019.07.032.
- Maxon, H.R. Quantitative radioiodine therapy in the treatment of differentiated thyroid cancer. J. Nucl. Med. 1999, 43, 313–323.
- Zidan, J.; Hefer, E.; Iosilevski, G.; Drumea, K.; Stein, M.E.; Kuten, A.; Israel, O. Efficacy of i131 ablation therapy using different doses as determined by postoperative thyroid scan uptake in patients with differentiated thyroid cancer. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1330–1336, doi:10.1016/j.ijrobp.2004.01.036.
- Haddad, R.I.; Nasr, C.; Bischoff, L.; Busaidy, N.L.; Byrd, D.; Callender, G.; Dickson, P.; Duh, Q.Y.; Ehya, H.; Goldner, W.; et al. Thyroid carcinoma, version 2.2018 featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2018, 16, 1429–1440, doi:10.6004/jnccn.2018.0089.
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid cancer in the pediatric population. Genes 2019, 10, doi:10.3390/genes10090723.
- Van Wyngaarden, M.; McDougall, I.R. What is the role of 1100 MBq (<30 mCi) radioiodine 131I in the treatment of patients with differentiated thyroid cancer? Med. Commun. 1996, 17, 199–207, doi:10.1097/00006231-199603000-00005.
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W.; Moreno-Reyes, R.; Van den Bruel, A.; Zira, C.; Feldt-Rasmussen, U.; et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. J. Endocrinol. 2006, 154, 787–803, doi:10.1530/eje.1.02158.
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. J. Med. 1994, 97, 418–428, doi:10.1016/0002-9343(94)90321-2.
- Schlumberger, M.; Pacini, F.; Wiersinga, W.M.; Toft, A.; Smit, J.W.A.; Franco, F.S.; Lind, P.; Limbert, E.; Jarzab, B.; Jamar, F.; et al. Follow-up and management of differentiated thyroid carcinoma: A European perspective in clinical practice. J. Endocrinol. 2004, 151, 539–548, doi:10.1530/eje.0.1510539.
- Biondi, B.; Fazio, S.; Carella, C.; Amato, G.; Cittadini, A.; Lupoli, G.; Saccà, L.; Bellastella, A.; Lombardi, G. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. Clin. Endocrinol. Metab. 1993, 77, 334–338, doi:10.1210/jcem.77.2.8345037.
- Matuszewska, G.; Roskosz, J.; Wloch, J.; Jurecka-Tuleja, B.; Hasse-Lazar, K.; Kowalczyk, B.; Jarzab, B. Evaluation of effects of L-thyroxine therapy in differentiated thyroid carcinoma on the cardiovascular system—Prospective study. Lek. 2001, 54, 373–377.
- Chow, S.M.; Yau, S.; Lee, S.H.; Leung, W.M.; Law, S.C.K. Pregnancy outcome after diagnosis of differentiated thyroid carcinoma: No deleterious effect after radioactive iodine treatment. J. Radiat. Oncol. Biol. Phys. 2004, 59, 992–1000, doi:10.1016/j.ijrobp.2003.12.023.
- Krassas, G.E.; Pontikides, N. Gonadal effect of radiation from 131I in male patients with thyroid carcinoma. Androl. 2005, 51, 171–175.
- Wichers, M.; Benz, E.; Palmedo, H.; Biersack, H.J.; Grünwald, F.; Klingmüller, D. Testicular function after radioiodine therapy for thyroid carcinoma. J. Nucl. Med. 2000, 27, 503–507, doi:10.1007/s002590050535.




